ABSTRACT
 The present study demonstrated the use of various PGR combinations for efficient in vitro regeneration of cv. kufri jyoti in kumaun hills. Best callus induction and proliferation was observed in MS medium supplemented with 13.59 µM 2,4-D alone and 2,4-D + kinetin (9.06 + 1.16 µM) out of different concentrations of 2,4-D (4.53 to 18.12 µM) alone and 2,4-D (0 to 18.12 µM) with kinetin (1.16 µM). Leaf explants were more efficient in producing callus as compared to internodes. Medium supplemented with BA + GA3 (8.88 µM + 1 µM) initiated shoot induction out of various combinations of BA (4.44 to 13.22 µM) and GA3 (1 µM) after 7 days of incubation with significantly high average number of shoots, average shoot length and average number of leaves per explant. MS medium supplemented with different concentrations of zeatin (4.56, 9.12 and 13.68 µM) with IAA (5.71 µM) and GA3 (8.49 µM) was tried for direct regeneration of shoots through nodes out of which zeatin + IAA + GA3 (13.68 µM + 5.71 µM + 8.49 µM) served to be the best combination and the raised plantlets were found to produce microtubers in a period of 8 to 10 weeks. 2.45 µM IBA in full strength basal MS medium induced highest number of roots. In addition to an efficient regeneration protocol, the microtuber production was also studied in the present piece of work. The research protocol may also be utilized for Agrobacterium tumefaciens mediated transformation towards the biotic and abiotic stress tolerant potato crop.
The present study demonstrated the use of various PGR combinations for efficient in vitro regeneration of cv. kufri jyoti in kumaun hills. Best callus induction and proliferation was observed in MS medium supplemented with 13.59 µM 2,4-D alone and 2,4-D + kinetin (9.06 + 1.16 µM) out of different concentrations of 2,4-D (4.53 to 18.12 µM) alone and 2,4-D (0 to 18.12 µM) with kinetin (1.16 µM). Leaf explants were more efficient in producing callus as compared to internodes. Medium supplemented with BA + GA3 (8.88 µM + 1 µM) initiated shoot induction out of various combinations of BA (4.44 to 13.22 µM) and GA3 (1 µM) after 7 days of incubation with significantly high average number of shoots, average shoot length and average number of leaves per explant. MS medium supplemented with different concentrations of zeatin (4.56, 9.12 and 13.68 µM) with IAA (5.71 µM) and GA3 (8.49 µM) was tried for direct regeneration of shoots through nodes out of which zeatin + IAA + GA3 (13.68 µM + 5.71 µM + 8.49 µM) served to be the best combination and the raised plantlets were found to produce microtubers in a period of 8 to 10 weeks. 2.45 µM IBA in full strength basal MS medium induced highest number of roots. In addition to an efficient regeneration protocol, the microtuber production was also studied in the present piece of work. The research protocol may also be utilized for Agrobacterium tumefaciens mediated transformation towards the biotic and abiotic stress tolerant potato crop.
Key words: In vitro, potato, callus, direct regeneration, microtubers.
Abbreviation:
ANOVA, analysis of variance; BA, benzyl adenine; GA3, gibberellic acid; IAA, indole acetic acid; MS, Murashige and Skoog (1962); PGR, plant growth regulators; 2,4-D, 2,4-dichlorophenoxy acetic acid.
Potato (Solanum tuberosum L.) is one of the world’s most economically important tuber crop belonging to the family Solanaceae. It plays an important role in the food chain, as it ranks 4th in importance after rice, wheat and maize (Solomon and Barker, 2001). Potato is a good, cheap source of carbohydrates, vitamins, minerals and proteins. It has multipurpose use in daily consumption and also industrial purpose (Hoque, 2010). cv. Kufri jyoti is well adapted to North and South Indian hills, parts of Bihar, Gujarat, Karnataka, Madhya Pradesh, Maharashtra, Punjab, Uttar Pradesh and West Bengal. It persists medium to long tuber dormancy, low storage losses and medium to high tuber dry matter. Appropriate combinations and concentrations of PGR in the culture media are required for rapid plant regeneration from explants (Ehsanpour and Jones, 2000a). In vitro regeneration of potato is easily done from different explants on MS medium supplemented with different PGR for diseases free good quality seeds and pathogen free planting materials (Hossain, 1994; Rabbani et al., 2001; Zaman et al., 2001). Successful in vitro plant regeneration of potato has been achieved from explants of different organs and tissues of potato such as leaf, stem, tuber discs and unripe zygotic embryos (Shirin et al., 2007). The success of plant biotechnology relies on several factors which include an efficient tissue culture system for regeneration of plants from cultured cells and tissues (Khalafalla et al., 2010). Tissue culture based potato multiplication has successfully been incorporated in high quality potato seed production programme (Srivastava et al., 2012).
Microtubers (in vitro developed tubers) are miniature seed potatoes and they represent an intermediary phase between in vitro plantlets and minitubers. The use of microtubers in storage and exchange of germplasm and seed potato production is advantageous (Seabrook et al., 1993; Rannali et al., 1994). Microtubers are the first generation of potato seed from tissue culture, being used to solve the problems of transplanting the plantlets from in vitro to in vivo conditions. They can be planted directly in the soil and they can be produced in any period of the year (Nistor et al., 2010).
Considering the main problems of potato cultivation in hills of Uttarakhand including biotic and abiotic stress, lack of seed agency who provide the quality seed potato and lack of technology intervention, the present study was undertaken to develop efficient protocol for in vitro regeneration of cv. kufri jyoti in kumaun hills directly through nodes and via callus using leaf and internodes as explants. This protocol may serve as a highly useful technique for crop improvement through Agrobacterium tumefaciens mediated transformation via rapid multiplication of plantlet production as well as virus free seed potatoes or microtuber formation.
Plant material
cv. Kufri jyoti was obtained from Government Breeding Garden, Kashipur, Uttarakhand and grown in pots (20 × 15 cm) containing soil and farmyard manure in a ratio of 3:1 over a period of 10 to 15 days. All the explants were taken from these donor plants for the present research work. Explants such as juvenile leaf, nodes and internodes were initially washed with Tween-20 and then with distilled water 3 to 4 times to remove the traces of the chemical applied. Thereafter they were treated with bavistin (fungicide) solution (0.5%, 15 min) to avoid fungal contamination. For surface sterilization explants were subjected to HgCl2 (0.1%, 1 min) and thoroughly washed with distilled water for 2 to 3 times under laminar airflow. Leaves were dissected into small pieces and trimmed, nodes and internodes were cut into small pieces (approx. 5 mm). After a quick dip in 70% alcohol explants were then washed with sterile distilled water.
Preparation of culture media and growth condition
Murashige and Skoog medium (1962) was used with 3% sucrose and solidified with 0.7% agar. For root development clarigel (0.24%) was used as solidifying agent for clear analysis. Plant growth regulators used were 2,4-D, zeatin, kinetin, IAA, GA3, BAP and IBA. All experiments were carried out in 250 ml jam bottles /flasks containing 50 ml of culture medium. The pH of media was adjusted to 5.8 using 1N NaOH prior to autoclaving at 121°C at 15 lbs pressure for 20 min. Cultures were incubated under 16 h photoperiod with photosynthetic photon flux density of 40 μ mol m-2 s-1 fluorescent lamps.
Callus induction and shoot regeneration
For callus induction juvenile leaf sections and internodes with cut ends were placed on MS medium with different concentrations of PGR like 2,4-D (4.53 to 18.12 µM) alone and 2,4-D (0 to 18.12 µM) with kinetin (1.16 µM). Callus initiated after 15 to 20 days of incubation. Calli were subcultured in every 15 days. Well differentiated calli were placed on MS medium supplemented with various combinations of BAP (4.44 to 13.22 µM) and GA3 (1 µM) for shoot regeneration. All cultures were maintained at 25 ± 2°C with 16 h photoperiod. Shoot regeneration initiated in 7 days.
Direct regeneration of shoots
For direct regeneration of shoots explants taken were nodes. Explants were cut into small sections of 2 to 5 mm size and inoculated in the MS medium supplemented with different concentrations of zeatin (4.56, 9.12 and 13.68 µM) with IAA (5.71 µM) and GA3 (8.49 µM) for shoot multiplication. Cultures were kept at 25 ± 2°C with 16 h photoperiod. Shoot induction initiated in 3 to 4 days of incubation.
Regeneration of roots and development of elite plantlets
When shoots grew upto a height of 3 to 4 cm, they were aseptically removed, separated from each other and subcultured on half and full strength MS medium with varying concentrations of IBA for root induction. Root development initiated after 4 to 5 days of incubation. The completely rooted plants (2 to 3 weeks) were taken out carefully and gently washed under running water to remove excess clarigel. They were then potted in thermocole cups (12 × 8 cm) containing soil and farmyard manure (3:1, v/v); covered with transparent polythene bags with small holes to maintain humidity. These plants were placed inside growth chamber under 16 h photoperiod with photosynthetic photon flux density of 40 μ mol m-2 s-1 fluorescent lamps at 25 ± 2°C temperature. Plants were watered regularly and gradually acclimatized over a period of 1 month. The polythene bags were then removed and the established plantlets were subsequently transplanted to earthen pots (20 × 15 cm) and kept in a polyhouse for further growth (Figure 1i, j and k).
Production of microtubers
Well grown plantlets obtained from direct regeneration of nodes were maintained in culture room at 16/8 h light/dark condition and observed for the production of microtubers .
Statistical analysis
All the experimental observations were recorded at regular intervals. Mean values of various treatments were analysed by using one way ANOVA (Analysis of Variance) for statistical significance. Effect of different concentrations of plant growth regulators were determined on average number of shoots, average shoot length, number of nodes and average number of leaves per explant.
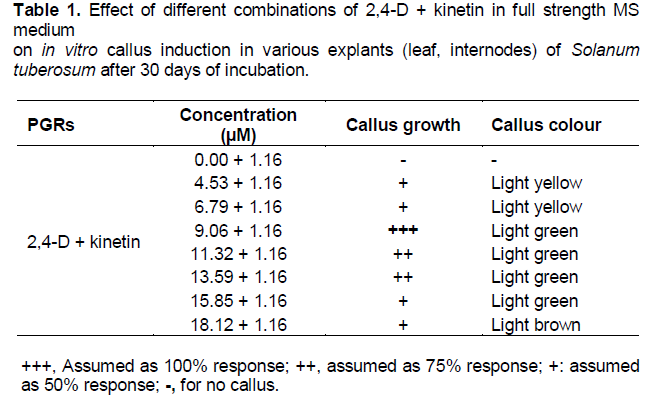
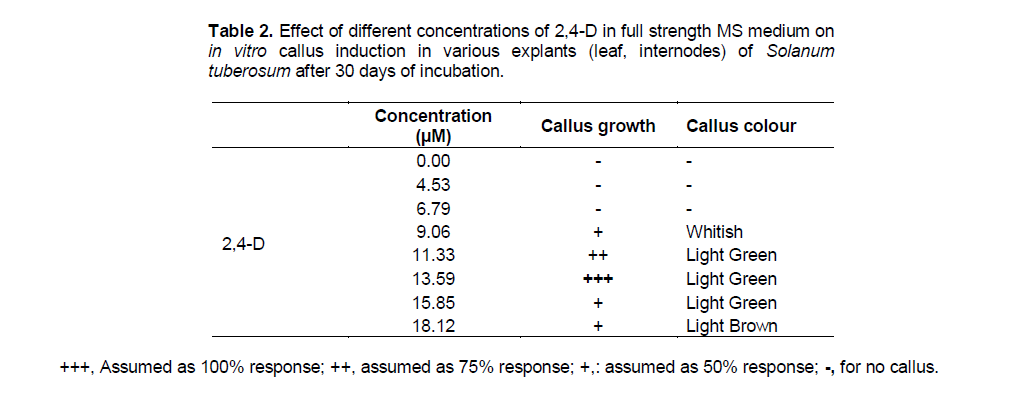
The explants showed callus formation in MS medium containing 2,4-D. Best callus induction and proliferation was observed after 15 to 20 days of incubation in MS medium with 13.59 µM 2,4-D alone and 2,4-D + kinetin (9.06 + 1.16 µM) (Figure 1a). The callus obtained was light green in colour. Increased concentration of hormones lead to browning of callus (Tables 1 and 2). This phenomenon was also supported by previous studies in other species. Auxin alone and in combination with cytokinin can produce callus but 2,4-D was found to be most effective for callus induction and proliferation (Shirin et al., 2007). But on the contrary, in the present study callus induction in leaf explants was more frequent in comparison to internodes as explants as observed after 30 days of incubation. Regular subculture of callus enhanced proliferation rate due to availability of nutrients before their exhaustion in the medium.
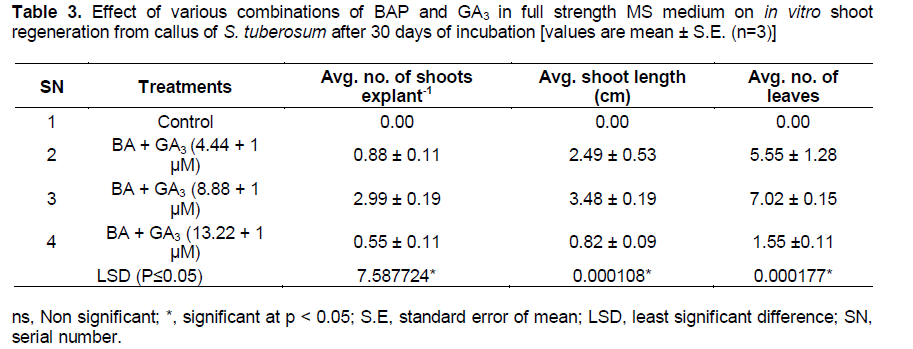
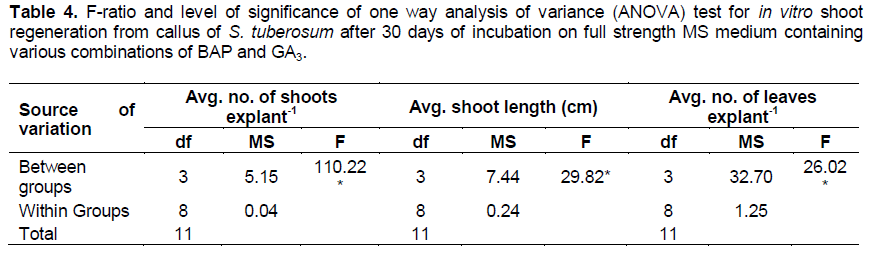
Shoot regeneration from the calli initiated in the medium supplemented with BAP + GA3 (8.88 + 1 µM) after 7 days of incubation (Figure 1b). GA3 has been reported to help in elongation of shoots. Combination of BA + GA3 (8.88 + 1 µM) produced significantly high average number of shoots, average shoot length and average number of leaves per explant as compared to other combinations (Table 3 and Figure 1c). The results of one way analysis of variance (ANOVA) showed that F- factor and P- value for most of the parameters were significant at 0.05% level (Table 4). Longest shoot obtained was 4.50 cm in height as observed after 20 to 25 days of incubation. This agrees with Haque et al. (2009) who observed the longest shoot by the treatment combination of BAP and GA3 in other species of the plant . On the other hand direct regeneration of shoots with highest average number of shoots, nodes and leaves per explant took place in the medium supplemented with zeatin + IAA + GA3 (13.68 µM + 5.7 µM + 8.49 µM) (Table 5 and Figure 1d). Longest shoot attained a height of 6 cm after 15 to 20 days of incubation. Profound shoot proliferation was obtained after 7 to 8 weeks (Figure 1e). Direct shoot regeneration is preferred since it reduces the possibility of somaclonal variation (genetic variation) common in plants regenerated from cultured cells or tissues (Misra and Datta, 2001; Dayal et al., 2003). Results of this experiment are also proved to be significant using ANOVA (Table 6). Observations recorded were observed for different parameters viz. average number of shoots, average shoot length, number of nodes and average number of leaves per explant.
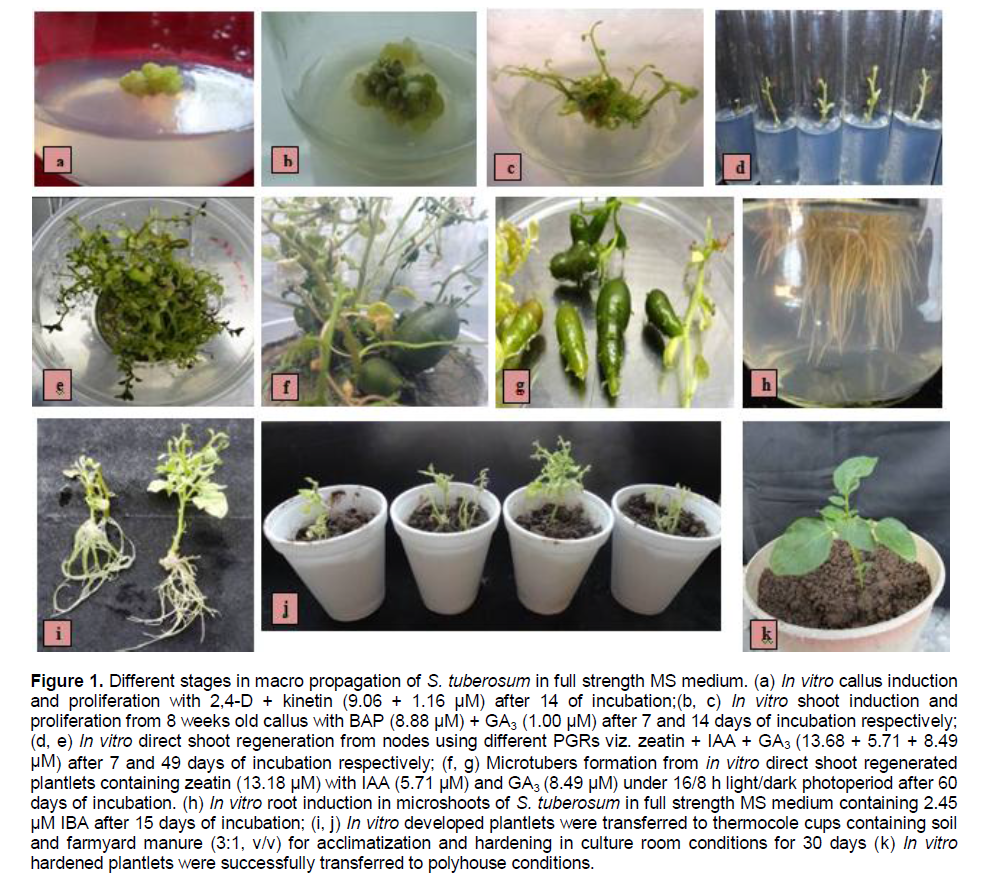
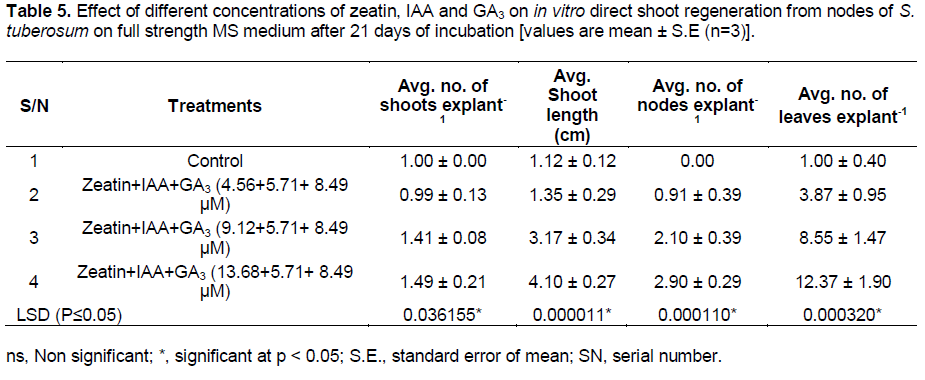
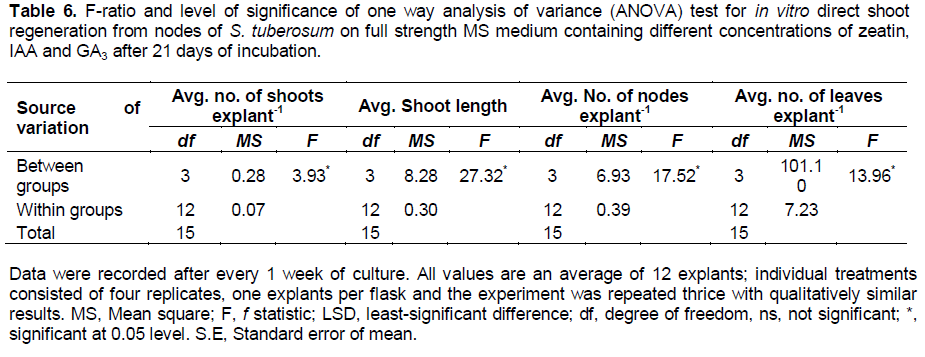
Plantlets were found to produce microtubers in a period of 8 to 10 weeks (Figure 1f and g). Cytokinins are believed to have strong promotive effects on tuberization, and to constitute major part of the tuberization stimulus, either alone or in combination with other substances (Pelacho and Mingo-Castel, 1991). The average weight of the tubers obtained was found to be 0.20 g with an average number of 3 tubers per explant. The microtubers obtained were green in colour. For root induction, out of different concentrations of IBA tried with basal MS medium and half strength basal MS medium, 2.45 µM IBA induced highest number of roots after 15 days of incubation in full strength basal MS medium (Figure 1h) as well as in half strength basal MS medium as compared to other concentrations. IBA has proved to be efficient in promoting root induction (Sakthivel and Manigandan, 2011). The mean value of in vitro rooting response for all the parameters at different PGR concentration showed that average number of root (43.50) and average root length (7.50 cm) was observed to be maximum with 2.45 μM IBA in full strength MS medium. Longest root attained the length of 8.5 cm. Data analysed for average number of roots and average root length for different concentrations of IBA used is represented in Table 7.
Callus is an unorganized mass of plant cells. Reliable callus induction and regeneration of viable plants is considered as a limiting step to the successful use of modern techniques in genetic improvement of the major crops (Murphy, 2003). In the present study, different PGR combinations were checked for in vitro callus induction in explants of Solanum tuberosum. Callus induction was found to be successful using different concentrations of 2,4-D alone and in combination with Kinetin. The auxin 2,4-D, by itself or in combination with cytokinins, has been widely used to enhance callus induction and maintenance. Moreover, many researchers observed 2,4-D as the best auxin for callus induction both in monocots and dicots (Chee, 1990; Mamun et al., 1996). Role of 2,4-D in callus induction has been widely accepted and utilized for potato, tomato and many medicinal plants (Ashakiran et al., 2011; Ahmed et al., 2012; Lakshmi and Reddy, 2012; Mehta et al., 2012).
On the basis of regular observation it was concluded that the source of explant has a direct effect on callus induction. Results showed that leaf explant were more efficient for callus induction with 100% response as compared to internodes which gave only 50% response. This may be due to the presence of more meristematic activity in leaves as compared to internodes. This result supported the previous study by Haque et al. (2009) who found that callus length was affected by different explants and that the leaf explants produced significantly highest callus length in contrast to the shoot tip which produced least results in case of potato cv. Diamant. The interaction effect between explant and concentration of growth regulators were found to have significant differences on callus length in different researches. This result was also proved to be significant by Dobranaszki et al. (1999) and Fomenko et al. (1998) who also observed significant effects of explants of potato on callus length. In the present study different concentrations of 2,4-D and Kinetin showed significant differences in callus growth and colour. Rate of callus induction increased with the increasing concentration of 2,4-D alone upto 13.59 µm and in the combination of 2,4-D and Kinetin upto 9.06 µm and 1.16 µm respectively. Further increase in concentration lead to decrease in callus growth and resulted in browning of callus. Callus initiation on cut ends of in vitro cultured explants of potato could be observed in all 2,4-D levels (Khalafalla et al., 2010). Similar findings were also reported by (Fiegert et al., 2000; Jayasree et al., 2001; Yasmin et al., 2003).
Both callus induction and plant regeneration from explant require appropriate combinations and concentrations of plant growth regulators in the culture media (Ehsanpour et al., 2000b). In the present research work, best results for shoot regeneration from callus of S. tuberosum was obtained by using a combination of 8.88 μM BAP and 1.00 μM GA3 with significantly high average number of shoots, shoot length and number of leaves per explant as compared to other combinations. BAP, Zeatin or Kinetin are known to help produce adventitious shoots. Martel and Carcia (1992) reported that both BAP and GA3 at higher concentrations were necessary for shoot formation of potato. Shoot regeneration responses vary with the potato cultivar but in most cases cytokinin helps to enhance shoot production (Ghaffoor et al., 2003). Generally a low ratio of auxin to cytokinin is required for adventitious shoot development in case of potato (Anjum and Ali, 2004).
A decrease in all the parameters of shoot regeneration occurred after increase in the concentration of BAP after 8.88 μM. Similar effects of increasing concentration of BAP on shoot regeneration of potato cv. Asterix were observed by Molla et al. (2011) who observed that the length of shoot increased with increasing BAP concentration up to 3 mg l-1 and then decreased. Role of GA3 in shoot elongation is well known and reported by many researchers. For rapid multiplication, addition of GA3 to the MS media has been reported to improve growth and development of shoots. Farhatullah and Abbas (2007) also have reported that dosage of 0.248 mg l-1 of GA3 in the MS medium boosted all morphological characters in in vitro raised potato plantlets. Ullah et al. (2012) also have reported that GA3 is involved in cell elongation and its addition in MS medium enhanced shoot growth in in vitro propagated plants of potato variety “Desiree”.
Direct regeneration system has an edge over regeneration after passing through callus phase to maintain the true-to-type nature of the regenerated plantlets and avoid somaclonal variation. Potato breeding programs can highly benefit from biotechnological tools, which are capable of surpassing some limitations found by traditional plant breeding methods and open new avenues for crop improvement. In the present study, attempts were made also made to induce direct regeneration of S. tuberosum. Explant used were nodes. Leaf discs and inter nodal tissues are the least responsive explants for direct regeneration. These explants underwent callus induction phase and then resulted in shoot regeneration indirectly in a study conducted by Hussain et al. (2005) on three potato cultivars viz., Cardinal, Altamash and Diamont. There are many advantages of taking nodal tissue as an explant, that is, a large number of aseptic plants can be obtained quickly and easily, and plants produced may remain true to type. Successful regeneration was obtained using hormonal combination of Zeatin, IAA and GA3 in a concentration of 13.68, 5.71 and 8.49 µM, respectively. Role of Zeatin in regeneration has been reported by Wendt et al. (2001) who found that the internode explant of potato cultivar Macaca treated with Zeatin showed higher regeneration rate than those treated with BAP.
Roots were induced in microshoots using different concentrations of IBA, out of which 2.45 μM concentration emerged to be best with maximum average number of root (43.50) and a maximum average root length of 7.50 cm in full strength MS medium. IBA has been shown as a potent root inducer in many studies conducted on various tomato cultivars (Chaudhry et al., 2010; Khalafalla et al., 2010; Sakthivel and Manigandan, 2011).
Microtubers of S. tuberosum were obtained after incubating directly regenerated shoots at 16/8 h light/dark condition after 8 to 10 weeks. Microtubers obtained were green in colour. The green colour might be due to the presence of alkaloid solanin which is produced under light conditions. Microtubers may vary in their shape, colour, weight, diameter, length etc. (Rannali, 2007). This study also supports the similar findings of Hoque (2010). The edible part of the plant is the tuber, which is formed at the end of underground stems called stolon. Potato produced more protein and calories per unit area per unit time and per unit of water than any other major plant food. In vitro tubers can be produced throughout the year and thus holds benefit over conventional tubers (Hoque, 2010).
The present regeneration protocol could be useful for rapid in vitro regeneration, multiplication and virus free seed, that is, microtuber production. This piece of work may also be utilized for transformation techniques for production of biotic and abiotic stress tolerant potato crop which may in turn contribute to overcome major obstacle in potato farming especially in the Kumaun hills towards quality and efficient production of this major cash crop.
The authors have not declared any conflict of interest.
ANOVA, analysis of variance; BA, benzyl adenine; GA3, gibberellic acid; IAA, indole acetic acid; MS, Murashige and Skoog (1962); PGR, plant growth regulators; 2,4-D, 2,4-dichlorophenoxy acetic acid.
REFERENCES
|
Ahmed S, Huq H, Zeba N, Hoque ME, Moon NJ (2012). Callus induction and plant regeneration of potato genotypes from meristem following hormonal treatments. J. Expt. Biosci. 3(2):67-74. |
|
|
Anjum MA, Ali H (2004). Effect of culture medium on direct organogenesis from different explants of various potato genotypes. Biotechnol. 3(2):187-193.
Crossref |
|
|
|
Ashakiran K, Sivankalyani V, Jayanthi M, Govindasamy V, Girija S (2011). Genotype specific shoots regeneration from different explants of tomato (Solanum lycopersicum L.) using TDZ. Asian J. Plant Sci. Res. 1: 107-113. |
|
|
|
Chaudhry Z, Abbas S, Yasmin A, Rashid H, Ahmed H, Anjum MA (2010). Tissue culture studies in tomato (Lycopersicon esculentum) var. moneymaker. Pak. J. Bot. 42(1):155-163. |
|
|
|
Chee PP (1990). High frequency of somatic embryogenesis and recovery of fertile cucumber plants. Hort. Sci. 25:792- 793. |
|
|
Dayal S, Lavanya M, Devi P, Sharma KK (2003). An efficient protocol for shoot regeneration and genetic transformation of pigeonpea (Cajanus cajan L.) using leaf explants. Plant Cell Reports 21: 1072–107.
Crossref |
|
|
|
Dobranaszki J, Takacs HA, Magyar TK, Ferenczy A (1999). Effect of medium on the callus forming capacity of different potato genotypes. Acta Agronomica Hungarica 47:59-61. |
|
|
|
Ehsanpour AA, Jones MGK (2000a). Evaluation of direct shoot regeneration from stem explants of potato (Solanum tuberosum L.) cv. Delaware by Thidiazuron (TDZ). J. Sci. Technol. Agric. 3: 47-54. |
|
|
|
Ehsanpour AA, Jones MGK (2000b). Evaluation of direct shoot regeneration from stem explants of potato (Solanum tuberosum L.) cv. Delaware by Thidiazuron (TDZ). J. Sci. Tech. Agric. 3: 47-54. |
|
|
|
Farhatullah ZA, Abbas JS (2007). In vitro effects of gibberellic acid on morphogenesis of potato explant. Int. J. Agric. Biol. 9: 200. |
|
|
|
Fiegert AK, Mix WG, Vorlop KD (2000). Regeneration of Solanum tuberosum L. Tomensa cv, Induction of somatic embryogenesis in liquid culture for the production of artificial seed. Landbauforschung Volkenrode. 50: 199-202. |
|
|
|
Fomenko TI, Reshetnikov VN, Malyush MK, Kondratskaya IP, Chumakova IM (1998). Conditions of development of callus tissues of potato in vitro. Vestsi Akademii Navuk Belarusi. Seria Biyalagichnykh Navuk. 4:97-105. |
|
|
Ghaffoor A, Shah GB, Waseem K (2003). In vitro response of potato (Solanum tuberosum L.) to various growth regulators. Biotechnol. 2:191-197.
Crossref |
|
|
|
Haque AU, Samad MA, Shapla TL (2009). In vitro callus initiation and regeneration of potato. Bangladesh J. Agric. Res. 34(3):449-456. http://dx.doi.org/10.3329/bjar.v34i3.3910.1111/j.1399-3054.1962.tb080 52.x 71 |
|
|
|
Hoque ME (2010). In vitro tuberization in potato (Solanum tuberosum L.). Plant Omics Journal 3(1): 7-11. |
|
|
|
Hossain MJ (1994) In vitro propagation of potato (Solanum tuberosum L.). J. Plant Tissue Cult. 4(1): 33-37. |
|
|
|
Hussain I, Muhammad A, Chaudhry Z, Asghar R, Naqvi SSN, Rashid H (2005). Morphogenic potential of three potato (Solanum tuberosum) cultivars from diverse explants, a prerequisite in genetic manipulation. Pak. J. Bot. 37(4): 889-898 |
|
|
Jayasree T, Pavan U, Ramesh M, Rao AV, Reddy KJM, Sadanandam A (2001). Somatic embryogenesis from leaf culture of potato. Plant Cell Tissue Organ Cult. 64: 13-17.
Crossref |
|
|
|
Khalafalla MM, Abd Elaleem KG, Modawi RS (2010). Callus formation and organogenesis of potato (Solanum tuberosum L.) cultivar almera. J. Phytol. 2(5):40–46. |
|
|
|
Lakshmi JB, Reddy JK (2012). Callus induction and Organogenesis in an Indian Box-wood (Gardenia latifolia Ait.). Sci. Res. Report. 2(1):07-12. |
|
|
|
Mamun ANK, Islam R, Reza MA, Joadar OI (1996). In vitro differentiation of plantlet of tissue culture of Samonea saman. Plant Tissue Cult. 6:1-5. |
|
|
|
Martel A, Carcia E (1992). In vitro formation of adventitious shoots on discs of potato (Solanuin tuberosum L. cv. Sebago) tubers. Phyton Buenos Aires. 53: 57-64. |
|
|
|
Mehta J, Ansari R, Syedy M, Khan S, Sharma S, Gupta N, Rathore R, Vaishnav K (2012). An effective method for high frequency multiple shoots regeneration and callus induction of Bacopa monnieri (L.) Pennel: An important medicinal plant. Asian J. Plant Sci. Res. 2 (5):620-626. |
|
|
Misra P, Datta SK (2001). Direct differentiation of shoot buds in leaf segments of white marigold (Tagates erecta L.). In vitro Cell Dev. Biol. Plant 37: 466–470. http://dx.doi.org/ 10.1007/s11627-001-0082-2
Crossref |
|
|
|
Molla MMH, Nasiruddin KM, Al-Amin M, Khanam D, Salam MA (2011). Effect of growth regulators on direct regeneration of potato. International Conference on environment and Industrial Innovation IPCBEE. 12. |
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15:473-497. http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x
Crossref |
|
|
|
Murphy DJ (2003). Agricultural biotechnology and oil crops – current uncertainties and future potential. Applied Biotechnology and Food Science Policy. 1: 25–38. |
|
|
|
Nistor A, Campeanu G, Atanasiu N, Chiru N, Karacsonyi D (2010). Influence of potato genotypes on "in vitro" production of microtubers. Romanian Biotechnological Letters 15: 5217-5324. |
|
|
Pelacho AM, Mingo-Castel AM (1991). Jasmonic acid induces tuberization of potato stolons cultured in vitro Plant Physiol 97:1253-1255. http://dx.doi.org/10.1104/pp.97.3.1253
Crossref |
|
|
|
Rabbani A, Askari B, Abbasi NA, Bhatti M, Quraishi A (2001). Effect of growth regulators on in vitro multiplication of potato. Int. J. Agric. Biol. 3(2): 181-182. |
|
|
Rannali P, Bizzari M, Borghi L, Mari M (1994). Genotypic influence on in vitro induction, dormancy length, advancing age and agronomical performance of potato microtubers (Solanum tuberosum L.). Annals of Appl. Biol. 125:161-172. http://dx.doi.org/ 10.1111/j.1744-7348.1994.tb04957.x
Crossref |
|
|
Rannali P (2007). The canon of potato science: 24. Microtubers Potato Res. 50:301–304. http://dx.doi.org/10.1007/s11540-008-9073-6
Crossref |
|
|
|
Sakthivel S, Manigandan V (2011). Tissue culture studies in tomatto (Lycopersicon esculantum, pkm1) from cotyledonary leaf explants. Int. J. Chem. Pharmaceut. Sci. 2(3): 22-25. |
|
|
Seabrook JEA, Coleman S, Levy D (1993). Effect of photoperiod on in vitro tuberization of potato (Solanum tuberosum L.). Plant cell, Tissue Organ Cult. 34:43-51. http://dx.doi.org/10.1007/BF00048462
Crossref |
|
|
|
Shirin F, Hossain M, Kabir MF, Roy M, Sarker SR (2007). Callus induction and plant regeneration from intermodal and leaf explants of four potato (Solanum tuberosum L.) cultivars. World J. Agric. Sci. 3(1):01-06. |
|
|
Solomon BRM, Barker H (2001). Breeding virus resistant potatoes (Solanum tuberosum): A review of traditional and molecular approaches. Heredity 86:17-35.
Crossref |
|
|
|
Srivastava AK, Diengdoh LC, Rai R, Bag TK (2012). Tissue culture- technology harnessed for potato seed production. Keanean J. Sci. 1:80-86. |
|
|
|
Ullah I, Jadoon M, Rehman A, Zeb T, Khan K (2012). Effect of different GA3 concentration on in vitro propagation of potato variety desiree. Asian J. Agric. Sci. 4(2):108-109. |
|
|
Wendt SN, Peters JA, Oliveira AC, Bobrowski VL, Costa FLC, Madruga CS, Vighi IL (2001). Plant regeneration and molecular characterization of potato cultivar Macaca obtained from gamma irradiated explants. J. New Seeds 3(2):17-37.
Crossref |
|
|
Yasmin S, Nasiruddin KM, Begum R, Talukder SK (2003). Regeneration and stablishment of potato plantlets through callus formation with BAP and NAA. Asian J. Plant Sci. 2(12):936-940.
Crossref |
|
|
Zaman MS, Quershi, Hasan AG, Din RU, Ali S, Khabir A, Gul N (2001). Meristem culture of potato (Solanum tuberosum L.) for production of virus free plantlets. Online J. Bio. Sci. 1: 898-899.
Crossref |
![]() The present study demonstrated the use of various PGR combinations for efficient in vitro regeneration of cv. kufri jyoti in kumaun hills. Best callus induction and proliferation was observed in MS medium supplemented with 13.59 µM 2,4-D alone and 2,4-D + kinetin (9.06 + 1.16 µM) out of different concentrations of 2,4-D (4.53 to 18.12 µM) alone and 2,4-D (0 to 18.12 µM) with kinetin (1.16 µM). Leaf explants were more efficient in producing callus as compared to internodes. Medium supplemented with BA + GA3 (8.88 µM + 1 µM) initiated shoot induction out of various combinations of BA (4.44 to 13.22 µM) and GA3 (1 µM) after 7 days of incubation with significantly high average number of shoots, average shoot length and average number of leaves per explant. MS medium supplemented with different concentrations of zeatin (4.56, 9.12 and 13.68 µM) with IAA (5.71 µM) and GA3 (8.49 µM) was tried for direct regeneration of shoots through nodes out of which zeatin + IAA + GA3 (13.68 µM + 5.71 µM + 8.49 µM) served to be the best combination and the raised plantlets were found to produce microtubers in a period of 8 to 10 weeks. 2.45 µM IBA in full strength basal MS medium induced highest number of roots. In addition to an efficient regeneration protocol, the microtuber production was also studied in the present piece of work. The research protocol may also be utilized for Agrobacterium tumefaciens mediated transformation towards the biotic and abiotic stress tolerant potato crop.
The present study demonstrated the use of various PGR combinations for efficient in vitro regeneration of cv. kufri jyoti in kumaun hills. Best callus induction and proliferation was observed in MS medium supplemented with 13.59 µM 2,4-D alone and 2,4-D + kinetin (9.06 + 1.16 µM) out of different concentrations of 2,4-D (4.53 to 18.12 µM) alone and 2,4-D (0 to 18.12 µM) with kinetin (1.16 µM). Leaf explants were more efficient in producing callus as compared to internodes. Medium supplemented with BA + GA3 (8.88 µM + 1 µM) initiated shoot induction out of various combinations of BA (4.44 to 13.22 µM) and GA3 (1 µM) after 7 days of incubation with significantly high average number of shoots, average shoot length and average number of leaves per explant. MS medium supplemented with different concentrations of zeatin (4.56, 9.12 and 13.68 µM) with IAA (5.71 µM) and GA3 (8.49 µM) was tried for direct regeneration of shoots through nodes out of which zeatin + IAA + GA3 (13.68 µM + 5.71 µM + 8.49 µM) served to be the best combination and the raised plantlets were found to produce microtubers in a period of 8 to 10 weeks. 2.45 µM IBA in full strength basal MS medium induced highest number of roots. In addition to an efficient regeneration protocol, the microtuber production was also studied in the present piece of work. The research protocol may also be utilized for Agrobacterium tumefaciens mediated transformation towards the biotic and abiotic stress tolerant potato crop.