ABSTRACT
Common bean (Phaseolus vulgaris L.) is an important source of food and income for majority of households in Sub-Saharan Africa. However, bean production in Uganda is being affected by drought which has resulted from recent changes in climate. Developing high-yielding and drought-tolerant bean cultivars would significantly contribute to increased and stable yields in drought-prone environments. However, prior research was not focused on breeding for drought tolerance in bean in Uganda. Thus, this study sought to elucidate the genetics governing the inheritance of drought tolerance in Ugandan bean genotypes, through establishing the mechanism of inheritance of this trait in the genotypes relevant to Uganda. Five drought-tolerant and three drought-sensitive genotypes were hybridized using a NCII mating design. The findings of the study indicated that drought tolerance is controlled by both additive and non-additive gene action with more predominance of additive gene effects for seed yield, pod weight, seed and pod and number. Further findings also revealed that the genotypes SEN 99 and NABE 15 are good combiners for drought tolerance.
Key words: Phaseolus vulgaris, drought, screening, combining ability, inheritance.
Climate change and food security are important issues challenging Uganda (NAPA, 2007) and Sub-Saharan Africa. Production of food crops is mainly dependent on natural rainfall and as such variety improvement for drought tolerance is key in coping with the negative impact of climate change on food security. Common bean is the most important crop legume in Uganda (Haggblade and Dewina, 2010), providing both food and income especially for the poor (Katungi et al., 2009) and it accounts for 7% of the national agricultural gross domestic product (CIAT, 2008). Thus, the crop’s adaptation to climate change requires immediate action.
Drought is becoming particularly more frequent and prolonged (NAPA, 2007) and is expected to have increasing negative effects on common bean production in Uganda (Kiwuka et al., 2012). It has been reported that the dry spells being experienced during the rainy season are sufficient to reduce agricultural production but these dry spells are expected to result into prolonged droughts in the future (NAPA, 2007) and this will have devastating effects on the yield of drought sensitive crops such as common bean.
According to Nielsen and Nelson (1998) yield reductions in common bean resulting from drought depend mainly on the severity and the period of drought occurrence. Reports by White and Singh (1991) have indicated overall common bean yield reductions in most production regions in the world as a result of drought. Singh (2007) quantified reductions in common bean seed yields to be as high as 88% depending on the cultivar and severity of the drought. In addition, Thornton et al. (2009) predicted that higher temperatures will affect the altitudinal range of adaptation of bean genotypes, reduce root growth and accelerate decomposition of soil organic matter, thereby aggravating drought stress.
With the present climate change, drought will continue to threaten the stability of Ugandan bean production (NAPA, 2007; Hepworth and Goulden, 2008) considering that less than 1% of the total arable land is irrigated (Kiiza, 2001). These effects are more profound with resource poor producers living in drought prone areas, who cannot afford to use irrigation (Wortmann et al., 1998). The development of high-yielding and drought-tolerant bean cultivars should significantly increase and stabilise yield in drought-prone environments. Considering the significant role that common bean plays in human nutrition and livelihood (CIAT, 2008), failure to address drought constraints might impact negatively on the livelihoods of the people living in drought prone areas of Uganda.
Previous attempts to improve the market-preferred Ugandan common bean genotypes for tolerance to drought were made by the National Bean Breeding Program in Uganda. Five drought-tolerant genotypes were obtained from CIAT and screened together with three market-preferred Ugandan bean genotypes. Results of the screening indicated possible existence of drought-tolerance in CIAT genotypes SEN 98, SEN 99 and SCR 48. However, there was no evidence for drought tolerance in the screened Ugandan genotypes (Amongi, 2013). Considering that these drought-tolerant genotypes are not adapted to Uganda’s agro-ecological zones, there exists a need to introgress drought tolerance into the Ugandan genotypes and also understands the inheritance of drought tolerance in these genotypes.
Common bean has a wide genetic base (Beebe et al., 2013; Kiwuka et al., 2012) with genetic differences reported in traits such as seed weight, leaf proline content, stay-green, root spread and depth, all of which play major roles in drought tolerance (Thomas, 1983; Badr, 2005). Previous studies have shown that drought tolerance in this crop is controlled by quantitative traits (Blum, 2002; Acquaah, 2007; Beebe et al., 2008, Mukeshimana et al., 2014). According to Thomas (1983) and Badr (2005), seed weight in bean is controlled by a large number of genes with both additive and dominance effects. In addition, Badr (2005) reported the role of partial dominance for total yield/plant where relatively low narrow sense heritability (< 60) estimates were obtained on a single plot basis. Similarly, Ramirez and Kelly (1998) found higher heritability (> 60) estimates on family mean basis for seed yield in a segregating population. In other drought related research on common bean, both additive and non-additive effects for seed yield and pod number per plant under drought stress have been reported (Asadi et al., 2010). Also, Makunde et al. (2007) found predominance of non-additive genes for seed yield. In this study, Ugandan market-preferred genotypes were crossed with the non-adapted drought-tolerant genotypes from CIAT to specifically, establish the mechanism of inheritance of drought tolerance in these crosses. The information generated will in turn be used to facilitate planning of an efficient breeding program for the improvement in the level of drought tolerance in common bean in Uganda.
Study area
The study was conducted in a screen house at the National Crops Resources Research Institute (NaCRRI) located in Namulonge, Wakiso District, 28 km north of Kampala (32° 34’E, 0° 32’N). The Institute’s elevation is 1150 m above sea level and it receives mean annual precipitation of 1300 mm. Its mean annual temperature is 22°C with annual minimum and maximum temperatures of 16 and 28°C, respectively. The temperature and humidity in the screen house ranged from 20 to 34°C and 45 to 96%, respectively. The water holding capacity of soil used in the study was 29 ml/100 g fresh soil.
Developing a breeding population
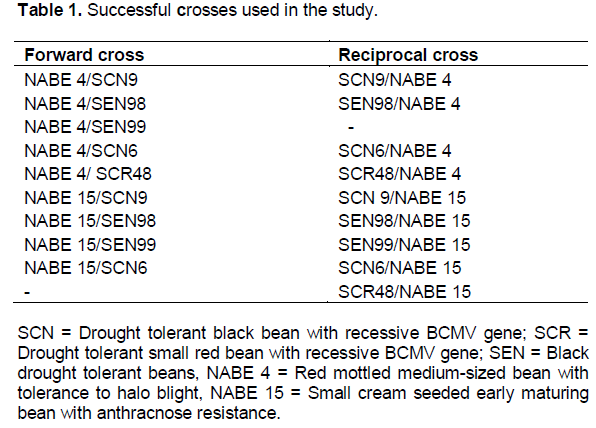
Eight genotypes were crossed in order to create a breeding population. Five exotic genotypes, three of which were confirmed as drought tolerant were obtained from CIAT, while the three market-preferred genotypes were provided by the National Bean Program in Uganda. The hybridization program utilized adapted (local) and non-adapted (exotic) drought-tolerant parents. Thus, a full North Carolina II (NC II) mating design, adapted to include reciprocals was used to produce 30 F1 families (Table 1). Wide application of NCII mating design is in studies of combining ability, heterosis and in estimating additive and non-additive gene effects. The NCII mating design is commonly used to estimate both general and specific combining ability of inbred lines (Acquaah, 2007).
Population advancement and screening for drought tolerance
The F1 plants for each of the 28 out of 30 successful NC II progenies were selfed to derive F2 seed. However, eight out of the 10 crosses of K132 with the five CIAT genotypes did not produce F2 seed because of inter gene pool incompatibilities. The successful crosses shown in Table 1 and the eight parents were phenotypically screened for drought tolerance for the following traits: leaf rolling, primary leaf lamina drooping, number of trifoliate leaves, dry pod and seed weights, number of pods per plant and number of seed per pod.
The genotypes were subjected to drought conditions in the screen house to determine their reactions to moisture stress using two watering regimes of either daily watering or watering after every four days. The critical watering interval was estimated based on the yield reduction observed by Amongi (2013). The water-stressed treatment was fully watered until 18 days after planting and thereafter, it was supplied with one litre of water in the late morning hours on the appropriate day. The well watered treatment was irrigated daily with one litre of water until physiological maturity. The experimental design used for this evaluation was a randomised complete block design in a split plot arrangement with only two replications due to limited seed. An experimental unit consisted of sixty Ten-litre dishpans each containing six plants, that is, four plants per cross and their two parents. Stress treatment was duplicated within a replication. Twenty (20) of the 60 dishpans were therefore well watered and 40 were imposed to drought stress. The stressed treatment was duplicated to increase the number of stressed plants in order to obtain reliable information on drought stress. Each dishpan contained 10 kg of sandy-clay-loam soil. Moisture meter that records a value of 1 to 5 when inserted in the soil was used in managing fluctuations of soil water.
Data collection
Data on potential drought stress indicators on growth and yield associated parameters were collected on a single plant basis. For growth parameters, data were collected on leaf rolling, primary leaf lamina drooping and number of trifoliate leaves. A 5-point scale where 0 = Not rolled / drooped leaf; 1 = shallow V-shaped leaves; 3 = deep V-shaped leaves; 5 = fully capped leaves / lamina fully collapsed and wrinkled, and 7 = tightly rolled leaves / lamina fully collapsed and dried was used to score for leaf rolling and laminate drooping (Amongi, 2013). When 90% of the pods had reached physiological maturity identified as a change in colour from green to yellow (Munoz-Perea et al., 2006), the number of pods per plant, seed number per pod, pod dry weight, and seed dry weight (g/plant) were recorded. The seeds were oven dried at 30°C for 3 weeks before recording seed weight (g). In addition, plants were also closely monitored for root rot infection caused by Fusarium solani f. sp. phaseoli through visual inspection of the stem base for necrosis.
Data analyses
Means of individual plant values for the 18 F2 populations and their seven parents were computed per replication for statistical analyses using the GenStat computer package (Release 14.1, PC/Windows 7; VSN International Ltd., 2011). Individual replication data were entered and subjected to general analysis of variance using the linear model shown:
Where,

, Mean of a specific cross; Y, Grand mean; GCA, general combining ability; Effect of the parent in the phenotypic mean of its crosses, i = female, j = male; SCA, specific combining ability; Phenotypic value of a specific cross compared to the value predicted from parental GCA values; B
k, Block effect; e
ijk, Error effect.
The error variance obtained from individual replication data analysis was used to test the significance of the sources of variations. The means of F2 progenies were subjected to general ANOVA and regression analysis using Genstat (Release 14.1 PC/Windows 7; VSN International Ltd., 2011) to determine the variance of general combining ability (GCA), specific combining ability (SCA), reciprocal and direction effects. SCA effects were calculated by subtraction of predicted means from observed means. In addition, the significance of SCA and GCA effects were tested using a standard t-test [t = effect / (standard error of the effect)]. Within watering regimes analyses were performed and to provide more understanding on the importance of the GCA, and SCA for the variables, their variance components which exclude the extraneous effect of replication unlike mean squares (variance) were estimated. These estimates were then used to calculate Baker’s ratio according to Baker (1978) and coefficients of genetic determination (estimate of heritability). Baker's ratio which estimates the relative significance between additive and non-additive effects (Baker, 1978) was calculated as:
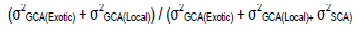
Where, σ2, Sample variance; (σ2GCA(Exotic) + σ2GCA(Local)), Additive gene effect; (σ2GCA(Exotic) + σ2GCA(Local)+ σ2SCA), Total genetic effect. Narrow sense coefficient of genetic determination (NS CGD ≈ h2), a proportion of the phenotypic variation attributed only to additive gene effects (Falconer and Mackay, 1996) was calculated as:
Where, e, Sample error; (σ2GCA(Exotic) + σ2GCA(Local)), Additive gene effect; (σ2GCA(Exotic) + σ2GCA(Local)+ σ2 SCA+ σ2e), Phenotypic effect. Broad sense coefficient of genetic determination (BS CGD ≈ H), a proportion of the phenotypic variation due to all genetic effects (Falconer and Mackay, 1996) was calculated as:
(σ2GCA(Exotic) + σ2GCA(Local)+ σ2 SCA) /(σ2GCA(Exotic) + σ2GCA(Local)+ σ2 SCA+ σ2e),
Where, (σ2GCA(Exotic) + σ2GCA(Local)+ σ2SCA), Total genetic effect; (σ2GCA(Exotic) + σ2GCA(Local)+ σ2 SCA+ σ2e), Phenotypic effect.
Drought intensity index (DII) was calculated as 1 - (Mean yield from stressed environment / Mean yield from well watered environment) (Ramirez-Vallejo and Kelly, 1998). Values of DII exceeding 0.70 indicates severe drought.
Phenotypic performance of F2 populations and parents
The analysis of variance of 18 F2 populations and their seven parents in varying watering regimes showed that their response to watering were significantly different (P ≤ 0.001) for all the parameters. However, their interactions with watering regimes were only significantly different (P ≤ 0.05) for primary leaf lamina drooping (Table 2).
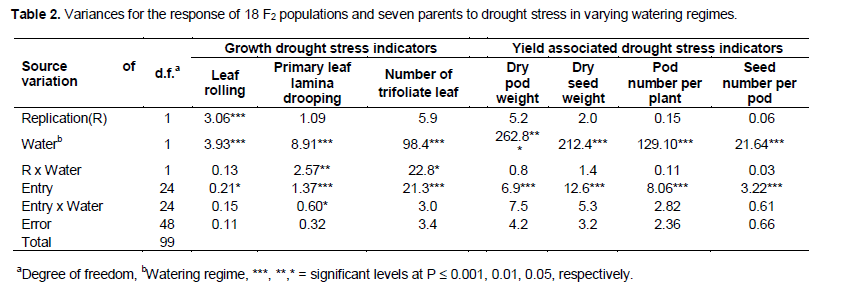
The populations and parents were significantly different (P ≤ 0.05) for all variables under water stress (Table 3). The F2 population, NABE 4 x SCN 9, had the lowest leaf lamina drooping and low leaf rolling but also the lowest number of trifoliate leaves and no pod production under water stress. However pod number per plant and seed biomass for the F2 populations, SEN 98 x NABE 15 and SCR 48 x NABE 15 were not only the highest but also greater than the mean of all F2 populations under water stress (Table 3). The F2 populations namely; NABE 15 x SEN 98, NABE 15 x SEN 99, SEN 99 x NABE 15 and SCR 48 x NABE 15 produced the highest number of trifoliate leaves, greater than the mean of all F2 populations. In addition, the F2 populations, NABE 15 x SEN 98 and SCR 48 x NABE 15 also produced high dry pod and seed weight, pod and seed number greater than the mean of all the crosses, unde water stress (Table 3).
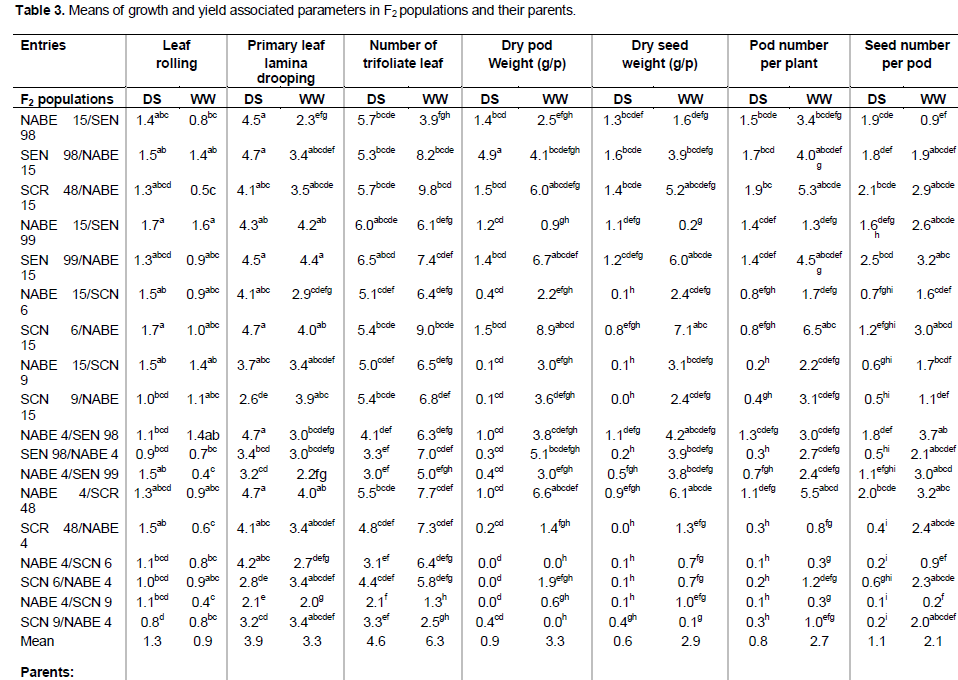
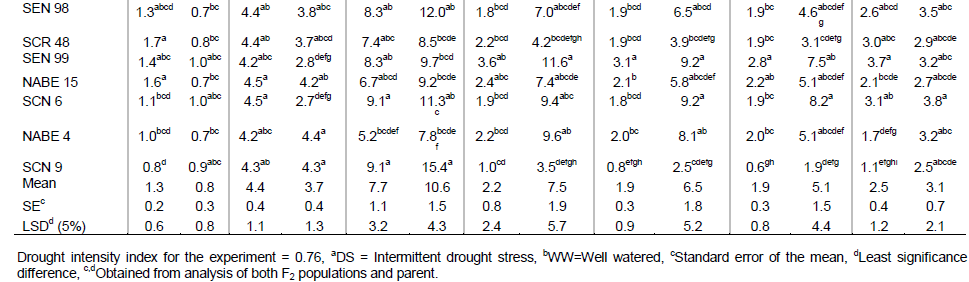
Contribution of the exotic verses local parent
The analysis of variance for the performance of F2 populations in each watering regime revealed that the F2 populations expressed more variation under water stress. It further showed that the variance of general combining ability (GCA) for both exotic and local parents differed significantly (P ≤ 0.01) for primary leaf lamina drooping (LD-P), seed weight, pod and seed number under drought stress (Table 4). The GCA variance for leaf rolling, trifoliate leaf number and pod weight were only significantly different (P ≤ 0.05) for local parents. No significant differences were observed for variance of specific combining ability (SCA) and reciprocal effect for yield associated variables.
However, direction effect was significant (P ≤ 0.01) for seed number and reciprocal effect on laminate drooping under water stress (P ≤ 0.05) (Table 4). The comparison of variance components of GCA revealed that GCA for local parents were higher than the GCA for exotic parents for leaf rolling, trifoliate leaf number and pod weight under water stress. The reverse is true for primary leaf lamina drooping, seed yield, pod and seed number (Table 4).
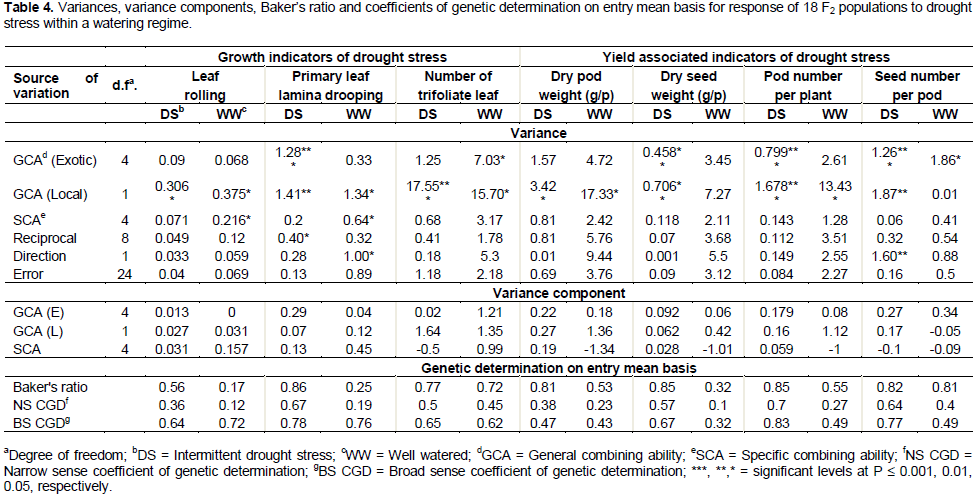
Heritability and Baker’s ratio
Baker’s ratios for genetic determination greater than 0.8 and 0.5 were reported under water stress treatment for yield associated variables and growth parameters, respectively. Under water stress, the NS-CGD ranged from 0.36 to 0.7 while BS-CGD ranged from 0.47 to 0.83 for all parameters (Table 4).
Combining ability effects
The parents with positive significant GCA effects for number of trifoliate leaf, dry pod and seed weight, pod number per plant and seed number per pod were defined as good combiners, whereas those with negative significant and non-significant GCA effects for these traits were designated poor combiners. This is because high values for these variables are desirable in a well performing progeny, whereas the opposite was true for leaf rolling, and primary leaf lamina drooping since lower values for these variables could imply less drought stress on a progeny. Genotype SCN 9 had the lowest significant (P ≤ 0.05) GCA effect for leaf rolling, primary leaf lamina drooping, seed weight, pod and seed number and it was followed by NABE 4 for all growth parameters under water stress. On the other hand, SCR 48 had high significant (P ≤ 0.05) GCA effect for primary leaf lamina drooping and on number of pod per plant and seed per pod. It also had high GCA effects pod and seed yield. The genotype, SEN 98 had the highest GCA effect for seed yield and pod weight and also a high significant (P ≤ 0.05) GCA effect for pod number (Table 5).
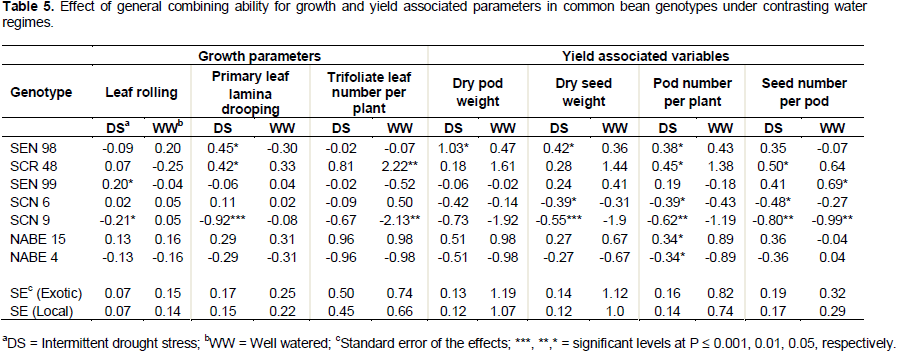
Considering the specific combining ability, F2 individuals of NABE 15 and SEN 98 had high positive SCA effects for trifoliate leaf number, pod weight (significant at P ≤ 0.05) and pod number under water stress. High positive SCA effects were in turn recorded for pod and seed weight and pod number for the cross SEN 99 x NABE 15. Furthermore, the cross NABE 15 x SCR 48, had relatively high SCA effects for trifoliate leaf number, and seed number under drought stress. The SCA effect was high for most drought stress indicators under well watered condition (Figure 1).
In any breeding strategy, germplasm diversity is of paramount importance when creating a breeding population (Kiwuka et al., 2012). Knowledge of the sources of resistance/tolerance and the gene action governing the trait of interest is particularly important in the improvement and selection of desired traits. This study was performed to investigate the mechanism of inheritance of drought tolerance in the crosses of Ugandan genotypes and CIAT drought-tolerant genotypes as a prerequisite in planning an efficient breeding program for drought tolerance in common bean in Uganda.
Phenotypic performance of F2 populations and parents
The drought intensity index (DII) for the experiment calculated basing on seed yield was 0.76 implying the water stress was high. Values of DII exceeding 0.70 indicates severe drought (Ramirez-Vallejo and Kelly, 1998). Nonetheless, high differences occurred between parents and crosses for all parameters suggesting diversity among genotypes. Generally, the parents performed better than the crosses for most yield associated indicators of drought stress because many F2 plants did not produce seed. Thus, there were not many elite recombinants. In addition, analysis of variance showed more contribution from the exotic genotypes for yield and associated parameters and more for growth parameters from the local genotypes.
Contribution of the exotic verses local parent
The relative magnitude of GCA and SCA variance or variance component provides information on the predominant type of gene controlling the inheritance of a trait (Baker, 1978). On the other hand, the relative magnitude of variance component of GCA male (exotic) and GCA female (local) provides information on the relative genetic contribution of the different categories of parents used in a cross. The significant differences reported in both GCA variance for exotic parents and GCA variance for local parents under water stress implies that gene action is additive and both exotic and local parents contributed towards drought tolerance or sensitivity for primary leaf lamina drooping (LD-P), seed weight, pod number per plant and seed number per pod.
In addition, the GCA variance for local parents were not only significant but also had the highest variance components for pod weight under water stress, implying that accumulation of assimilates in pod wall was mainly contributed by the local parent. There was more contribution by the exotic parents for LD-P, seed weight, seed number per pod and pod number per plant basing on the relative magnitude of variance components under water stress.
Heritability and Baker’s ratio
The predominant role of additive genes in the inheritance of seed yield under water stress is supported by previous studies (Venkatraman et al., 2007; Badr, 2005; Asadi et al., 2010). However, contrary to the findings of these studies, the SCA variance of parents for seed biomass yield was not significant although its variance component was high, possibly implying statistical inadequacy due to small sample size. High SCA variance like that obtained in leaf rolling (LR) indicates presence of non-additive genes (Baker, 1978) which results in low heritability and thus delayed selection till advanced generations at F6 or F7. Seed weight exhibited high estimates for narrow sense (≥ 0.50) and broad sense (≥ 0.72) coefficients of genetic determination on entry mean basis as did LD-P, number of trifoliate leaves, pod and seed weight, pod and seed number. The result on seed weight is supported by findings of Ramirez and Kelly (1998). Despite the high narrow sense heritability reported in these traits, increased replication and multi-location testing would be necessary to effectively select for drought tolerance because it is quantitative inherited (Teran and Singh, 2002; Beebe et al., 2008) and high genotype x environment has been reported (Beebe et al., 2013). In addition, these traits also recorded a high Baker’s ratio (≥ 0.77) under drought stress implying predominance of additive genes. The genetic superiority observed in one generation would, therefore, be largely passed on to subsequent generations. It also means that the value of the F2 individuals can be predicted from the mid parent. Baker’s ratio of 1 implies total influence of additive genes (Baker, 1978). For a self-pollinated crop like bean, a trait with high Baker’s ratio means that the genes controlling that trait can be fixed by the breeder in advanced generations, a time when non-additive genes have been lost.
Combining ability effect
Combining ability effects are effective genetic information used in planning the next phase of breeding programs. From this study, the lowest negative significant GCA effects recorded for SCN 9 with respect to LR and LD-P under water stress are desirable indicators of drought tolerance. Genotype SCN 9 has the potential to produce more progenies that can withstand high levels of water stress before showing LR and leaf lamina drooping signs. Similarly, the low negative non-significant GCA effect for LR and LD-P recorded for SEN 98 and SEN 99 respectively, the high positive significant GCA effects for pod and seed biomass, and pod number per plant recorded for SEN 98 in addition to the high positive GCA effects for seed weight, pod number per plant and seed number per pod obtained for SEN 99 are the desirable
effects for producing more drought-tolerant progenies. This is in agreement with Franco et al. (2001) findings where they reported that crosses involving parents with higher estimates of general combining ability for traits where high values are desirable should be potentially superior for the selection of lines in advanced generations.
Given the high SCA effects recorded in F2 individuals of NABE 15 and SEN 98 for trifoliate leaf number and pod weight and in SEN 99 x NABE 15 for pod and seed dry weight and pod number per plant would indicate that the means of these F2 individuals were higher than predicted for the mentioned indicators of drought stress. These effects imply that genotypes SEN 99 and NABE 15 could be considered as good combiners for use in future drought breeding programs in common bean.
This study generated knowledge on the genetic inheritance of drought tolerance in a chosen set of common bean parents. The role of additive genes was noted to be greater than non-additive gene action in most parameters under drought stress. This implies that weight and number of seeds and pods, number of trifoliate leaves and laminate drooping can be fixed in advanced generations. In comparison, it is worth noting that non-additive genes; dominance or epistasis played a significant role in the inheritance of leaf rolling under water stress which suggests that this trait would be lost in advanced generations. Genotypes SEN 99 and NABE 15 were noted to be good combiners because they had a high SCA effect resulting from a higher mean for seed yield, pod weight and pod number than predicted.
In addition, genotypes SEN 98, SEN 99 and SCR 48 had high positive GCA effects for yield associated variables. Thus, these genotypes should be useful donors to improve drought tolerance. The best progenies from the cross made between NABE 15 x SEN 99, SEN 98 x NABE 15 and SCR 48 x NABE 15 (Figure 1) will be further screened and advanced and could possibly be released as new drought tolerant bean genotypes with traits that are preferred in Ugandan markets by local consumers.
The authors have not declared any conflict of interest.
REFERENCES
|
Acquaah G (2007). Principles of plant genetics and breeding. Blackwell Publishing Ltd. First Edition, pp. 121–392. |
|
|
|
Amongi W (2013). Inheritance of tolerance to intermittent drought stress in selected common bean (Phaseolus vulgaris L.) genotypes. MSc. Thesis, Makerere University, Kampala, Uganda, pp. 1-44. |
|
|
|
Asadi B, Bihamta MR, Dori HR (2010). Genetic analysis of drought tolerance in white bean. Seed. Plant Improv. J. 26-1(4):469-484. |
|
|
|
Badr LAA (2005). Inheritance and nature of drought tolerance in common bean (Phaseolus vulgaris L.). MSc. Thesis, Zagazig University, Benha Branch, Egypt, pp. 1-20. |
|
|
Baker RJ (1978). Issues in diallel analysis. Crop Sci. 18:533-536.
Crossref |
|
|
|
|
Beebe SE, Rao IM, Cajiao I, Grajales M (2008). Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 48:582–592.
Crossref |
|
|
|
|
Beebe SE, Rao IM, Blair W, Acosta-Gallegos JA (2013). Phenotyping common beans for adaptation to drought. Font. Physiol. 4:1-20.
Crossref |
|
|
|
Blum A (2002). Drought tolerance - Is it a complex trait? Field screening for drought tolerance in crop plants with emphasis on rice. In: Field screening for drought tolerance in crop plants with emphasis on rice. Saxena, N.P. and O'Toole, J.C. (Ed.), pp. 17-22. International Workshop on Field Screening for Drought Tolerance in Rice. ICRISAT, 11-14 December 2000, Patancheru, India. |
|
|
|
CIAT (2008). The impact of improved bush bean varieties in Uganda. Highlights - CIAT in Africa. p. 43.
|
|
|
|
Falconer DS, Mackay TFC (1996). Introduction to Quantitative Genetics. 4th Ed. Longman group. New York. |
|
|
Franco MC, Cassini STA, Rodrigues OV, Vieira C, Tsai SM, Cruz CD (2001). Combining ability for nodulation in common bean (Phaseolus vulgaris L.) genotypes from Andean and Middle American gene pools. Euphytica, 118:265-270.
Crossref |
|
|
|
|
|
GenStat (2011). GenStat Release 14.1 (PC/Windows 7). GenStat Procedure Library. Release PL22.1. VSN International Ltd. Rothamsted, UK. |
|
|
|
Haggblade S, Dewina R (2010). Staple food prices in Uganda: Prepared for the COMESA policy seminar on "Variation in staple food prices: causes, consequence, and policy options". Maputo, Mozambique, 25-26 January 2010 under the African Agricultural Marketing Project (AAMP), pp. 4-5. |
|
|
|
Hepworth N, Goulden M (2008). Climate Change in Uganda: Understanding the implications and appraising the response. LTS International, Edinburgh, pp. 3-4. |
|
|
|
Katungi E, Farrow A, Chianu J, Sperling L, Beebe S (2009). Common bean in Eastern and Southern Africa: A situation and outlook analysis. International Centre for Trop. Agric. (CIAT), pp. 1-26. |
|
|
|
Kiiza N (2001). Rainfall Reliability for Crop Production." Unpublished Project Report (Oct 2001). Department of Civil Engineering, Makerere University, Uganda. |
|
|
|
Kiwuka C, Bukenya-Ziraba R, Namaganda M, Wasswa MJ (2012). Assessment of common bean cultivar diversity in selected communities of central Uganda. Afr. Crop Sci. J. 20(4):239–249. |
|
|
|
Makunde GS, Beebe S, Blair MW, Rochirwa R, Lungu D (2007) Inheritance of drought tolerance traits in Andean x Andean and Andean x Mesoamerican F2 populations. Annu. Rep. Bean Improv. Coop. 50:159-160. |
|
|
Mukeshimana G, Butare L, Cregan PB, Blair MW, Kelly JD (2014). Quantitative Trait Loci associated with drought tolerance in common bean. Crop Sci. 54:923-938.
Crossref |
|
|
|
|
Munoz-Perea GC, Teran H, Allen RG, Wright JL, Westermann DT, Singh SP (2006). Selection for drought resistance in dry bean landraces and cultivars. Crop Sci. 46:2111-2120.
Crossref |
|
|
|
|
|
NAPA (National Adaptation Programmes of Action) (2007). Climate change: Uganda National Adaptation Programmes of Action, pp. 1-71 |
|
|
Nielsen DC, Nelson N (1998). Black bean sensitivity to water stress at various growth stages. Crop Sci. 38:422–427.
Crossref |
|
|
|
|
Ramirez-Vallejo P, Kelly JD (1998). Traits related to drought resistance in common bean. Euphytica 99:127–136.
Crossref |
|
|
|
|
Singh SP (2007). Drought resistance in the race durango dry bean landraces and cultivars. Agron. J. 99:1219–1225.
Crossref |
|
|
|
|
Teran H, Singh S (2002). Comparison of sources and lines selected for drought resistance in common bean. Crop Sci. 42:64-70.
Crossref |
|
|
|
|
|
Thomas CV (1983). Genetic, morphological and physiological studies of drought and heat resistance in tepary bean (Phaseolus acutifolius A. Gray) and common bean (P. vulgaris L.). Dissertation Abstracts International, B, 44(2):410. |
|
|
Thornton PK, Jones PG, Alagarswamy A, Andresen J (2009). Spatial variation of crop yield responses to climate change in East Africa. Glob. Environ. Change 19:54-65.
Crossref |
|
|
|
|
Venkatraman SH, Yadav SS, Kumar J (2007). Heterosis and combining ability for biomass and harvest index in chickpea under drought-prone, short-duration environment. Euphytica 157:223–230.
Crossref |
|
|
|
White J, Singh SP (1991). Physiology of yield potential and stress tolerance. In A. van Schoonhoven and O. Voysest (ed.) Common bean: Research for crop improvement. CABI, Walingford, UK, and Centro Internacional de Agricultura Tropical, Cali, Colombia, pp. 501–560. |
|
|
|
Wortmann CS, Kirkby RA, Eledu CA, Allen DJ (1998). Atlas of common bean (Phaseolus vulgaris L) production in Africa. CIAT, Cali, Colombia, p. 133. |
|
|
|
Walinga I, van Vark W, Houba VJG, van der Lee JJ (1989). Plant Analysis Procedures, Part 7. Department of Soil Science and Plant Nutrition, Wageningen Agricultural University, Syllabus 1989, pp. 197-200. |
|
|
|
Zhang P, Jaynes JM, Potrykus I, Gruissem W, Puonti-Kaerlas J (2003). Transfer and expression of an artificial storage protein (ASP1) gene in cassava (Manihot esculenta Crantz). Transgenic Res. 12:243-250. |
|
|
|
Ziska LH, Runion GB, Tomecek M, Prior SA, Torbet HA, Sicher R (2009). An evaluation of cassava, sweet potato and field corn as potential carbohydrate sources for bioethanol production in Alabama and Maryland. Field Crops Res. 33:1503–1508. |
 , Mean of a specific cross; Y, Grand mean; GCA, general combining ability; Effect of the parent in the phenotypic mean of its crosses, i = female, j = male; SCA, specific combining ability; Phenotypic value of a specific cross compared to the value predicted from parental GCA values; Bk, Block effect; eijk, Error effect.
, Mean of a specific cross; Y, Grand mean; GCA, general combining ability; Effect of the parent in the phenotypic mean of its crosses, i = female, j = male; SCA, specific combining ability; Phenotypic value of a specific cross compared to the value predicted from parental GCA values; Bk, Block effect; eijk, Error effect.