ABSTRACT
Infectious diseases like tuberculosis, malaria and the human immunodeficiency virus are both preventable and curable but cause about half of the deaths in the developing world. They affect people in the prime of their productive lives, inflicting a very heavy impact on development, opportunities and livelihood. Poverty is the end result with an enormous toll on the economy of developing nations. As such, the community goes for the cheapest medications that work and therefore can report on the effectiveness of drugs as an effect of many activities upstream that cause parasite unresponsiveness to therapy. The aim of this study was to investigate the relationship between community auto-medication and molecular markers of drug resistance in malaria to detect and provide evidence for when the community feels a drug is not or no longer effective. This would propose a better management policy towards decreasing drug resistance. This would be achieved by promoting proper therapy-seeking habits and thus ensure a higher quality of life and a higher productive capacity of workers. A survey was first carried out in 8 localities to observe various aspects of therapy-seeking behaviour. The knowledge and practice of malaria management and prevention were quite poor, favouring the prevalence of drug resistant parasites. To establish an index for community-sensing of drug resistance, the prevalence of the mutation of the dhfr gene at position 108 in parasites was used in which Y = -2.5X + 48, where Y = prevalence of mutations (Pm) and X = level of self-prescription. This was confirmed by the extrapolation of the self-prescription of Fansidar (12%) against a value of 18%. This study shows that the therapy-seeking habits of a community can be used to demonstrate the prevalence of mutations to Fansidar and hence possibly to other antimalarial drugs presenting resistance.
Key words: Therapy-seeking behaviour, auto-medication, community-sensing, molecular markers, surveillance indicators, drug resistance.
Abbreviation:
AluI, Arthrobacter luteus I; ApoII, Arthrobacter protophormiae II; Bsr I, Bacillus stearothermophilus I; CDC, Centers for Disease Control and Prevention; CTE, Cameroon Tea Estate; dhfr, dihydrofolate reductase gene; DNA, deoxyribonucleic acid; dNTP, deoxynucleotide triphosphate; HaeIII, Haemophilus aegyptius III; HindIII, Haemophilus influenzae III; HIV, human immunodeficiency syndrome; P. falciparum, Plasmodium falciparum; PCR, polymerase chain reaction; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; Pfcrt, Plasmodium falciparum chloroquine resistance transporter gene; Pm, prevalence of mutations; PR, prevalence of resistance; RDT, rapid diagnostic tests; RFLP, restriction fragment length polymorphism; Taq, Thermophilus aquaticus; UV, ultraviolet; WBC, white blood cell; WHO, World Health Organization.
Highly endemic mostly in the tropics, malaria is caused by protozoan parasites of the genus Plasmodium, 5 species of which affect humans (Kantele and Jokiranta, 2011). The seriousness and gravity of this vector-borne disease cannot be overemphasized, given that about 3.4 billion people are at risk, of which 1.2 billion are at high risk. This led to an estimated 207 million clinical cases which resulted in about 627 000 deaths in 2012, 90% of which occurred in sub-Saharan Africa (WHO, 2013). Malaria is a huge socio-economic problem in tropical countries. Its treatment and control require large sums of money, both for individuals and for the government. The socio-economic consequences at the personal level are numerous and result in reduced productivity as a whole. A striking correlation has been shown between malaria and poverty. The fact that malaria-endemic countries also have lower rates of economic growth adds to the burden, hence the conclusion that where malaria prospers most, human societies have prospered least. Its effects on fertility, population growth, saving and investment, worker productivity, absenteeism and school performance, premature mortality and medical costs have been cited as some of the ways by which malaria impedes development (Rollback Malaria, 2013).
Chemotherapy remains the best intervention measure against the malarial parasite (Sanz et al., 2012). However, the occurrence and increasing resurgence of resistant strains of the parasites and vectors to drugs and insecticides respectively have greatly weakened the use of these tools as the main strategies for the control of the disease. This makes the need for new and alternative control measures a matter of utmost importance and urgency which has therefore not brought the desired dramatic decrease in prevalence. Presently, combination therapy is the most effective measure against uncomplicated malaria (Nosten and White, 2007). The uptake of interventions is tricky in poor settings and the low rates are as a result of preference for cheap or community-wide perceived effectiveness of certain therapies. This prompted the need to study the therapy-seeking behaviour and practices in an area known for anti-malaria drug resistance.
The aim of the study was therefore to relate therapy-seeking behaviour to prevalence of biochemical markers of resistance by establishing the frequency of mutations to Fansidar-metabolising drugs. It is believed that this will set baseline data for monitoring programmes and for educating populations on habits that would disfavour drug resistance. It is assumed that the study will provide tools for the monitoring of resistance and thus contribute to a better understanding of the epidemiology of malaria in view of better management policies.
Study populations
An overall of 498 persons in urban and rural settings responded to the questionnaire in the towns of Yaounde, Djuttitsa, Kumba, Limbe, Buea, Nkambe and Ndu. For the molecular analyses, a temporary malaria laboratory was set up in the absence of a diagnostic laboratory at the Djuttitsa Health Centre. Blood samples were collected by venupuncture from consenting individuals visiting the health clinics with complaints of fevers. Written informed consent was obtained from all the participants after an explanation of the aim and methods of the study according to the Declaration of Helsinki.
Surveys on therapy-seeking behaviour
A questionnaire designed to determine therapy-seeking behaviour was administered and either filled out by the participants or interpreted into the local languages and filled out by trained field staff. Commonly used anti-malarial drugs were displayed for the participants to identify which ones they had previously taken or prefer.
Sample collection
Two drops of blood were collected by finger prick to prepare thick and thin smears for microscopy. For the other analyses, more drops of blood were then collected on filter paper, air dried, put in mini-grip plastic bags containing silica gel and preserved at room temperature. The haematocrit and complete blood cell count were also determined. Anthropometric data including weight, temperature, age, gender and medication previously taken or preferred were recorded.
Microscopy
Thick and thin blood smears were dried and the thin smear fixed in methanol for 15 s. Both smears were stained with Eosin (Solution I) followed by Methylene Blue (Solution II) for 15 s each. The slides were then rinsed with distilled water, dried and observed under a microscope at a magnification of 1000. The thick smear was used for the diagnosis of malaria and the thin blood smear for speciation. Parasitaemia was determined by:
Parasitaemia (μl-1) = Number of parasites counted × Estimated WBC count (8,000) / Number of WBC counted (200 where WBC is the white blood cell.
DNA extraction and molecular genotyping
Parasite DNA was extracted from filter paper whole blood samples by the Chelex method and stored at -20°C. Genotyping for prevalence of mutations was done by Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP). DNA amplification was done using 1X PCR buffer, 2.5 mM MgCl2, 1.0 mM dNTPs, 1.7 pmols of primers, 1 unit/µl of Taq DNA Polymerase, DNA-free water and DNA at a final reaction volume of 25 μl. Deoxynucleotide triphosphates (dNTPs) were used at a final concentration of 0.5 μl. The dhfr gene mutation was genotyped using two sets of primers: for the outer PCR, Forward 5'-TTTATGATGGAACAAGTCTGC-3' and Reverse 5'- AGTATATACATCGCTAACAGA-3'; and for the nested PCR, Forward 5'-GAAATGTA ATTCCCTAGATATGgAATATT3' and Reverse 5'-TTAATTTCCCAAGTAAAACT ATTAGAgCTTC-3’. The PCR was carried out for the outer PCR at 94°C for 3 min (primary denaturation), followed by 40 cycles of 94°C for 1 min (denaturation), 50°C for 2 min (primer annealing), 72°C for 2 min (primer elongation), and a final extension of 72°C for 10 min. For the nested PCR: 94°C for 2 min (initial pre-heating), followed by 35 cycles of 94°C for 1 min, 45°C for 1 min (primer annealing), 72°C for 2 min (primer elongation), and a final extension of 72°C for 10 min.
A RFLP was performed to detect the presence of mutations on the dhfr gene with 5 μl of each sample digested either with AluI or BsrI restriction enzymes respectively to determine which of the “S” wild type or “N” mutants were present. The HB3 sensitive strain of Plasmodium falciparum served as the positive control. The digested amplicons from the nested PCR were analyzed by agarose gel electrophoresis using a 3% agarose gel. The resulting bands were visualized over a UV transilluminator.
Statistical analyses
A database was created for all the surveys using the Epi Info 2000 statistical software. Frequencies of occurrences or responses generated were used to describe the self-prescription trends. Chi-square analysis was used to compare groups of responses in order to obtain the levels of significance.
Survey analysis
The predominant age range was 21 to 30 and the rest were mainly older. The general state of health reported by the participants showed that malaria was a common chronic disease from which some suffered from time to time. Fever was the main symptom, sometimes accompanied by body pains, nausea, rigours, dizziness and fatigue. Auto-medication was the method preferred by more than 80% of the respondents both before and/or after testing. Self-prescription of anti-malarials was also accompanied by anti-fever drugs. Two-thirds of participants would really decide to go to health facilities only when other treatments failed. A variety of anti-malaria drugs were consumed by the participants but there was no outstanding medication of preference though Quinine-sulfate ranked first followed by Maloxine and Fansidar. One-quarter of the participants also preferred Quinine-sulfate as the choice drug for prophylaxis. More than half of the respondents said they completed their drug prescription while many stopped when they felt better. A small proportion of the respondents (6%) in Djuttitsa practiced the use of bednets in the prevention of malaria. There was a high tendency for self-prescribing of malaria and/or fever medications among the participants once they were ill, even without being sure what the problem was (Table 1). Most of the respondents took their drugs to completion. For a few, fever was the main disease symptom observed by the medical personnel, closely followed by headache, then body pains.
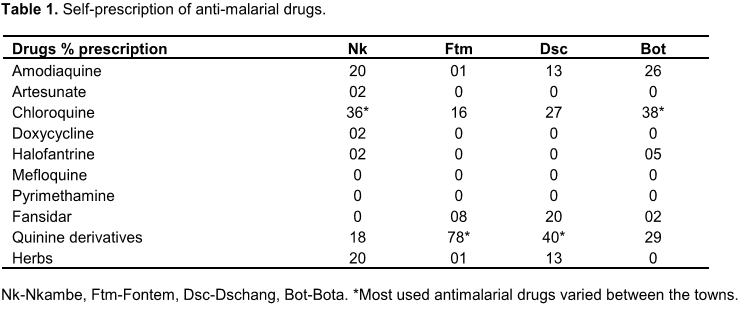
When tested for malaria, P. falciparum was found to be the predominant species (98%) and the only other species was Plasmodium malariae. The results shown in Table 1 also demonstrate that chloroquine and quinine derivatives were the most self-prescribed antimalarial drugs in the towns indicated.
Prevalence of mutations
The frequency of mutations so far established for chloroquine and Fansidar demonstrated a high prevalence of mutations on the pfcrt gene and variable levels on the dhfr gene.
The results in the BsrI digestible and the AluI & BsrI digestible boxes were used to determine the relationship between self-prescription and frequency of mutations (Table 2).
Presence of mutants and mixed parasite populations
Band migration distances were measured and recorded and product sizes were determined from a standard curve. The prevalence of mutations (Pm) was quantified as the absence of a digested product for AluI or the presence of a confirm mutation by digestion with BsrI.
From the PCR and RFLP experiments, the electrophoregram showed that there were some mixed type reactions of both sensitive and mutant parasites (Figure 1). The prevalence of mutations on dhfr 108N was at 18% for 108N and 3% represented the mixed type of S108N.
Extrapolation of the prevalence of mutation Dhfr 108N
Data obtained from mutational studies of the genes involved in folate metabolism in Plasmodium provided us with a measure of self-reporting and the presence of mutations in a given locality. The correlation between self-prescription and prevalence of mutations (Figure 2) resulted in establishing an equation for the best straight line through the data points. The straight line was established to be:
Y = -2.5x + 48
where Y = prevalence of mutations and x = self-prescription of Fansidar.
From the investigation on the Djuttitsa population, the level of Fansidar intake by self-prescription/preference was reported to be 12%. By applying this to the equation, this gave a value of Y = -2.5x 12 + 48 = 18.0% for the prevalence of the dhfr 108N mutation. By the PCR and RFLP experiments, a value of 21% was found for the mutations in Djuttitsa (cf 18%).
This study demonstrated that within margins of error the habits of a people can be indicative of the extent to which drugs might be perceived as effective or not. Sensing equation has been established for this purpose and used it to demonstrate the closeness of scores with field data. Previously, in an attempt to establish an index of resistance, Diourte et al. (2003) had defined Pm to be Pm/PR = I, where PR = prevalence of resistance. However, current data shows that it is not possible for it to be an index because of variations in prevalence and practice.
The microscopic analysis of the blood samples collected from subjects in one of the sites (Djuttitsa) gave a prevalence of 76.47% attributable to human and environmental factors which enhanced the breeding of mosquitoes. Environmental management and the hygiene of the inhabitants to avoid mosquito bites were not very encouraging. Very little sensitization on basic methods of prevention of transmission went on at all levels and could account for the poor knowledge of the prevention guidelines that emphasize the use of insecticide-treated bednets (CDC, 2015). Cameroon has also been reported to be highly endemic for malaria due to several socio-economic, environmental and climatological factors (Mbenda et al., 2014). Though the general state of health was declared good, the presence of malaria was such that participants could habitually assume from the symptoms that one was coming down with the disease. This was probably because of the frequency of the attacks and may explain why so few went for laboratory diagnosis, thereby making self-prescription and auto-medication common practice. Self-medication in malaria is supported by government agencies in Cameroon only in places that are quite inaccessible. Previously, in malaria-prone areas, if fever did not subside in 5 days after taking routine antibiotics, patients were encouraged to take Chloroquine. However, this medication has been taken off the shelf and is used only for other conditions like rheumatoid arthritis. It is also no longer used because of its monotherapeutic composition as opposed to the current recommended combined therapy.This intake of drugs when not needed could also be a fundamental cause of drug resistance, with the body getting used to drugs that are not necessary (Talisuna et al., 2004). The population therefore needs to be educated on the absolute necessitytotestbeforetreatmentandto complete treatment regardless of how they feel. In endemic areas, effective malaria management relies on accurate diagnosis and prompt treatment of suspected or confirmed infected individuals based on microscopic diagnosis or RDT testing of blood samples (Reyburn et al., 2007; Azikiwe et al., 2012). This is particularly important for the most vulnerable population groups, such as young children and non-immune populations, in whom life-threatening falciparum malaria can develop and be
rapidly fatal within hours (WHO, 2015).
A few medications were outstanding, and preference depended on the local practices. This was because the drug sellers had no formal training and therefore had erroneous, little or no idea of the physical and chemical methods of drug preservation, dosage, toxicity and compatibility, but supplied them to patients without complying with accepted specifications. The level of Fansidar intake by self-prescription and preference was 12% in Djuttitsa. By extrapolating on the graph, this corresponded to a mutational prevalence (Pm) of the dhfr gene in malaria parasites of 18%. This implied that resistance to Fansidar was low in this area. This level was similar to that found in Fontem (20%), but higher than that of Dschang (7%) and Yaounde (12%) during the same period (Basco and Ringwald, 2000). This difference between Djuttitsa and neighbouring Dschang could be due to the difference in self-prescription of the drug, being at 12 and 20%, respectively. From this, it was apparent that the higher the self-prescription, the lower the Pm.
Low drug resistance in the area could be attributed to the development of partial immunity to the illness, because of the constant presence of infection. It could secondarily be attributed to the low self-prescription of the drug. Mackinnon and Hastings (1998) stated that the rate of change of frequency of drug resistance is primarily a function of the number of people receiving that particular treatment. Ndo et al. (2011) also demonstrated that resistance to anti-malarial drugs is likely to occur with large scale anti-malaria drug use, self-prescription and inadequate doses from unreliable sources. Mbacham (1998) and Laxminarayan et al. (2006) observed that human habits in therapy matters such as inadequate dosing, incomplete courses and indiscriminate and inappropriate drug use have contributed to the emergence and spread of resistant strains.
The investigation was geared towards contributing to a better understanding of the epidemiology of malaria and the possibility of proposing public health management policies through establishing a relationship between a bio-diagnostic marker and a community-related index. This was achieved by relating self-reporting of auto-medication to molecular markers of drug resistance. There was an inverse relationship between self-prescription habits in an area and the prevalence of drug resistance markers, thereby providing a community sensor equation for predicting drug resistance from behavior.
The authors have not declared any conflict of interest.
REFERENCES
|
Azikiwe CCA, Ifezulike CC, Siminialayi IM, Amazu LU, Enye JC, Nwakwunite OE (2012). A comparative laboratory diagnosis of malaria: microscopy versus rapid diagnostic test kits. As. Pac. J. Trop. Biomed. 2(4):307-310.
Crossref
|
|
|
|
Basco LK, Ringwald P (2000). Molecular epidemiology of malaria in Yaounde, Cameroon VII. Analysis of recrudescence and re-infection in patients with uncomplicated malaria. Am. J. Trop. Med. Hyg. 63(5):215-221.
|
|
|
|
|
Centers for Disease Control and Prevention (CDC) (2015). Insecticide-treated bed nets for malaria prevention. Global Health – Division of Parasitic Diseases and Malaria.
|
|
|
|
|
Diourte Y, Djimde A, Doumbo OK, Sagara I, Coulibaly D, Dicko A, Diallo M, Diakite M, Cortesa JF, Plowe CV (2003). Pyrimethamine-Sulfadoxine efficacy and selection for mutations in Plasmodium falciparum Dihydrofolate Reductase and Dihydropteroate Synthase in Mali. Am. J. Trop. Med. Hyg. 60(3):475-478.
|
|
|
|
|
Kantele A, Jokiranta TS (2011). Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin. Infect. Dis. 52(11):1356-1362.
Crossref
|
|
|
|
|
Laxminarayan R, Bhutta ZA, Duse A, Jenkins P, O'Brien T, Okeke IN, Pablo-Mendez A, Klugman KP (2006). Drug resistance: risk factors (Drug use in humans). Drug resistance; Disease Control Priorities in Developing Countries (Second Edition), ed. New York: Oxford University Press.
|
|
|
|
|
Mackinnon MJ, Hastings IM (1998). The evolution of multi-drug resistance in malaria parasites. Trans. Roy. Soc. Trop. Med. Hyg. 92:188-195.
Crossref
|
|
|
|
|
Mbacham WF (1998). Malaria, an ancient scourge: Myths, mistakes and management. Biodiag. Therapy. 2:4-10.
|
|
|
|
|
Mbenda HGN, Awasthi G, Singh PK, Gouado I, Das A (2014). Does malaria epidemiology project Cameroon as 'Africa in Miniature'? J. Biosc. 39:727-738.
Crossref
|
|
|
|
|
Ndo C, Menze-Djantio B, Antonio-Nkondjio C (2011). Awareness, attitudes and prevention of malaria in the cities of Douala and Yaoundé (Cameroon). Parasit. Vect. 4:181-187.
Crossref
|
|
|
|
|
Nosten F, White NJ (2007). Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77(6):181-192.
|
|
|
|
|
Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJM (2007). Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: Randomized trial. Brit. Med. J. 334:403.
Crossref
|
|
|
|
|
Roll Back Malaria (2013). World Health Organization. Rollback Malaria Partnership Annual Report. Geneva, Switzerland.
|
|
|
|
|
Sanz LM, Crespo B, De-Cozar C, Ding XC, Llergo JL, Burrows JN, Garcia-Bustos JF, Gamo F-J (2012). P. falciparum in vitro killing rates allow to discriminate between different antimalarial mode-of-action. PLoS ONE 7(2):e30949.
Crossref
|
|
|
|
|
Talisuna AO, Bloland P, D'Alessandro U (2004). History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17:235-254.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2013). Factsheet on the World Malaria Report 2013.
|
|
|
|
|
World Health Organization (WHO) (2015). Guidelines for the Treatment of Malaria. Third Edition. ISBN 978 92 4 154912 7. pp. 1-317.
|
|