ABSTRACT
One of the major biotic stresses that limits tef yield is insect pests attack. Of these tef, shoot fly is sporadically important insect pest in various tef growing areas of Ethiopia. Evaluation of diversity of tef germplasm in Ethiopia for the reaction tef shoot fly might give a chance to get host plant resistance. The main objective of this study was to assess the genetic variation among tef genotypes for their reaction to tef shoot fly. Field experiment was conducted in 2015 cropping season at Maysiye, Northern Ethiopia. The genotypes were planted in triple lattice design with three replications category of reaction of the tested tef genotypes for their reaction to shoot fly attack was adopted from the rating scale of the IRRI for rice. The study revealed that acc. 17 WJ was found to be the only genotype evaluated as resistant to tef shoot fly Atherigona hyalinipennis species. While the standard checks Quncho and Kora were grouped under the susceptible category, generally 10 tef genotypes were moderately resistance, 19 genotypes were moderately susceptible, 16 genotypes were susceptible, and three genotypes were highly susceptible to tef shoot fly attack. Tef damage (dead heart and white headed) due to tef shoot fly ranged from 4.20 to 36.96%, respectively. The yield loss also varied from 3.58 to 97.83 kg ha-1. Infestation was high from the beginning of August to the end of September. Besides using the varieties grouped under resistance and moderately resistance category and chemical application at early stage infestation would be recommended to avoid yield loss. Further study over seasons and environments would be important to have a valid conclusion
Key words: Shoot fly resistance, time of infestation, yield loss, tef genotypes.
Tef (Eragrostis tef (Zucc.) Trotter, is one of the major staple cereals of Ethiopia. Ethiopia is the center of both origin and diversity for tef (Vavilov, 1951). It occupies more than three million hectares of land and considered to be a healthy food as its gain is gluten free (Spaenij-Dekking et al., 2005) and the crop is very important in the overall national food security of the country (Kebebew et al., 2013). In spite of the fact that tef has numerous merits and considerable economic significance in Ethiopia, the national average grain yield of tef is relatively low, about (1575 kg ha-1) (CSA, 2015). However, Tareke et al. (2013) reported that the tef yields of 4000 and 2500 kg ha-1 on research fields and on farmers’ fields, respectively. Tef’s major yield limiting factors are the low yield potential of tef landrace, lack of cultivars tolerant to lodging, drought and pests (Assefa et al., 2011). One of the major biotic stresses that limits tef yield is insect pests attack. Among the 40 insect pest species recorded on tef, tef shoot fly (Atherigona hyalinipennis Van Emden) is sporadically important insect pest in various tef growing areas (Sileshi, 1997). Tef productions in areas with erratic rainfall distribution like northern part of Ethiopia is severely affected by tef shoot fly infestation. This might be due to the conducive climate for the reproduction of the tef shoot fly, weakening of tef plant due to drought effect and perhaps changes in agronomic practice (fertilizer application, use of improved varieties). Tef is infested by six shoot fly species that belonged to three families and three genera. Of these the three species belonged to the family Muscidae and the genus Atherigona. A. hyalinipennis, Atherigona Lineata (Adams) ssp. and Atherigona longifolia Van Emden are the family which belongs to Muscidae. The second family is the Anthomyiidae of the Delia flavibasis species. Whereas, the third family Chloropidae flies, Oscinella nartshukiana Beschovski and Oscinella sp. n. dimidiofrit were another group of shoot flies that caused dead heart in tef. The species A. hyalinipennis was previously reported as pest of tef by Ebba (1969) and Sileshi (1997). Taxonomic history, description, identification key and host plants of these Atherigona spp. Is found in Deeming (1971). The other tef shoot fly species is D. flavibasis (Stein in Becker), which belongs to the family Anthomyiidae, is also a new record on tef under field condition. In areas depending up on season and location shoot fly damage on tef ranged from 6.96 to 37.60% in Tigray region and 2.98 to 22.87% in Awi zone (DZARC, 2004). Moreover, in north Wollo and Wag-hmra zone damaged tef panicles in 25×25 cm quadrant were in the range of 2 to 4% (Bayeh, 2004). However, tef shoot fly damage may not always lead to yield loss. Thus, where rain fall is plenty, tef compensates for shoot fly damage and grain yield from insecticide unsprayed tef was greater than grain yield from insecticide sprayed tef (Bayeh et al., 2009). In spite of the economic implications of tef shoot fly damage on tef in Tigray region particularly in Mekoni, Axum and Wukro districts (DZARC, 2002; Bayeh et al., 2008), development of host plant resistance were not attempted. Host plant resistance can play a major role in minimizing the extent of losses and is compatible with other tactics of pest management, including the use of natural enemies and chemical control (Kumar et al., 2008). Therefore, having the diversity of tef in Ethiopia and evaluating them for the reaction tef shoot fly can lead to get a material host plant resistance. The main objective of this study was to assess the genetic variation among tef genotypes for the reaction to tef shoot fly.
Description of the study area
The field experiment was carried out at Axum Agricultural Research Center (AxARC) during 2015 main cropping season at the substation Maysiye (14° 6’43’’ North and 38° 36 ’41’’ East, altitude of 2200 masl) in Tahitaey Maichew district, in central zone of Tigray, Ethiopia. The substation is located at 17 km west of Axum town. The annual rainfall received by the experimental site during the main cropping season was 613.92 mm. Moreover, the mean average annual minimum and maximum temperature was 12.16 and 26.78°C, respectively. The experimental material consisted of 49 genotypes of which 32 released tef varieties, 12 promising lines, three accessions collected from tigray region and two local landraces obtained from Deber Zeit Agricultural Research Center (DZARC), Axum Agriculture Research Centers and farmers, respectively used as standard checks. The tef seeds were sown in the third week of July, 2015.
Experimental design and management
The experiment was laid out in 7×7 triple lattice designs. Each tef seeds from each genotype was sown in three rows of 2 m length spacing at 0.2 m inter row spacing plots, blocks and replications were spaced at 1, 0.5 and 1.5 m, respectively. In accordance with the recommended tef seed rate of 10 kg/ha (AxARC, 2013/2014), 1.2 g of seeds per plot was hand-drilled in the rows. Fertilizer rates of 60 kg N and 40 kg P2O5 ha-1 was used (Seyfu, 1997). The source of nitrogen was urea and di-ammonium phosphate (DAP) was the source of phosphorus. DAP was applied once at the time of sowing, while urea was applied in split after germination. The first urea application was made two weeks after seed germination and the second split was applied two weeks later after the first application. All other cultural crop management practices were applied as per the recommendation for tef production. To ensure uniform distribution of tef shoot fly infestation, fish meat (dried and powdered fish meat) was broadcasted at a rate of 15 g/plot in two phases on all entries. The first fish meal application was made at the first appearance of the tef shoot fly damage symptom in early vegetative stage and the second was made during heading stage. As reported by Jotwani and Young (1972), the fish meal was used to ensure the infestation on sorghum shoot fly resistance materials.
Data collection
The number of infested plants was estimated by counting the plants with dead heart at the interval of three days starting from the first appearance of damage symptom. At each count, the infested plant was tagged with thread. White heads were counted or noted at pre harvesting (physiological) maturity. The numbers of infested and non-infested productive tillers were also counted from randomly selected ten plants. At harvesting, total population were counted (including tillers raised from infested and non-infested plants) and the sum of dead heart plus the white head per plot used for determining percent damage.

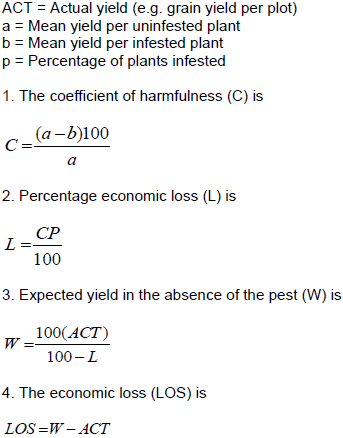
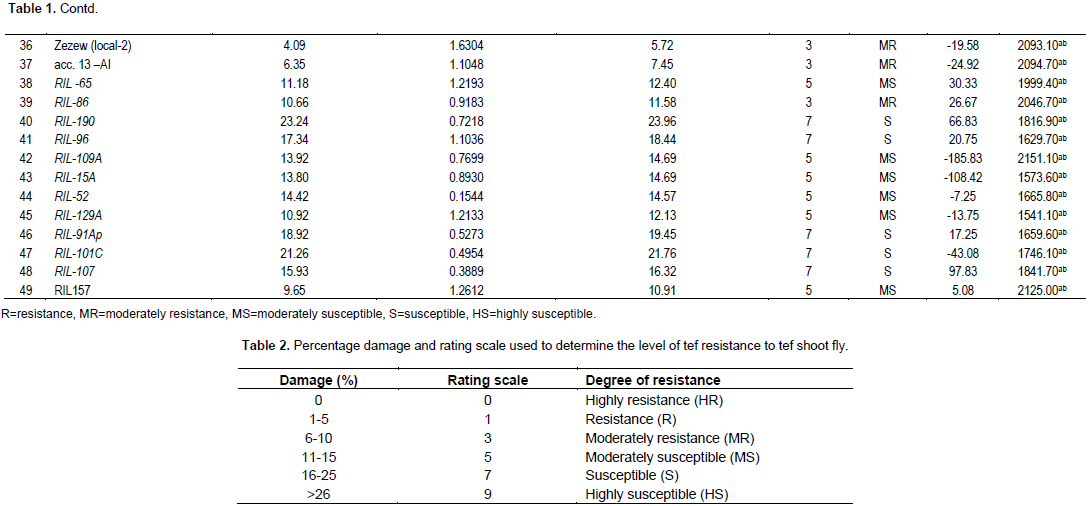
The actual mean of dead heart plus white headed percentage used for classification of the tef genotypes under different categories. This categorizing way was taken by adopting the rating scale of the International Rice Research Institute (IRRI), viz. Standard Evaluation System (SES) for rice (Table 2) (Visalakshmi et al., 2014). Grain yield was measured form infested and none infested plants each from ten main plants with their tillers. The plants were hand threshed, weighed and the weight was divided by the total number of productive tillers of the ten sample plants. The grain yield data from infested and non-infested plants was used for yield loss estimation. Yield loss due to the tef shoot fly damage was estimated using the analytical methods of yield loss as stated by Judenko (1972) formula:
Reaction of tef genotypes to the tef shoot fly infestation is presented in Table 1. Based on the damage (%) of dead heart plus white heads, only one accession viz. acc. 17 WJ was grouped under resistant category based on the damage of dead heart plus white heads. In general, 10, 19, 16, and 3 tef genotypes were grouped as moderately resistant, moderately susceptible, susceptible and highly susceptible to tef shoot fly attach, respectively. As plant resistant to insect pest is known to be due to antibiosis, tolerance and antixenosis (Abro et al., 2003), the mechanism of résistance observed for 17 WJ in the current study requires further investigation in order to reach at concrete conclusion . The tef shoot fly infestation started from two weeks after emergence up to the end of heading. This trial was sown on 22 July, 2015 and the shoot fly infestation started on 10 August, 2015 when the seedlings reached three leaf stages. The infestation continued up to September 4, 2015. The pattern of tef shoot fly infestation was less at early seedling stage, it slowly increased as the season progresses and finally become slow. Mostly, the shoot fly affects seedling and newly raised tillers and in few intensity at heading. DZARC (1983) reported late sown tef is infested by the tef shoot fly, while early sown tef is infested only if there is dry spell. Conditions like late sowing date, poor germination and production of tillers at later stage increased the level of infestation. During the crop growth sparsely populated varieties compensated for lost plants by producing more tillers. However, late produced tillers were severely affected by tef shoot fly. Some tef varieties such as Melko might be inherently susceptible to tef shoot fly and as a result they had high level of infestation.
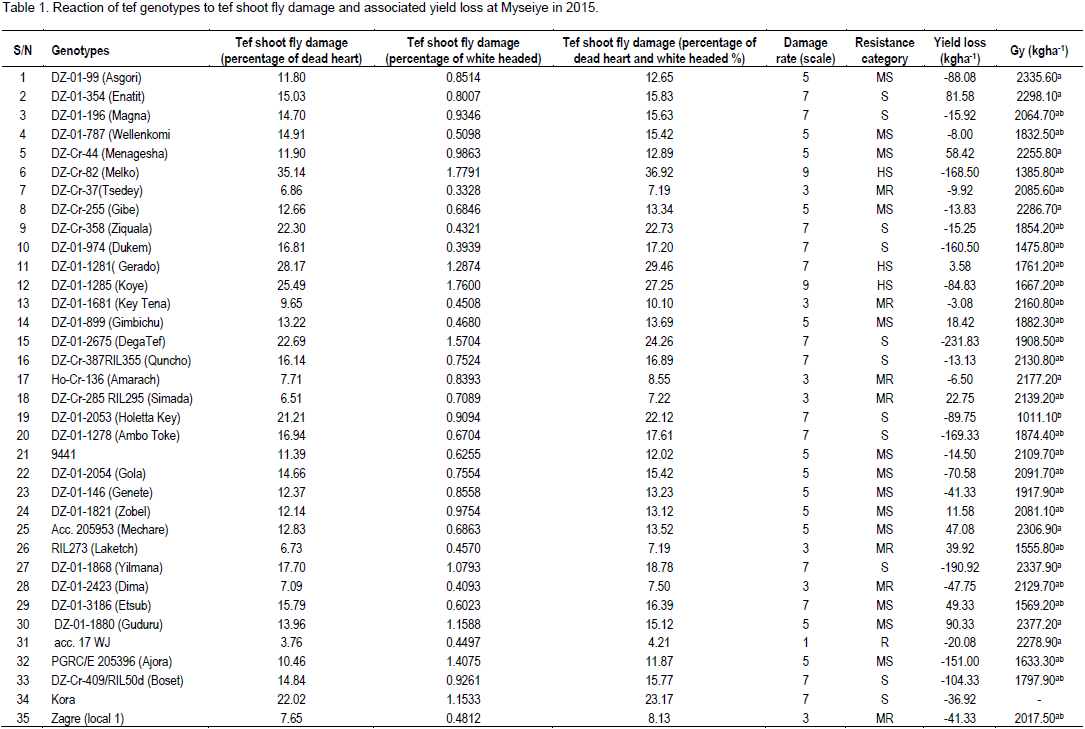
Moreover, use of yield enhancing inputs like chemical fertilizer and manure might also increases the infestation of tef shoot fly. Corbeels et al. (2000) and Berhane et al. (2015) had similar observation on the effect of inputs for tef production that stimulates the appearance of shoot fly. In general, dead hearts were more prevalent in fertile parts than in the waterlogged or less fertile parts of the tef field (Tesfaye and Zenebe, 1998). This input increase the infestation due to making the plant softy favorable for food and to lay egg. The damage due to tef shoot fly ranged from 4.21% from acc.17 WJ to 36.92% for Melko per plot. The results are in agreement with the previous reports of 6.96 to 37.60% damage in tef due to tef shoot fly infestation in Tigray regional state (DZARC, 2002). The standard checks like Quncho and Kora have been grouped under the susceptible category. However, the local check was categorized with moderately susceptible. Three varieties, namely, Melko, Gerado and Koye had the highest damage rate. The majority of the genotypes were classified as moderately susceptible and susceptible. Tef shoot fly is becoming economic important insect pest in the last three to five years in the study area. The estimated yield loss for genotypes had positive and negative sign (Table 1). The positive value indicates actual yield loss due the tef shoot fly infestation. Whereas, the negative value indicates yield from shoot fly infested plants was greater than the yield from non-infested plants. Compensation of lost parts is one mechanism of resistance to insect pests. The yield loss ranged from 3.58 for Gerado to 97.83 kg ha-1 for RIL-107. The yield loss had a negative and positive sign, which indicates the loss yield due to the infestation had positive sign, whereas the positive sign showed that the infestation of the fly on the tef plant can increase the yield over the un-infested plant. Therefore, some of tef genotypes increased their yield due to tef shoot fly infestation.
The yield increased due to tef shoot fly infestation which was minimum, 3.08 kg ha-1 for Key Ten and maximum, 231.83 kg ha-1 for Dega Tef. Insects like shoot flies are known to encourage more tiller production at low level of infestation and when moisture is not limiting. Even though, it stimulates grain yield on the infested individual, it does not enhance the genetic make of the tef genotypes. However, these genotypes which were over-compensatory would be used for breeding programme. On the bases of yield loss, 16 and 3 genotypes were susceptible and highly susceptible, respectively. Susceptible indicates because they were not able to compensate the damaged parts by the tef shoot fly. However, at Haramaya (Alemaya) tef is sown after sorghum and the shoot fly population that built-up on sorghum might have caused severe damage (378 to 522 kg ha-1) (Sileshi, 1997). As far as the yield loss due to tef shoot fly is concerned, out of the total ten moderately resistant tef genotypes, only Simada and lakech had positive yield loss, implying that there is an actual yield loss due to this particular insect pest. Despite they had low damage percent; the yield loss indicated that these genotypes were susceptible. On the contrary, out of 19 moderate susceptible genotypes, only eight genotypes showed positive yield loss. However, the remaining genotypes showed negative yield loss. Therefore, these genotypes were not grouped under the susceptible category due to the yield gain. In general, the evaluation based on the damage percent alone cannot give full information on resistance and susceptibility of the tef genotypes to tef shoot fly. Therefore, looking on both damage percent and yield loss would be the accurate method for the categorization of genotypes. Genotypes having resistance and moderately resistance genotypes would better to have at least 0 to negative yield loss. Whereas, moderately susceptible, susceptible and highly susceptible genotypes by the damage percent also must have yield loss value of greater than 0. In general, in tef growing areas, with moisture limitation tef shoot fly infestation causes yield losses. Therefore, providing resistant variety (acc.17 WJ) and chemical application is recommended for these areas.
CONCLUSION AND RECOMMENDATIONS
The acc. 17 WJ was a resistance for tef shoot fly as the result revealed. While, the standard checks were categorized or grouped under the susceptible category. Therefore, the acc. 17 WJ is recommended for the tef shoot fly resistance. Tef damage (dead heart and white headed) due to tef shoot fly ranged from 4.20 to 36.96%. Consequently, the yield loss also varied from 3.58 to 97.83 kg ha-1. Although tef shoot fly infestation caused yield losses in some genotypes, it had increased grain yield in some other tef genotypes. The evaluated 49 tef genotypes were classified into resistant, moderately resistant, moderately susceptible, and susceptible and highly susceptible (Table 1). Duration of tef shoot fly infestation determined between the beginnings of August to the end of September. Conditions favorable to tef shoot fly are the erratic rainfall, inherently susceptibility of tef genotypes, late produced productive tillers and inputs (chemical andmanure fertilizers), which are good environment for the reproduction and growth of the fly. Therefore, the genotypes which exhibited resistant and moderately resistant tef shoot fly damage would be important for the production of tef. In areas where the mentioned condition is faced and the infestation of shoot fly occurred, the application of chemical at early stage is recommended. Moreover, evaluation of the tef genotypes at multi-location for further investigation is better.
The authors have not declared any conflict of interests.
REFERENCES
|
Abro GH, Lakho GM, and Syed TS (2003). Relative resistance of some rice cultivars to yellow, Scirpophagaincertulas and pink, Sesamia inference stem borers. Pak. J. Zool. 35:85-90.
|
|
|
|
Assefa K, Yu JK, Zeid M, Belay G, Tefera H, Sorrells ME (2011). Breeding tef [Eragrosttef (Zucc.) trotter]: conventional and molecular approaches. Plant Breed. 130:1-9.
|
|
|
|
|
Axum Agriculture Research Center (AxARC) (2014). Axum Agriculture Research Center annual research report for the period of 2013/14. Axum Tigray, Ethiopia.
|
|
|
|
|
Bayeh Mulatu (2004). Estimates of Yield losses Caused by Major Insect Pests' of Main Crops Grown in North Wollo and Wag-Hamera Zones. Working paper, Integrated Pest
|
|
|
|
|
Bayeh M, Biruk W, Gezahegne G, Belay Eshetu (2009). Shoot fly on Tef panicles in South West and West Shewa Zones: Results of Survey, Yield Loss Assessment and Insecticide Control. Paper presented at the 16th Annual conference of the Plant Protection Society of Ethiopia, August 13-14, Addis Ababa, Ethiopia.
|
|
|
|
|
Bayeh M, Mekasha C, Tafa J, Tesfaye B, Yeshitela M, Asmare D, Bayuh B, Biruk W (2008). Review of research outcomes on insect pests of economic importance to major small cereals. pp. 325-374. In: Abraham Tadesse (ed.) Increasing Crop Production through Improved Plant Protection Vol. 1. Proceedings of the 14th Annual Conference of the Plant Protection Society of Ethiopia (PPSE), pp. 19-22.
|
|
|
|
|
December 2006, Addis Ababa, Ethiopia.
|
|
|
|
|
Berhane M, Zenebe A, Ibrahim F (2015). Farmer's Perception of Productivity and Profitability of Organic and Conventional tef [Eragrostistef (Zucc.) Trotter] Production: 77 Tigray, Northern Ethiopia. J. Agric. Sci. Food Technol. 1(7):88-93.
|
|
|
|
|
Central Statistical Agency (CSA)(2015). Agricultural Sample Survey for2013/14.Statistical
|
|
|
|
|
Corbeels M, Abebe S, Mitiku H (2000). Farmers' knowledge of soil fertility and local management strategies in Tigray, Ethiopia. Managing Africa's Soils, No. 10
|
|
|
|
|
Deeming JC (1971).Some species of Atherigona Rondani (Diptera, Muscidae) from northern Nigeria, with with special reference to those injurious to cereal crops. Bull. Entomol. Res. 61:133-190.
Crossref
|
|
|
|
|
Debre Zeit Agricultural Research Center .Crop (DZARC) (1983). Debre Zeit Agricultural Research Center. Crop Protection Annual Research Report.
|
|
|
|
|
DebreZeit Agriculture Research Center (DZARC) (2002). DebreZeit Agricultural Research Center. Annual Research report for the period for the Period 1977-1983. Addis Ababa University, Ethiopia.
|
|
|
|
|
Zerihun Tadele (eds.) Achievements and Prospects of tef Improvement; Proceedings of the Second International Workshop, November 7-9, 2011, DebreZeit, Ethiopia. Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia; Institute of Plant Sciences, University of Bern, Switzerland. Printed at Stampfli AG, 3001 Bern, Switzerland. ISBN: 978-3-033-03818-9.
|
|
|
|
|
Tesfaye B, Zenebe W (1998). Barley fly incidence on tef in Tigray. In: Beyene Seboka and AberaDeressa (eds). Agricultural research and technology transfer attempts and achievements in north Ethiopia. Proceedings of the Fourth Technology Generation, Transfer, and Gap Analysis Workshop, 18-21 March 1997, Bahir Dar, Ethiopia, pp. 173-179.
|
|
|
|
|
Vavilov I (1951). The origin, variation, immunity and breeding of cultivated plants. Translated from the Russian by K. S. Chester, Ronald Press Co. New York, USA.
Crossref
|
|
|
|
|
Visalakshmi VN, Hari S, Jyothula DPB, Raju MRB, Ramana MKV (2014). Screening of rice germplasm for resistance to yellow stem borer Scirpophaga80incertulaswalker. Int. J. Plant Anim. Environ. Sci. 4(1).
|
|