Full Length Research Paper
ABSTRACT
Identification of durum wheat variety adapted to drought environment is required to expand durum wheat cultivation to lowland areas in order to meet the growing demand of the crop for industrialization. The objective of the study was to evaluate and identify durum wheat genotypes tolerant to terminal drought, using morpho-agronomic traits. One hundred and forty four durum wheat (Triticum turgidium var durum) genotypes were grown in lattice design replicated twice under non-drought and drought stressed conditions, induced at anthesis stage at Debre-Zeit experimental station in 2017 during dry season. Analysis of variance showed that significant differences for all the traits, except days to heading and anthesis and between normal and stress conditions and also among studied genotypes as well as interaction effects of moisture environment and genotypes. Drought significantly affected reduction of all traits, except number of days to heading and spikelet number. In average, drought reduced grain yield (48.3%), grain filling period (41.7%), grain yield per spike (29.6%) and 1000 grain weight (18.3%) and number of kernels per spike (16.3%). LRPL-31, MCD-1-21 and ICA# showed superior performance under drought environment whereas Ude, the cultivated variety found the best under non-drought condition. Six genotypes, namely, 55, 30, 31, 91 15 and 58 were found among the top 10% high yielding genotypes and showed superior performance in both stress and non-stress conditions.
Key words: Anthesis, drought tolerance, drought stress, grain yield, Triticum turgdium.
INTRODUCTION
Wheat is the major important cereal crop after maize, tef and sorghum in terms of area coverage and production in Ethiopia. Both bread and durum wheats are extensively cultivated, in different agro-ecology and proportion in the country. Wheat is cultivated on over 1.69 million hectares with annual production of about 4.6 million tons (CSA, 2019). Although the crop is among the major food security crops and covers large cultivation area, its national productivity is about 2.74 tones ha-1 (CSA, 2019) which is below the world average (3.2 tons/ha).
Agricultural system in Ethiopia is rain fed and characterized by uneven distribution and uncertainty during reproductive stage, leading to terminal drought stress which results in low productivity. Wheat production mainly concentrated in the highlands, where rainfall is high and wheat has disease and quality problems besides competing with other crops. Thus, the need of producing addition wheat has become the major critical concern to meet the continuous increasing demand in the country. The expansion of wheat production to lowland environment is limited by lack of tolerant wheat varieties developed for drought in the target area. Drought is one of the most common environmental stresses that limit durum wheat production in much wheat growing environment. Drought usually affects plant growth and development at different growth stages leading to crop losses (Acevedo et al., 2009; Akram, 2011; Arash, 2013). Several studies proved that greater yield reduction occurred during the reproductive stage (Jinmeng et al., 2018) than vegetative stages. Nimai et al. (2019) reported the mean yield advantage of 28 to 37% higher on drought tolerance varieties than drought sensitive wheat during reproductive stages. The reduction of grain growth period from 39 to 33 days after anthesis illustrated by Abdul Karim et al. (2000) also proved that evaluation of wheat genotypes would be better for identification of the right genotypes for drought stress. Genetic variability, correlation on quantitative traits and drought effect on wheat yield and agronomic traits was reported by several authors. Drought affects both grain and biomass yield (Ameer et al., 2009; Garcia et al., 2003; Leilah and Al-Khateeb, 2005; Khan et al., 2010), photosynthesis translocation and partitioning (Muhammad et al., 2014; Wenhui et al., 2020), number of kernels and kernel weight (Simane et al., 1993; Solomon et al., 2003). Furthermore, Campos et al. (2004) illustrated the importance of field evaluation as the best methods for identifying drought tolerance under rain-fed and irrigation condition and would help screen the genotypes uniformly to terminal drought during the reproductive stages.
While wheat germplasm has been developed for drought affected areas in other areas of the globe, very little effort has been directed to develop or adapt wheat to drought in the Ethiopian lowlands by the local breeding programs.
Based on the actual knowledge status it was assumed that deployment of large number of collections from different sources and evaluation for drought stress under field condition to be an effective breeding strategy for the development of tolerant genotypes targeting the lowlands drought prone environment is critical for expanding wheat area and production.
The purpose of the study was undertaken to assess the variation in durum wheat genotypes for terminal drought stress and identify lines to be used in the future breeding program.
MATERIALS AND METHODS
Description of experimental site
The field trial was conducted at Debre-Zeit Agricultural Research Center (DZARC) located at 8° 41’36” latitude and 39° 03’17’ longitude with altitude of 1880 m above sea level (masl). The station categorized as mid highland in sandy clay soil can potentially represent the lowland wheat growing environments of Ethiopia. According to the data obtained from the Agricultural and Nutritional Research Laboratory of DZARC (2018), the soil of the experimental site is characterized by sandy clay texture with pH of 7.3, and organic carbon, total N, electrical conductivity and soil cation exchange capacity of 1%, 0.08%, 0.12 Ds/m and c100 meq/100 g of soil, respectively.
Experimental genotypes
One hundred and forty four durum wheat genotypes were used in the study. Global wheat collections developed for dry environment, landraces collected from different parts of the country and obtained from Ethiopian biodiversity institute, local breeding lines and improved cultivars were included (Table 1). The results for 90 of 144 genotypes tested were presented since some of the genotypes did not adapt to the stress environment during the season.
Experimental design and trial management
The plant materials were grown from January 12 to May 24, 2017 during the dry season. The genotypes were arranged in 12 ×12 simple lattice designs with two replications. Each genotype was grown in two rows of 2.5 m length and 0.20 m width with total plot area of 1 m2. Seeding rate and planting date were used as per the recommendation. During seeding 50 kg/ha urea (46% N) as N source and 100 kg/ha DAP (46% P2O5) as source of phosphorus were applied. At the beginning of tillering, the remaining 50 kg/ha urea (46% N) was applied by top dressing. To reduce the influence of biotic factors under both conditions, weeds were controlled manually as per needed and tilt-250 with rate of 150 ml/ac fungicides were sprayed twice during the season to prevent the genotypes from stem and leaf rust infections.
Moisture treatment
The stress environment was created by growing the genotypes during the dry season when no or very limited rainfall is expected. Seeds were sown on January 12 and harvest was done on May 15 during which small amount of rain were received in very few days. Since drought stress is the only effect examined on genotypes, all the crop management practices followed was the same. Under both drought and non-stressed conditions, furrow conventional irrigation method was supplied every day to the genotypes. Soil moisture depletion was detected using gravimetric methods.
In the drought stress treatment, genotypes were fully irrigated every five days until 50% of genotypes headed and then irrigation was stopped until physiological maturity. The genotypes in non-drought condition were fully watered using furrow irrigation every five days until physiological maturity. Irrigation was applied when the soil moisture depletion was reduced to about 75% field capacity during the growing period.
Data collection
The plants were measured for the following traits: (ii) Days to heading (DH), taken as the number of days from sowing until 50% of the plants in the plot have at least one emerged spike; (ii) Days to maturity, based on number of days from sowing to physiological maturity of at least 90% of the plants in plot; (iii) Grain filling period was computed by subtracting the number of days to heading from the number of days to maturity; (iv) Grain filling rate was determined as the ratio of final grain yield to the days from anthesis to physiological maturity; (v) Plant height was also measured from five randomly selected plants per plot and the average were recorded; (vi) Flag-leaf length (FL), measured at heading on five random samples taken from each genotype; (vii) Number of spikelet per spike and number of grains per spike, from the average grain number in ten spikes taken from random plants in the plots; (ix) Number of spikelet per spike, from the average number of spikelet in ten spikes taken from random plants in the plots; (x) 1000 seed weight, from the average weight of 100 grain samples multiplied by 10; (xi) Grain yield was measured from net plot area of 0.8 m2, after drying and cleaning of grain and adjusted to approximately to 12.5% moisture content; (xii) Above ground biomass determined by measuring dried above ground biomass from net plot area of 0.8 m2; (xiii) Straw yield per plot determined by subtracting grain yield from dried above ground biomass; (xiv) Harvest index determined as the proportion of grain yield to the overall aboveground biomass; and (xv) Grain weight per spike was taken from ten randomly selected spikes.
Estimation of traits due to drought stress effect in non-drought and to drought was calculated by percent reduction percentage (%R) using the formula where the means for each genotype under stress and non-stress conditions.
Data analysais
The Statistical Analysis System (SAS) version 9.1 (SAS, 2002) was employed for individual and combined variance and means comparisons. Homogeneity of error variances between drought and non-drought was made before the combined analysis of variance was carried out using F max test according to Gomez and Gomez (1984).
RESULTS AND DISCUSSION
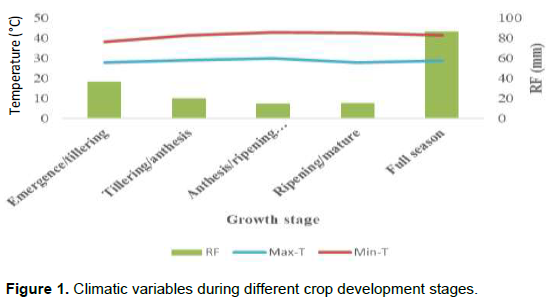
Rainfall and temperature recorded (Figure 1) during the cropping season showed the characteristic of the conditions in which the genotypes were screened. There were no extreme records and large differences in both minimum and maximum mean temperatures during the cropping season indicating that drought was the only effect the genotypes experienced. The total rainfall amount and distribution differed in different crop development stages. The amount of rainfall received and the number of rainy days were only 86.6 mm and 11, respectively. The number of drought days from anthesis to ripening (hard dough stage) was about 42 days. Thus screening for drought stress was managed very well and drought intensity reached about 42% and high enough to disorder the rank of the genotypes under stress and non- stress conditions.
Analysis of variance
The main effect variance analysis for the drought condition revealed significant effect of genotypes for the grain filling rate, plant height, number of kernels per spike, thousand grain weight and grain yield. Under non-drought, there was significant effect for the grain yield thousand seed weight and grain filling rate (Table 2). The combined analysis of variance was conducted based on F max test, which uses the error variance as homogenous when the ratio of the error mean squares is not greater than 7 (Gomes, 2000).
The combined analysis of variance showed significant differences at 0.01% for the environment for all traits studied except days to heading and days to anthesis indicating that drought stress affected the expression of traits. The genotypes effect also was significant for all traits. The results showed that genotypes differed in response of drought treatment. The interaction effects of genotypes and moisture environment for all traits were also detected except for days to anthesis (Table 3).
Response of genotypes to drought
An interaction between durum wheat and drought stress treatment on grain wheat was observed indicating that the existence of genetic variation among the genotypes and water deficit is the limiting factor for the experiment. The genotypes produced different yield across the drought treatments and the analysis was carried out independently for stress and non-stress environments.
Under drought condition, the two top yielding genotypes were the landraces LRPL-31 and MCD-1-21 followed by the exotic genotype ICA#73 but these are not significantly different from the standard check variety Ude (Table 4). These two landraces displayed the highest grain yield and produced about 10.3 and 22.2% mean yield advantages over Ude and Assasa (cultivars for drought stress environment), respectively. This implies that exploring locally existed collection probably have stress related adaptive traits than exotic germplasm.
Similar results were reported by Ayed et al. (2021) in 11 durum wheat evaluated under rain fed and irrigated conditions in Tunisia to determine the extent of drought tolerance. Similarly, ICA#73, an exotic genotype, developed for dry environment was found high yielding than the cultivated variety (Ude). These genotypes also showed superior performance under irrigated condition indicating that their ability to make use of available water to increase grain yield. Similar findings indicated the existence of genetic variability between durum genotypes for grain yield and yield components on durum wheat (Ayed et al., 2021; Blum et al., 2001; Solomon et al., 2003a; Simane et al., 1993; Bogale and Tesfaye, 2016) and bread wheat (Habtamu et al., 2016; Desalign et al., 2001; Sial et al., 2010).
Grain yield of genotypes under non-stress conditions are shown in Table 5. The best genotypes in non-drought condition were Ude, semi dwarf cultivated varieties followed by 203762 and MCD-1-21. In addition to non-drought condition, these genotypes respond similarly under stress environment and could be used as source of gene for drought stress breeding program.
Effect of drought on yield and agronomic traits
The mean minimum and maximum values and the percentage reduction of traits mean in non-stress and stress condition are shown in Table 6. Drought affected most of the traits studied and showed the highest effect on grain yield, grain filling rate, grain yield per spike, grain weight and kernel number per spike which reduced 48.3, 41.7, 29.6, 18.8 and 16.3%, respectively.
Drought showed an average reduction of grain yield of 2.17 tons/ha (48.3%) compared to non-drought stress condition. Grain yield per spike was between 0.91 and 2.45 g with a mean of 1.59 g for non-stress and from 0.59 to 1.85 g with a mean of 1.12 g for stress. The mean of grain yield per spike was 29.6% higher in the non-stress than the stress environment. Genotype 59 and 13 had the highest and the lowest grain yield per spike, respectively in non-stress, whereas genotype 118 and 128 showed the highest and the least in the stress condition in the respective order. The grain yield reduction due to drought was comparable to the works of Darzi-Ramandi et al. (2016) and Sahar et al. (2016) who reported a 49 9 and 42% yield reduction, respectively. The probable reasons for significant effect of severe drought stress on grain yield is associated to photosynthesis, translocation and partitioning of carbohydrate reserves leading to decrease in production. Drought caused 18.8% reduction of 1000 grain weight. Genotype 117 showed the highest 1000 seed weight and genotype 13 the lowest in non-stress condition. Genotype 33 and 43 showed the highest and the lowest 1000 seed weight, respectively in the stress environment.
Grain filling rate was also highly affected by stress with reduction of about 41.7% compared to non-stress. Grain filling period ranged from 3.7 (Genotype 121) to 18.5% (Genotype 30), which was among the highest yielding genotypes under stress. Similarly, the lowest grain filling period was obtained from genotypes 35 and 79 which gave the maximum record under non-drought stress.
The reduction in performance due to drought stress observed was consistent with the previous work in wheat that drought stress induced from anthesis to maturity resulted in remarkable reduction in yield and yield related traits (Solomon et al., 2003; Bogale and Tesfaye, 2016). Terminal drought shortens grain filling duration and grain filling rate compared to non-stress (Table 6). This result is supported by Muhammad et al. (2014) who reported that grain filling rate under drought affected due to reduced photosynthesis, accelerated leaf senescence, and sink limitations. Similarly, Mahpara et al. (2018) emphasized that drought after heading results in reduced grain weight by shortening the time between fertilization and maturity.
Pearson correlation analysis
The Pearson correlation coefficient between grain yield and yield related traits under drought and non-drought are shown in Table 7. Grain yield was negatively correlated with days to heading, anthesis date and days to maturity but it showed a non-significant and positive association with grain filling duration although the association was weak under moisture stress.
Grain yield showed a positive and significant correlation with plant height under both non-moisture and moisture stress conditions. Thousands kernel weight, kernel number, above ground biomass, harvest index, kernel number per spikelet and grain weight per spike had similar trend and they were positively and significantly correlated with grain yield under both moisture stress and non-moisture stress conditions. These findings were consistent with the Khan and Naqvi (2012) works of previous authors (Solomon et al., 2003; and Simane et al., 1993) suggesting that the supply of metabolites to grain development through dry matter reallocation would be expected under moisture stress.
The correlation of grain filling rate with grain yield was strongly positive and highly significant at 0.01% under stress (0.83) than non-stress (0.41) indicating that indirect selection for improving grain yield through these traits would be effective under terminal stress environment. The result is consistent with the work of Bogale and Tesfaye, (2016). Grain filling rate was significant at 0.01% and had strong positive association with grain yield (0.41) than grain filling period (0.38). Similarly, Dias and Lindon (2009) indicated strong relationship between grain yield and grain filling rate and the relative advantage of grain filling rate than the duration of grain filling period in increasing the rate of photosynthate translocation to grains as one of the mechanisms to confer stress tolerance to wheat. Therefore, it is likely to predict that genotypes 30 and 31 with high grain filling rate performed better than the other genotypes. These results reflected that the two traits probably could be genetically improved separately or indirectly. The correlation of thousand seed weight and grain number was significant at 0.05%, with values 0.25 and 0.22, respectively. The moderate response of thousand seed weight of wheat genotypes for genetic and environmental factor was presented by Soares et al. (2021). The negative association between grain yield and these phenological traits found in this study were similarly reported by authors (Gonzalez et al., 2007) indicating the importance of earliness as drought tolerance mechanism. f earliness as drought Spikelet number per spike showed positive but non-significant correlation with grain yield under non-moisture stress while it showed a negative and significant association with grain yield under stressed condition. This could be due to the fact that moisture stress induced after the plants reached its maximum growth probably influence grain number on wheat. Khan and Naqvi (2012) spikelet numbers and grains number may be used as an effective selection criterion for increasing grain yield of wheat under different moisture levels. Similarly, Dorion et al. (1996) reported no or little effect of drought on the number of spikelet per spike.
Path coefficient analysis
The correlations were analyzed further by the path coefficient technique, which partition the correlation coefficient into direct and indirect effects via alternative traits. Grain yield was performed and influenced by different traits. The direct and indirect effects of grain yield traits under non-stress and stress conditions are shown in Tables 8 and 9, respectively. The path coefficient analysis showed that the direct effect of grain filling rate on grain yield under both non-stress and stress condition were very high and positive (0.97) and (0.76) in respective order. The strong and positive correlation of grain filling and grain yield and its importance to be used as good selection trait than duration (Jones et al., 1979). This indicates that there were little or no indirect effects of these traits and the relationship between grain yield and grain filling rate was direct under non-stress and stress environments. Singh and Chaudhary (1979) suggested that if the correlation coefficient between a causal factor and the effect is almost equal to its direct effect, the correlation explains the true relationship and direct selection through this trait is effective. Ashene and Kinde (2016) also obtained similar result on durum wheat suggesting that selection of wheat genotypes based on grain filling rate under non-stress environment would be beneficial for increasing wheat grain yield.
CONCLUSIONS
Drought caused significant reduction in yield and its related traits. Grain yield reduction reached about 48.3% due to the effect of drought. Grain filling rate was also highly affected by drought stress. LRPL-31, MCD-1-21 and ICA#73 are potentially useful and could be considered as source of genes to improve drought tolerance in the breeding program whereas the released cultivar Ude would be utilized for irrigated as well as good rainfall environment. Genotypes 55, 30, 31, 91 15 and 58 were identified among the top 10% high yielding genotypes and showed superior performance in both stress and non-stress environments.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors thank the Ethiopian Institute of Agricultural Research (EIAR), Agricultural Growth Program II (AGP II) and International Center for Agricultural Research Institute (ICARDA) for providing technical and financial supports for the execution of the field experiment. The authors also acknowledged the support provided by Wondo-Genet Research Center and Debre-Zeit Research Center. Research and technical staff members of the crop research process of Debre-Zeit Research Center are highly acknowledged for assisting the field activities.
REFERENCES
|
Acevedo E, Silva P,Silva H (2009). "Wheat Growth and Physiology," FAO. Corporate Repository pp. 1-24. |
|
|
Akram M (2011). Growth and Yield Components of Wheat under Water Stress of Different Growth Stages. Bangladesh Journal of Agricultural Research 36(3):455-468 |
|
|
Ameer AM, Markh GS, Mahar AR, Abro SA and Kanhar NA (2009). Effect of Water Stress on Yield and Yield Components of Wheat (Triticum aestivum L) Varieties. Pakistan Journal of Botany 41(3):1301-1310. |
|
|
Arash N, Zakaria HP, Golam F (2013). Drought Tolerance in Wheat. Available at the Scientific World Journal 12 p |
|
|
Bogale A, Tesfaye K (2016). Relationship between grain yield and yield components of the Ethiopian durum wheat genotypes at various growth stages. Tropical and Sub-tropical Agro-ecosystems 19(1):81-91. |
|
|
Ayed S, Othmani A, Bouhaouel I, Teixeira da Silva JA (2021). Multi-Environment Screening of Durum Wheat Genotypes for Drought Tolerance in Changing Climatic Events. Agronomy 11(5):875. |
|
|
Blum A (1996). Crop response to drought and interpretation of adaptation. Plant Growth Regulation 20(2):135-148. |
|
|
Cattivelli L, Rizza F, Badeckc F, Mazzucottelli W, Mastrangelo, E Franciaa AM.,Marca E Tondellia A, Stanca AM (2008). Drought tolerance improvement in crops plants: An integrated view from breeding to genomics. Field Crops Resource 105(1-2):1-14 |
|
|
Campos H, Cooper M, Habben JE, Edmeades GO, Schussler JR (2004). Improving drought tolerance in maize: a view from industry. Field Crops Research 90(1):19-34. |
|
|
Central Statistical Agency (CSA) (2019). "Agricultural Sample Survey 2018/19, Area and Production for Major Crops. Private Peasant Holdings," Vol. 1, Statistical Bulletin. |
|
|
Darzi-Ramandi H, Najafi-Zarini H, Shariati VJ, Razavi K, Kazemitabar SK (2016). Screening Iranian bread wheat lines under different water regimes using yield based drought tolerance indices. SABRAO Journal of Animal Breeding and Genetics 48(4):491-503. |
|
|
Desalign D, Bededa G, Zewdie A, Solomon G (2001). Drought Tolerance of Some Bread Wheat Genotypes in Ethiopia. Africa Crop Science Journal 9(2):385-392. |
|
|
Dias AS, Lidon FC (2009). Evaluation of grain filling rate and duration in bread and durum wheat under heat stress after anthesis. Journal of Agronomy and Crop Science 195(2):137-147. |
|
|
Dorion S, Lalon DES, Saini HS (1996). Induction of male sterility in wheat (Tritcum aestivum L.) by meiotic stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology 111(1):137-145. |
|
|
Fischer RA, Maurer R (1978). Drought resistance in spring wheat cultivars: I. Grain yield response, Australian Journal of Agricultural Research 29(5):897-907. |
|
|
Garcia L, Moral F, Rharrabti Y, Villegas D, Royo C (2003). Evaluation of Grain Yield and its Components in Durum wheat under Mediterranean Conditions: An Ontogenic Approach. Agronomy Journal 95(2):266-274. |
|
|
Guilherme Filgueiras Soares Walter Quadros Ribeiro Júnior Lucas Felisberto PereiraCristiane Andréa de Lima Daiane dos Santos Soares Onno Muller Uwe Rascher Maria Lucrecia Gerosa Ramos. |
|
|
Soares GF, Ribeiro WQ, Pereira LF, Lima CA, Soares DD, Muller O, Rascher U, Ramos ML (2020). Characterization of wheat genotypes for drought tolerance and water use efficiency. Crop Science (Piracicaba, Braz.) 78(5). (2021). |
|
|
Gomez K, Gomez A (1984). Statistical Procedures for Agricultural Research 2nd edition, John Wiely and Sons inc., New York. |
|
|
Gonzalez A, Isaura M, and. Luis A (2007). Response of genotypes to terminal soil moisture stress: phenology, growth and yield. Australian Journal of Agricultural Research 58(1):29-37. |
|
|
Habtamu A, Tadesse D, Liu H, Yan G (2016). Performance of Ethiopian bread wheat (Triticum aestivum L.) genotypes under contrasting water regimes: potential sources of variability for drought resistance breeding. Australian Journal of Crop Science 10(3):370-376. |
|
|
Jinmeng Z, Shiqiao Z, Min C , Hong J Xiuying Z , Changhui P , Xuehe Lu Minxia Z and Jiaxin J (2018). Effect of Drought on Agronomic Traits of Rice and Wheat: A Meta-Analysis. International Journal of Environmental Research and Public Health 15:839, |
|
|
Jones DB, Peterson ML Geng S (1979). Association between Grain Filling Rate and Duration and Yield Components in Rice. Available: |
|
|
Karim Md A, Hamid A, and Rahman S 2000. Grain Growth and Yield Performance of Wheat under Subtropical Conditions: II. Effect of Water Stress at Reproductive Stage Cereal Research Communications 28(1):101-17 |
|
|
Khan N, Naqvi FN (2012). Correlation and Path Coefficient Analysis in Wheat Genotypes under Irrigated and Non-Irrigated Conditions. Asian Journal of Agricultural Sciences. ISSN : 2041-3890 |
|
|
Khan AJ, Azam F, Ali A (2010). Relationship of morphological traits and grain yield in recombinant inbred wheat lines grown under drought conditions. Pakistan Journal of Botany 42(1):259-267. |
|
|
Koocheki A, Yazdansepas RA, Mahmadyorov U, Mehrvar MR (2014). Physiological-based Selection Criteria for Terminal Drought in Wheat (Triticum aestivum L.) Journal of Agriculture, Science and Technology 16(5):1043-1053 |
|
|
Kwabena D, Daniel A, Hussein M, Asrat A, Blair MW (2016). Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought adaptation in Ethiopia. The crop Journal 4(5):367-376. |
|
|
Leilah AA, Al-Khateeb SA (2005). Statistical Analysis of Wheat Yield under Drought Conditions .Journal of Arid Environments 61(3):483-496. |
|
|
Mahpara F, Ahmed Z, Aslam M, Zaynab M (2018). Drought Effect and Tolerance Potential of Wheat: A Mini Review. International Journal of Nanotechnology and Allied Sciences 2(2):16-21. |
|
|
Muhammad F, Hussain M, Kadambot H, Siddique M (2014). Drought Stresses in Wheat during Flowering and Grain-filling Periods. Critical Reviews in Plant Sciences 33(4). |
|
|
Nimai S, Pierre S, Matthew JP, Mikhail AS (2019). Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe.Journal of Experimental Botany 70(9):2549-2560, |
|
|
Plaut Z., Butow CW Blumenthal CS, Wrigley CW (2004). Transport of dry matter developing wheat kernels and contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Research 86(2-3):185-198. |
|
|
Reza M, Abdolvahab A (2017).Evaluation of Durum Wheat Genotypes based on Drought Tolerance Indices under different levels Drought Stress. Journal of Agricultural Sciences 62(1):1-4. |
|
|
SAS Institute Inc. 2004. SAS/STAT ® 9.1 User's Guide. Cary, NC: SAS Institute Inc. |
|
|
Sahar B, .Nasserlehaq N, Ahmed B, Hassan O, Wuletaw Tadesse (2016). Effective Selection criteria for screening drought tolerant and high yielding bread wheat genotypes Universal Journal of Agricultural Research 4(4):134-142. |
|
|
Simane B, Streak PC, Nachit MM, Peacock JM (1993). Ontogenetic analysis of yield and yields components and yield stability of durum wheat in water limited environments Euphytica 71(3):211-219. |
|
|
Sial MA, Mohammed UD, Mangrio SM, Simair AA (2010). Screening of Wheat Genotypes for Water Stress Tolerance. Pakistan Journal of Biotechnology 7(1):137-143. |
|
|
Singh RK, Chaudhary BD (1979). Biometrical methods in quantitative genetic analysis.Biometrical methods in quantitative genetic analysis. |
|
|
Solomon KF, Labuscagne MT, Bennie ATP (2003). Responses of Ethiopian Durum Wheat (Triticum turgidum var durum L.) Genotypes to Drought Stress, South African Journal of Plant and Soil 20(2):54-58. |
|
|
Vahamidis K, Andreas J, Economou G (2019). Grain number determination in durum wheat as affected by drought stress: An analysis at spike and spikelet level. Annals of Applied 174(2):190-208. |
|
|
Zhao W, Liu L, Shen Q, Yang J, Han X, Tian F, Wu J(2020) Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 12(8):2127. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0