ABSTRACT
A study was conducted to establish the epidemiology of Campylobacter species in the four agricultural zones of Sokoto. A total of 798 (506 cloacal and 292 fecal) swabs from poultry and humans respectively were screened and analyzed using standard culture isolation technique and biochemical characterization. A total of 152 (30%) and 160 (55%) were positive for Campylobacter spp. in poultry and humans respectively. The prevalence rates of 53, 28, and 18% were for Campylobacter coli, Campylobacter lari and Campylobacter jejuni in poultry while 39, 37 and 24% were for C. coli, C. lari and C. jejuni in humans, respectively. The prevalence rate of 30% was recorded in both chicken and guinea fowl, while 14, 56 and 50% were found in pigeon, ducks and turkey, respectively. The prevalence rates were slightly higher in males than females in both poultry and humans. There was no significant statistical association (P>0.05) between prevalence rate and species. The prevalence in agricultural zones revealed 42, 39, 28 and 13% in Gwadabawa, Isah, Sokoto and Tambuwal, respectively in poultry, while in humans, 65, 25, 50 and 70% were recorded in the same order. There was no significant statistical association (P>0.05) between prevalence rate and sex, but the association between prevalence and zones were statistically significant (P<0.05) in both poultry and humans. Poultry in the state have been shown to harbor Campylobacter spp. and may serve as reservoir of infection for humans. Humans independent of age and sex, were infected with Campylobacter spp.. The transportation of poultry together with humans in the same truck while moving birds from different locations to live bird markets should be discouraged. Adequate environmental sanitation and strict hygiene measures should be implemented in the poultry slaughter slabs and processing units in the state.
Key words: Campylobacter species, poultry, humans, agricultural zones, Sokoto State, Nigeria
Campylobacter species (formerly Vibrio fetus) were first associated with diseases of cattle and sheep at the beginning of 20th century. They are small curved or spiral-shaped gram negative bacilli that exhibit rapid darting and spinning motion (WHO, 2002). They are neglected zoonotic disease agents with an increased frequency of isolation from man, food, water, animals and their products (Salihu et al., 2010; Ugboma et al., 2013).
The economic loss due to Campylobacter infection poses a challenge to food and livestock industries as they usually colonize the gastrointestinal tract of birds causing diarrhea, less feed conversion ratio, decrease egg production and mortality in day old chicks (Butzler, 2004; Ruiz-Palacios et al., 1981). The rate of infection in poultry is affected by seasons and the type of production system (Kapperud et al., 1993; Wallace et al., 1997). Thermophilic Campylobacter spp., mainly Campylobacter jejuni and to a lesser extent Campylobacter coli have been recognized as the most common bacteriological causes of gastroenteritis in animals and humans worldwide (Jones et al., 1931). The gastroenteritis caused by these species is associated with abdominal pain and discomfort which sometimes persist even after the diarrhea has stopped (Jones et al., 1931). Humans acquire infection through handling and consumption of undercooked poultry meat. However, in the developing countries more cases of infection in children have been associated with poor hygiene (Coker et al., 2002). Other risk factors include the consumption of food and water contaminated with untreated animal or human waste in addition to close proximity and contacts with farm animals (Coker and Adefeso, 1994; Cools et al., 2003; Lindmark et al., 2009). Cost implication of treatment, in addition to increased resistance of these Campylobacter spp. to antimicrobial agents has been a concern in public health and disease control (Hein et al., 2003; Tollefson et al., 1999).
The aim of this study was to establish the prevalence of Campylobacter species in poultry and humans in the four agricultural zones of Sokoto State, Nigeria.
Ethical approval
The research was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. Ethical clearance was obtained from the Ministry of Health, Usman Farouk Secretariat Sokoto, Sokoto State.
The study area
The study was carried out in Sokoto State, which is located in the extreme Northwestern Nigeria and lies between the latitudes 12°N to 58°N and longitudes 4.8°E to 6.54°E with annual average temperature of 28.3°C. The 23 Local Government Areas have been grouped into 4 agricultural zones (MANR Sokoto, 2000). The state shares boundaries with Zamfara State to the East, Republic of Niger to the North and Kebbi State to the West.
Sample size determination
The minimum sample size for this study was determined by the formula N=Z2p(1-p)/d2 (Thrusfield, 2005), where N=Sample size; Z=the score for a given interval which is 1.96 (S.E) at 95% confidence interval; P= known or estimated prevalence; d=5% level of precision. The prevalence rate of 38.8% in birds in Sokoto was used for poultry (Salihu et al., 2009) while 20% was estimated for humans. With the known prevalence, the minimum calculated sample size (n) required for the study in birds was 1.962 × 0.39 × 0.61/0.052=365, while the minimum sample size required for humans was 1.962 × 0.20 × 0.80/0.052=245.
Sampling in poultry
A minimum of one Local Government Area (LGA) was randomly selected from each of the four Agricultural zones of the state. Visits were made to live bird markets in each of the selected LGAs to seek approval and cooperation from the authorities of the market union and estimate the number of birds that were presented for sales and slaughter during the market days. For each of the live bird market, visits were made once in every 2 weeks to avoid repeat sampling as birds presented for sales are usually transported from one live bird market to another and at least 40% (2 in every 5) of birds counted were sampled at each visit. In zone that has slaughter slab/processing points, cloacal swabs were collected outside the two weeks that samples were routinely collected from the market to avoid sampling same birds twice at both sales and slaughter unit.
Sampling in humans
Visits were made to the randomly selected hospitals in the Local Government Areas (LGAs) selected from each agricultural zone. Introductory letters from the hospital service management board were presented to the Chief Medical Officers of the selected hospitals. Convenient sampling technique was used in the collection of faecal swabs after the assigned health workers have explained the purpose of the study to the patients and such consented.
Sample transportation and processing
The samples were placed in Amies transport media (CMO425, Oxoid), kept cold with the use of ice pack (Butzler, 2004) and transported to the Veterinary Public Health Laboratory, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto for analyses. Samples were plated directly onto modified Charcoal Cefaperazone Deoxycholate Agar (mCCDA) and incubated at 42°C for 48 h under microaerophilic condition generated by Campygen® (Oxoid, BR0056) in the anaerobic jar (Butzler, 2004).
Identification of Campylobacter spp.
The plates were examined for typical Campylobacter colonies, characterized by creamy or white, greyish, moist, flat or slightly raised extending along the streak line, or regular circular discrete colony (Atabay and Corry, 1998). All the distinct pure colonies were gram-stained and isolates were identified to species level using the standard Campylobacter spp. phenotypic identification tests (Atabay and Corry, 1998; Barrett et al., 1988; Quinn et al., 1994).
Statistical analysis
The results obtained were presented in tables and percentages. Chi-square (c2-test) was used to determine any significant statistical association between Campylobacter prevalence in poultry and humans with some categorical variables such as species, sex, and agricultural zone.
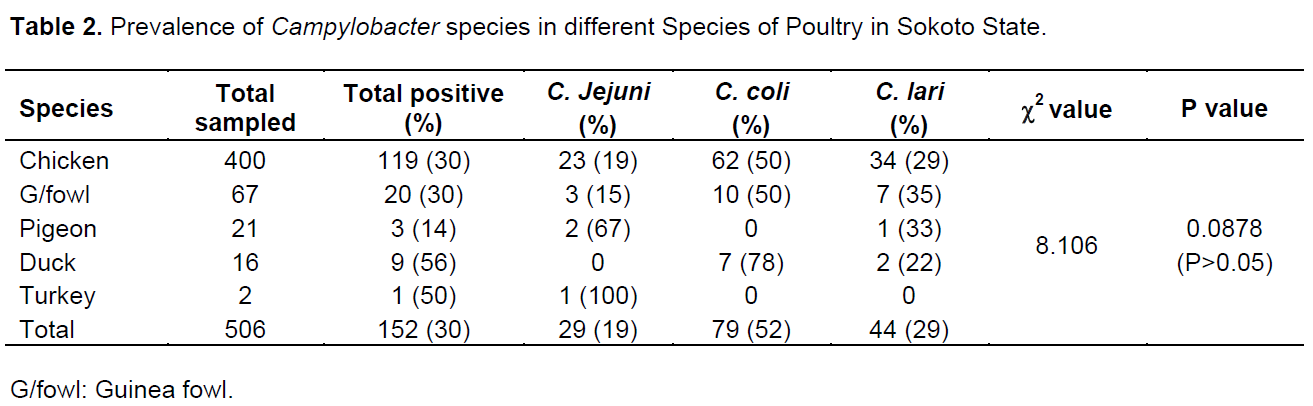
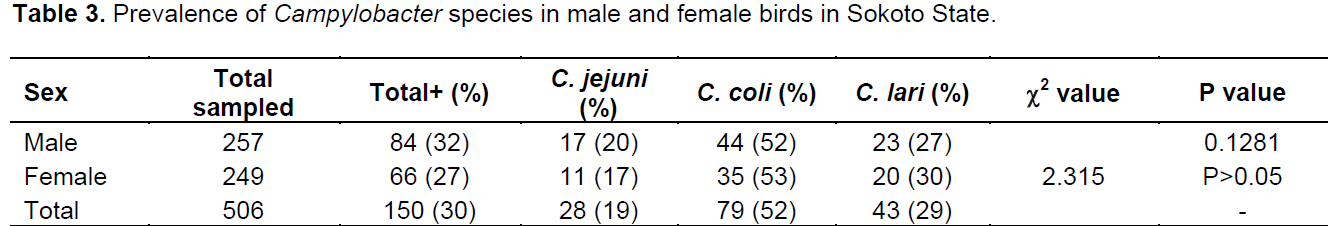
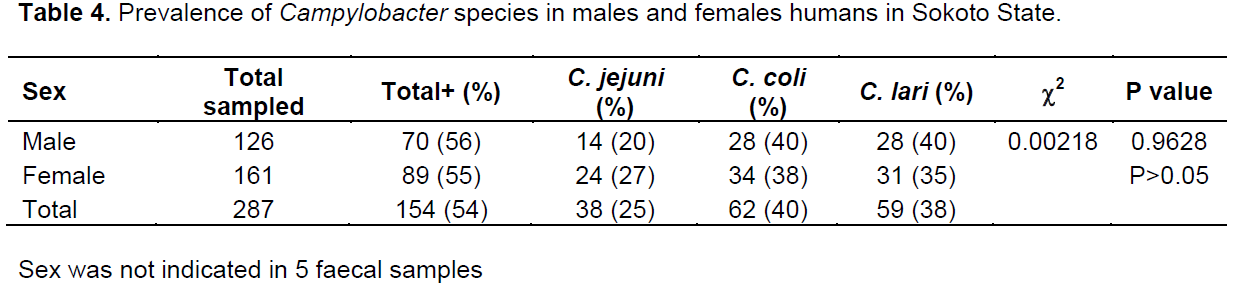
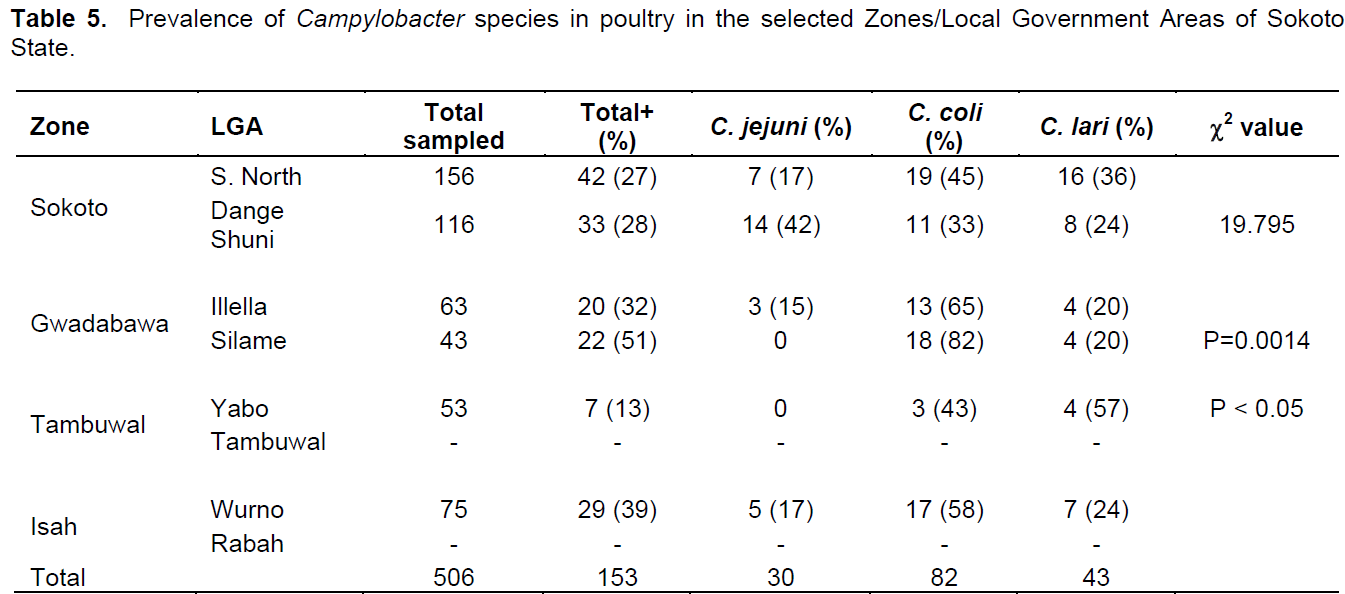
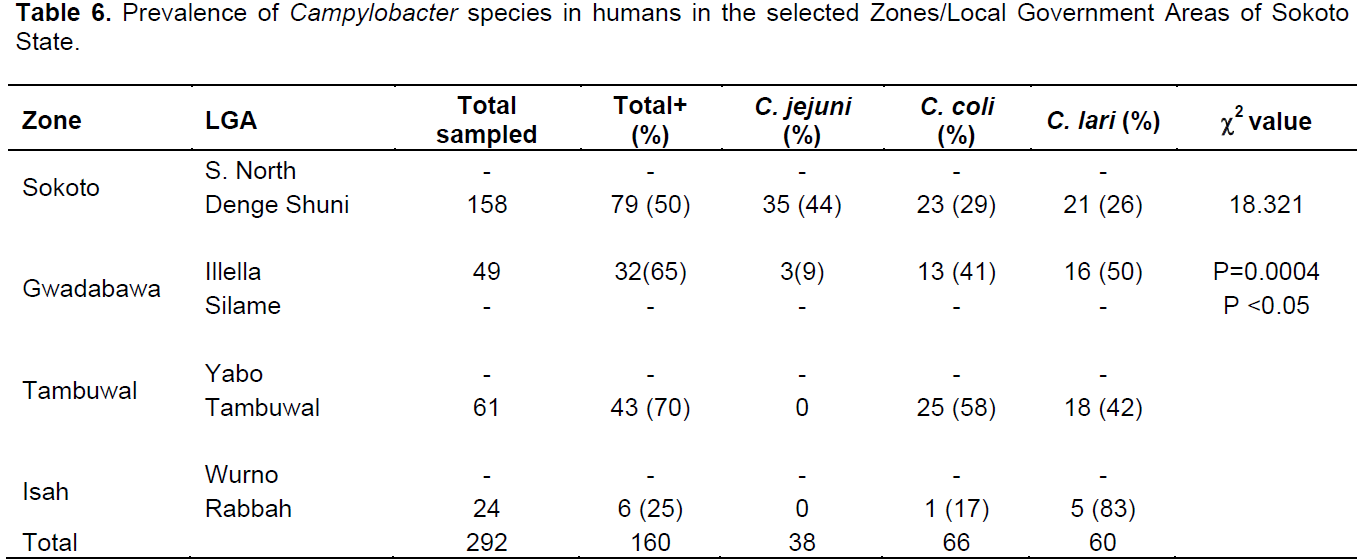
Out of the 798 samples analyzed, 506 were from poultry and 292 from humans. A total of 312 samples were positive for Campylobacter spp.; 152 (30%) and 160 (55%) in poultry and humans, respectively. The prevalence rates of 52, 29 and 19% were for Campylobacter coli, Campylobacter lari and C. jejuni, respectively in poultry while 39, 37 and 24% were for C. coli, C. lari and C. jejuni, respectively in humans (Table 1). The prevalence rate of 30% was recorded in both chicken and guinea fowl while 14, 56 and 50% were for pigeon, ducks and turkey, respectively (Table 2). In chicken, C. coli had the prevalence rate of 50% which is higher than 29 and 19% recorded for C. lari and C. jejuni, respectively. C. coli also recorded high rates in guinea fowl and ducks with 50 and 78%, respectively (Table 2).In poultry, 84 (32%) and 66 (27%) prevalence rates were recorded for males and females, respectively while in humans, 70 (56%) and 89 (55%) were positive for males and females, respectively (Tables 3 and 4). C. coli had a higher prevalence rates than other species of Campylobacter in both male and female poultry. In humans, the same prevalence rates were recorded for C. coli and C. lari in males while C. coli had higher rate than others in female (Table 4). The zonal prevalence revealed 42, 39, 28 and 13% in Gwadabawa, Isah, Sokoto and Tambuwal zones, respectively in poultry while in humans, 65, 25, 50 and 70% were recorded in the same order (Tables 5 and 6). There was no significant association (P>0.05) between prevalence rate, species and sex in both poultry and humans, but the association (P<0.05) between prevalence and zone were statistically significant.
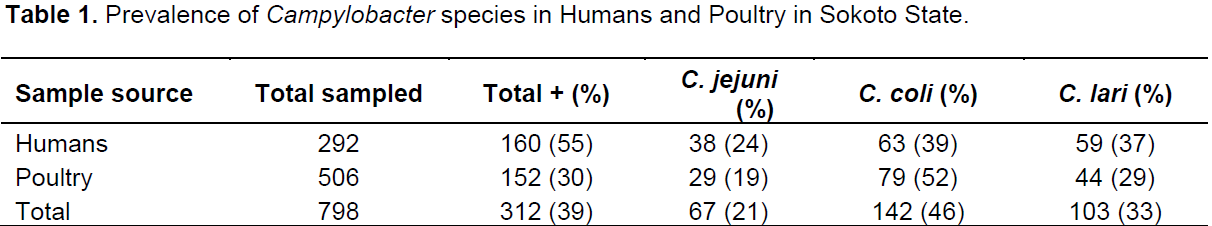
The prevalence of Campylobacter spp. in both poultry and humans has been established in the study area. The 30% prevalence rate in poultry was lower than 38.8% recorded in indigenous chicken in Sokoto by Salihu et al. (2009). The reduced rate could be an indication of increased awareness and improved environmental sanitation at backyard poultry houses at different homes, live bird markets and poultry farms. The prevalence rate in poultry was also in agreement with that of Uaboi-Egbenni et al. (2008) that recorded 33% prevalence rate in Lagos. However, it differs with the high prevalence rates of 94.2 and 89% recorded by Workman et al. (2005) and Georgios et al. (2004) in chicken meat and faeces, respectively. The similarities and variations in the prevalence rates could be a reflection of environmental contamination, however, other factors such as stock density, season, feeding regimen and geographical location have been proposed to account for significant differences and similarities in the isolation rates (Mary et al., 2004; Stern, 1994).
The rate of prevalence among species of poultry was high in ducks, which is a water fowl. Ducks are known to tip up on the surface of shallow water or submerge completely and swim under the water in search of food. They get infected especially when the ground water is contaminated with Campylobacter spp. (Savill et al., 2001). The low prevalence rate recorded in chicken might be linked to the free range system which is common in the study area as coprophagy which enhances bird to bird spread is limited. This can be supported by findings of Robino et al. (2010) with a Campylobacter spp. prevalence rate of 78.4% in intensively reared poultry and 18.3% in small scale rural poultry farming in Italy. The prevalence rates in pigeon, turkey and guinea fowl also revealed the possibilities of infection through feeds as they usually feed on insects, fruits, seeds and flowers which have been suggested as potential routes of infection in poultry (Waldenstrom et al., 2002). The interaction of these birds among themselves and with human communities suggests the possibilities of infection and transmission to humans (Shane, 1992; Waldenstrom et al., 2002). The higher prevalence of C. coli than other species in poultry in this study agreed with the findings of Wieczorek et al. (2012) that revealed 58.9% as C. coli and 41.1% as C. jejuni. Other reports on the higher isolation rate of C. coli compared to C. jejuni have also been reported (Kurincic et al., 2005; Lynch et al., 2011). However, the findings disagreed with the higher isolation rate of C. jejuni than other species in the work of Salihu et al. (2009) that reported 72.9% of the total isolate from chicken as C. jejuni and Cuiwei et al. (2001) who recorded the prevalence rate of 53.6, 41.3 and 5.1% for C. jejuni, C. coli and other species, respectively. Such differences have been attributed to several factors, including isolation method, sample size, seasonal variation and geographical location (Allos, 2001; Stanley et al., 1998). Since contact and consumption of contaminated improperly cooked poultry meat has been attributed to the occurrence of gastroenteritis in humans, the prevalence rate in poultry may have contributed to the prevalence in humans as over 80% of human population in the state are engaged in agriculture (Corry and Atabay, 2001).
The prevalence rate of 55% in humans disagreed with 78.4% recorded in Salihu (2009) study in the same study area and that of 87% among livestock workers as recorded by Saenz et al. (2000) in Spain. Salihu et al. (2009) collected and analyzed samples from a risk group (Poultry processors) while the focus of this study was on diverse groups which included people attending outpatient and ante natal clinics in the hospitals. C. coli had higher rate of 39% which contradicts the findings of workers who observed C. jejuni as the most common species of Campylobacter in humans (Ohanu and Offune, 2009; Salihu, 2009). C. coli also had high rate of 51% in chicken which was in agreement with the record of 58.9% as C. coli and 41.1% as C. jejuni by Wieczorek et al. (2012). It has been observed that C. lari is mostly found in wild birds and its isolation has remained low both in humans and poultry (Benjamin et al., 1983). However, the isolation rate for C. lari in poultry in this study was in agreement with that of 28% by Baserisalehi et al. (2007) in Iran. Furthermore, the lower isolation rate of C. lari to C. coli in this study was in agreement to the work of Uaboi-Egbenni et al. (2008) who reported a zero rate of C. lari and 14.2% for C. coli. The prevalence of Campylobacter spp. may be dependent on the sample size and weather conditions of different areas as some species grow optimally during the hot temperature and high humidity. Other species such as Campylobacter hyointestinalis, Campylobacter sputorum and Campylobacter fetus not found in the study were likely due to the high temperature of birds that do not support their survival, agents in the selective medium such as cefoperazone that might have hindered their growth and unsuitable temperature at 42°C used in the isolation (Martin et al., 2002).
The prevalence rates as recorded in the agricultural zones can be used as a reflection of environmental contamination in the areas. The high prevalence rates in poultry and humans recorded in Gwadabawa zone can be linked to the presence of large live bird market at Niger/Nigeria border town of Illella located in this zone whereby poultry that were transported unchecked into the country may have served as carriers of infection for humans.
High and low prevalence rates for humans and poultry, respectively in Tambuwal zone suggest the possibilities of other source of infection in humans other than poultry (Cools et al., 2003; Ugboma et al., 2013). However, genetics studies are needed to further link the isolates from poultry and humans. There was no much difference in prevalence rates in male and female birds and humans which is in agreement with the findings of Samuel et al. (2004) that recorded similar rates suggesting no sex preference in Campylobacter infection.
The study has established the prevalence of Campylobacter spp. in both poultry and humans in Sokoto State. Poultry and humans were infected independent of species and sex while different prevalence rates were recorded in different agricultural zones. Hence, human activities like the transportation of poultry together with humans in the same truck while moving birds from different Local Government Areas to live bird markets should be discouraged. Adequate environmental sanitation and strict hygiene measures such as washing of hands after handling of live birds, raw poultry meat especially for those that work in poultry slaughter slabs should be implemented to avoid the spread of Campylobacter infection in the state.
The authors have not declared any conflict of interests.
The research was funded by ION being part of his PhD thesis. Dr. E. B. Ibitoye was appreciated for his help in the statistical analysis of the data. The corporation of the chairmen and members of Live Bird Sellers Associations in Sokoto and the assistance of Chief Medical Officers and other health workers of the hospital used for sample collection are highly appreciated. We also thanked the Laboratory Staff of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto for their assistance in the laboratory throughout the course of the study.
REFERENCES
|
Allos BM (2001). Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206.
Crossref
|
|
|
|
Atabay HL, Corry JEL (1998). The isolation and prevalence of Campylobacters from the dairy using a variety of methods. J. Appl. Microbiol. 84:733-740.
Crossref
|
|
|
|
Barrett TJ, Patton CM, Morris GK (1988). Differentiation of Campylobacter species using phenotypic characterization. Lab. Med. 19:96-102.
Crossref
|
|
|
|
Baserisalehi M, Bahador N, Kapadnis BP (2007). Isolation and characterization of Campylobacter spp. from domestic animals and poultry in South of Iran. Pakistan J. Biol. Sci. 10(9):1519-1524.
Crossref
|
|
|
|
Benjamin J, Leaper S, Owen RJ, Skirrow MB (1983). Description of C. laridis, a new species comprising the nalidixic acid resistance thermophilic Campylobacter (NARTC) group. J. Curr. Microbiol. 8:231-238.
Crossref
|
|
|
|
Butzler JP (2004). Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10: 868-876.
Crossref
|
|
|
|
Coker AO, Adefeso AO (1994). The changing patterns of Campylobacter jejuni/coli in Lagos, Nigeria after ten years. East Afr. Med. J. 74:437-440.
|
|
|
|
Coker AO, Isokpehi RD, Thomas RN, Amisu KO, Obi CL (2002). Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237-244.
Crossref
|
|
|
|
Cools I, Ubttendaele C, Caro C, D'Haese E, Neils HJ, Debevere J (2003). Survival of Campylobacter jejuni strain of different origin in drinking water. J. Appl. Microbiol. 94:886-892.
Crossref
|
|
|
|
Corry JEL, Atabay HI (2001). Poultry as a source of Campylobacter and related organisms. J. Appl. Microbiol. 90:96S-114S.
Crossref
|
|
|
|
Cuiwei ZB, De Villena J, Sudler R, Shaohua Zhao E, White DG (2001). Prevalence of Campylobacter spp., Escherichia coli, and Salmonella Serovars in Retail Chicken, Turkey, Pork, and Beef from the Greater Washington, D.C., Area. Appl. Environ. Microbiol. 67(12):5431-5436.
Crossref
|
|
|
|
Georgios K, Dang DB, Mariannr L, Mogens M, Henrick B, Pieter T, Claus BVC (2004). Use of PCR analysis and DNA microarrays for detection of Campylobacter jejuni and Campylobacter coli from chicken feaces. J. Clin. Microbiol. 42(9):3985-3991.
Crossref
|
|
|
|
Hein C, Schneck M, Knogler G, Feierl P, Pless J, Kofer R, Achmann R, Wagner M (2003). Campylobacter jejuni isolation from poultry and human in Styria, Austria: epidemiology and ciprofloxacin resistance. Epidemiol. Infect. 130(03):377-386.
|
|
|
|
Jones FS, Orcutt M, Little RB (1931). Vibros (Vibrio jejuni, n.sp) associated with intestinal disorder of cows and calves. J. Exp. Med. 53(6):853-863.
Crossref
|
|
|
|
Kapperud G, Skjewe E, Vik L, Hauge K, Lysaker A, Aalmen L, Ostroff S, Potter, M (1993). Epidemiological Investigation of risk factor for Campylobacter colonization in Norwegian broiler flocks. Epidemiol. Infect. 111:245-255.
Crossref
|
|
|
|
Kurincic M, Berce I, Zorman T, SmoleMozina S (2005). The prevalence of multiple antibiotic resistances in Campylobacter spp. from retail poultry meat', Food. Food Tech. Biotech. 43:157-163.
|
|
|
|
Lindmark H, Boqvist S, Ljungstro M, Ågren P, Bjo ̈rkholm B, Engstrand L (2009). Risk Factors for Campylobacteriosis: an Epidemiological Surveillance Study of Patients and Retail Poultry. J. Clin. Microbiol. 47, 8:2616–2619.
Crossref
|
|
|
|
Lynch OA, Cagney C, McDowell DA, Duffy G (2011). Occurrence of fastidious Campylobacter spp. in fresh meat and poultry using an adapted cultural protocol. Int. J. Food Microbiol. 150:171–177.
Crossref
|
|
|
|
Martin KW, Mattick KL, Harrison H, Humphrey TJ (2002). Evaluation of selective media for Campylobacter isolation when cycloheximide is replaced with amphotericin B. Lett . Appl. Microbiol. 34:124-129.
Crossref
|
|
|
|
Mary EP, Christiansen L, Steen MW, Madsen EH, Wegener HC (2004). Effects of Climate on Incidence of Campylobacter spp. in Humans and Prevalence in Broiler Flocks in Denmark. Appl. Environ. Microbiol. 70(12):7474–7480.
Crossref
|
|
|
|
MANR Sokoto (2000). Ministry of Agriculture and Natural Resources. Sokoto, Sokoto State, Nigeria.
|
|
|
|
Ohanu ME, Offune J (2009). The prevalence of Camplobacter in Childhood diarrhea in Enugu State of Nigeria. J. Comm. Dis. 41(2):117-120.
|
|
|
|
On SL, Holmes B (1992). Assessment of enzyme detection tests useful in identification of campylobacteria. J. Clin. Microbiol. 30(3):746-9.
|
|
|
|
Quinn PJ, Carter ME, Markey B, Carter GR (1994). Campylobacter species', In: Clinical Veterinary Microbiology. Wolfe publishing, an imprint of Mosby-year Book Europe Limited, London. pp 268-272.
|
|
|
|
Robino P, Tomassone L, Tramuta C, Rodo M, Giammarino M, Vaschetti G, Nebbia P (2010). Prevalence of Campylobacter jejuni, Campylobacter coli and enteric Helicobacter in domestic and free living birds in North-Western Italy. Schweiz Arch Tierheilkd 152(9):425-431.
Crossref
|
|
|
|
Ruiz-Palacios GM, Escamilla E, Torres N (1981). Experimental Campylobacter diarrhea in chickens. Infect. Immun. 34:250-255.
|
|
|
|
Saenz Y, Zarazaga M, Lantero M, Gastanares MJ, Baquero F, Torres C (2000). Antibiotic resistance in Campylobacter strains isolated from Animal, Foods and Humans in Spain in 1997-1998. Antimicrob. Agents Chemother. 44:267-271
Crossref
|
|
|
|
Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, McGivern T, Kassenborg H, Reilly K, Kennedy M, Angulo F, Tauxe RV (2004). Epidemiology of sporadic Campylobacter infection in United States and declining trend in incidence food net 1996-1999. Clin. Infect. Dis. 38(3):165-174.
Crossref
|
|
|
|
Salihu MD (2009). Epidemiological studies of Campylobacter in food animals in Sokoto State. Doctoral thesis. Usmanu Danfodiyo University Sokoto. pp. 131-132.
|
|
|
|
Salihu MD, Junaidu AU, Magaji AA, Abubakar MB, Adamu AY, Yakubu AS (2009). Prevalence of Campylobacter in poultry meat in Sokoto Northwestern Nigeria. J. Public Health Epidemiol. 1(2):041-045.
|
|
|
|
Salihu MD, Junaidu AU, Magaji AA, Rabiu ZM (2010). Study of Campylobacter in Raw cow milk in Sokoto state Nigeria. Br. J. Dairy Sci. 1(1):1-5.
|
|
|
|
Savill MG, Hudson JA, Ball A, Klena JD, Scholes P, Whyte RJ, Mccormick RE (2001). Elucidation of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 91:38-46.
Crossref
|
|
|
|
Shane SM (1992). The significance of Campylobacter jejuni infection in poultry. a review. Avian Pathol. 21:189-213.
Crossref
|
|
|
|
Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K (1998). The seasonal variation of thermophilic Campylobacter in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480.
Crossref
|
|
|
|
Stern NJ (1994). Mucosal Competitive exclusion to diminish colonization of chickens by Campylobacter jejuni. J. Poult. Sci. 73:402-409
Crossref
|
|
|
|
Thrusfield M (2005). Estimation of disease prevalence; In: Veterinary Epidemiology 2ndedn. Blackwell science. Oxford. pp 182-187
|
|
|
|
Tollefson L, Fedarka-cray JP, Angullo FJ (1999). Public Health aspects of antibiotic resistance monitoring in the USA. Acta Vet. Scand. 92:67-75.
|
|
|
|
Uaboi-Egbenni PO, Okolie PN, Adesanya OD, Omonigbehin E, Sobande AO (2008). Epidemiological studies of the incidence of pathogenic Campylobacter spp. amongst animals in Lagos metropolis. Afr. J. Biotechnol. 7(16):2852-2956.
|
|
|
|
Ugboma AM, Salihu MD, Magaji AA, Abubakar MB (2013). Prevalence of Campylobacter species in ground water in Sokoto, Sokoto State, Nigeria. Vet. World (6):285-287.
|
|
|
|
Waldenstrom J, Broman T, Carlsson I, Hasselquist D, Achterberg RP, Wagenaar JA, Olsen B (2002). Prevalence of Campylobacter jejuni, C. lari and C. coli in different ecological guids and taxa of migrating birds dagger. Appl. Environ. Microbiol. 68:5917-5917.
Crossref
|
|
|
|
Wallace J, Stanley K, Currie J, Diggle P, Jones J (1997). Seasonality of thermophilic Campylobacter population in chicken. J. Appl. Microbiol. 82: 224-230.
Crossref
|
|
|
|
Wieczorek K, Szewczyk R, Osek J (2012). Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter jejuni and C. coli isolated from retail raw meat in Poland. Vet. Med. Czech. 57(6):293–299.
|
|
|
|
Workman NS, Mathison EG, Lavoie CM (2005). Pet dods and chicken meat as reservoir of Campylobacter spp. in Barbados. J. Clin. Microbiol. 43(6):2642-2650
Crossref
|
|
|
|
World Health Organization (2002). The increasing incidence of human campylobacteriosis', Report and proceeding of a W.H.O. consultation of experts Copenhagen, Denmark, 21-25 November 2000. WHO/CDS/CDR/APH publication 2001.7.
|