ABSTRACT
In this study, near infrared (NIR) spectroscopy was performed to establish a non-destructive method to predict the harvesting maturity, fruit ripening and storage ability of Ca-chitosan treated baby kiwifruit. Destructive measurements of firmness, dry matter (DM), soluble solids content (SSC), and acidity was performed. The calibration range investigated for dry matter content (DM) and SSC using NIR reflectance spectrums were observed at 729-975 nm wavelengths. NIR predictions of those quality factors were calculated using the modified partial least square regression method. The predicted R2 value for DM and SSC was 0.73, and the standard error deviation (SED) value was greater than 2. The correlation between the actual value and predicted model of DM were r = 0.74. The correlation between the predicted DM content and the actual SSC, using SSC model was r = 0.65. The correlation between the predicted value of SSC and the actual value of SSC (baby kiwifruits ripen with ethylene) was r = 0.48, which was lower than the actual SSC model. Further, Ca-chitosan pre-harvest treatment on baby kiwifruit showed considerable effects on baby kiwifruit quality. The actual DM content of untreated fruits was 21.4% and it was 22.3% in Ca-chitosan treated fruits. Also, the predicted DM content was significantly high in Ca-chitosan treated fruits (22.7%) compared to untreated fruits. NIR spectroscopy is an effective and efficient method to measure DM and SSC to determine the fruit harvest maturity hence, date of harvest and storability for quality baby kiwifruits from the marketing point of view.
Key words: Ca-chitosan, firmness, harvest index, maturation, nondestructive measurement.
Actinidia arguta, a very promising species in genus Actinidia also known as baby kiwi, hardy kiwi, kiwi berry, or mini kiwi, is currently highly appreciated fruit by consumers for its delicious taste and health-promoting properties (Latocha, 2017). Moreover, the consumers are highly concerned on fruit quality indicators such as ripeness, firmness, dry matter (DM), soluble solids content (SSC) and acidity (Wang et al., 2015). Kiwifruits are highly perishable and have a short shelf-life of 1-2 weeks depending on SSC, cell respiration and microbial spoilage. The decrease of flesh firmness and acidity, and conversion of starch to sugar are the prominent changes in kiwifruits during their maturation and ripening (Lee et al., 2012). The physiological, chemical and sensory changes that occurred during storage reduce the shelf life and quality of kiwifruits. The shelf life of kiwifruits can be increased considerably using edible coating materials such as polysaccharides, proteins, lipids and plant extracts. The coating materials decrease the gas exchange, oxidative reaction rates, respiration and moisture losses as well as suppress the physiological disorders. Chitosan combined with calcium chloride (CaCl2; Ca-chitosan) can be used as an edible coating to increase shelf life of kiwifruits. Chitosan is a high molecular weight cationic polysaccharide produced by chemical deacetylation of the chitin found in arthropod exoskeletons. Chitosan has been actively used as an edible coating material for increasing the shelf life of fruits due to its edible, biocompatible, antimicrobial and nontoxic nature. Chitosan possesses excellent film-forming properties and inhibits the growth of a wide range of fungi and trigger defensive mechanisms in fruits (Kaya et al., 2016; Drevinskas et al., 2017). Calcium improves structural integrity and makes the cell wall less accessible to the enzymes that cause softening and controls fruit ripening, softening and decay. Further, calcium application enhances tissue resistance to fungal attack, maintains cell turgor, membrane integrity, tissue firmness and delays membrane lipid catabolism, maintains textural quality, hence extending shelf life of fruits (Kazemi et al., 2011; Shiri et al., 2016). Therefore, application of chitosan coating together with calcium might have positive effects on postharvest quality of kiwifruits.
Moreover, the firmness, DM, SSC and acidity are much important parameters in kiwifruit quality evaluation, but they currently require the destructive measurements. Hence, the development of reliable, nondestructive, and rapid method is required for the quality evaluation of kiwifruits. Near infrared (NIR) spectroscopy has been introduced as a rapid and nondestructive method to measure the internal fruit quality and potential replacement of existing methods which are highly subjective and time consuming (Maniwara et al., 2014; Wang et al., 2015). Further, the portable, handheld NIR spectroscopic instruments have drawn wide attention due to their potential for in-situ measurements, which enables the growers to obtain nondestructive measurements on pre-harvest maturation rates of fruits. However, to the best of our knowledge, no research has been reported in literature about NIR spectroscopy method used to predict the harvesting maturity, fruit ripening and storage ability of baby kiwifruit [(A. arguta) cv. Saehan] and about the combined effect of Ca-chitosan coating on postharvest qualities of baby kiwifruits. Hence, in this study, near infrared (NIR) spectroscopy was performed to establish a non-destructive method to predict the harvesting maturity, fruit ripening and storage ability of Ca-chitosan treated baby kiwifruit.
Fruit material
Baby kiwifruits [(A. arguta) cv. Saehan] from the National Institute of Forest Science in Suwon, Republic of Korea were harvested at 108 days after full bloom (DAFB). Two consecutive harvests were collected on 24th September (1st harvest) and 8th (2nd harvest) October, in 2015. First harvest was used to predict the harvesting maturity based on NIR-spectroscopy and they were not pre-treated with Ca-chitosan. The second harvest consisted of pre-harvest Ca-chitosan treated kiwifruits and was used to measure the fruit quality parameters based on NIR-spectroscopy. All samples were packaged immediately in a plastic clamshell container (KMD-501, Go Pack, Republic of Korea), where twenty fruits were allotted per container and moved to the Laboratory of Fruit Science, Gyeongsang National University, Republic of Korea.
NIR method
NIR spectra of kiwifruit were measured using NIR-spectroscopy system F-750 spectrophotometer (Felix, WA, Camas, USA) with an internal white reference shutter to normalize collected data. The spectrophotometer scanned absorbance at 3 nm sampling wavelength intervals and partial least squares regression (PLSR) was performed on the second derivative of absorbance spectra. Owing to the temperature dependence of the spectral response (Peinado et al., 2006; Cozzolino et al., 2007), kiwifruits were stabilized at temperatures of 1, 15, and 25°C, prior to spectra measurements and a set of three representative scans for each sample was obtained.
Sample preparation and measurement of DM and SSC
A total number of 100, even size fruits without wounds or mechanical damages were selected from the 1st harvest on 24th September, 2015 and stored at three different temperatures, that is, low temperature (1°C), medium temperature (15°C), and maximum temperature (25°C), respectively for 1 h. Three different temperatures were selected due to the temperature dependence of the spectral response and kiwifruits were stabilized at 1, 15 and 25°C, prior to spectra measurements. The spectra were nondestructively measured using F-750 at each temperature before making 2 cm thick destructive fruit cores at the scanned location. For DM reference values, fruit cores were oven dried for 48 h at 65°C. Fresh weight and dry weight was measured using a Voyager Pro balance (Ohaus Voyager, United Kingdom) with a scale at 0.001 mg resolution. The DM reference value of each fruit was correlated with the original spectral to create a calibration for F-750. For SSC calibration, another set of 100 baby kiwifruits was prepared and stored by following the same procedure described previously. Thereafter, fruits were ripened with 50 ppm exogenous ethylene (12 h, 18°C) in an environmental chamber. After 4-5 days, the SSC of ripened fruits were measured. The SSC reference value of each fruit was correlated with the original spectral to create calibration for F-750 which allows the pre-harvest estimation of SSC after ripening.
Calcium chitosan treatment and fruit storage conditions
High molecular weight chitosan (500,000 MW) and CaCl2 (77%) were purchased from JS Logistics, Sejong and Samchun Pure Chemical Co., LTD., Pyeongtaek, Republic of Korea, respectively. Coating solutions were prepared by dissolving 2% chitosan and 2% CaCl2 in 0.2 M acetic acid solution with 0.02% surfactant. Pre-harvest Ca-chitosan treatment was done for the baby kiwifruits through dipping method at three times, that is, 17th, 24th September and 1st October, 2015. The treated fruits were harvested on 8th October, 2015 and stored in a plastic container (2.1 L vessel, HPL826M, LocknLock, China) at 5°C and 95% RH for 7 and 14 days. After 7 days of storage, 10 fruits were subjected to ripening with 50 ppm ethylene for 5 days and SSC measurements were recorded. Similarly, after 14 days of storage, the rest of fruits were also subjected to ripening with 50 ppm ethylene for 5 days and SSC measurements were recorded.
Soluble solids content (SSC), titratable acidity (TA), and firmness
The SSC was measured using kiwifruit juice extractions from nine biological replicates. The extracted fruit juice was filtered through 4 layers of cheesecloth prior to analysis using PAL-1 Refractometer (Atago Co. LTD, Tokyo, Japan). Similarly, nine biological replicates were used for TA and firmness measurements. The titratable acidity (TA) of fruit juice was assayed by titration with 0.05 mol∙L-1 NaOH using a professional benchtop BP3001 pH meter (Trans Instruments, Singapore). The TA content was expressed as citric acid equilibrium. The fruit firmness was measured using a rheometer (RHEO TEX SD-700, Sun Scientific Inc, Japan) with a round flat probe of 3 mm in diameter on a horizontal axis and the probe was inserted to the fruit up to 3 mm depth. The measurements were performed at the crosshead speed of 120 mm∙min−1 at room temperature (20 ± 2°C).
Data analysis
All fruit samples were harvested randomly, and analysis was performed on three biological replicates. The data analysis was done with SAS 8.2 statistical software (SAS Inst., Cary. N.C., USA), following the analysis of variance (ANOVA) and Tukey’s T-test. PLSR calibrations were done with the F-750 Model Builder software (Felix Instruments, WA, Camas, USA).
NIR spectra calibration and prediction of physiochemical parameters
The NIR reaction wavelength range for soluble solids content (SSC) of kiwifruit has been reported as 800 1,100 nm (Lee et al., 2012) and for measuring carbohydrate content the wavelength range varies from 880, 900-930, and 970 nm. The spectra collected in the present study showed variations among fruit specimens (in 100 fruit samples) (Figure 1). The calibration range investigated for dry matter content (DM) and SSC using nondestructive predictions from F-750 Model Builder software (Felix Instruments) was observed at 729 - 975 nm wavelengths. The predicted R2 value for DM and SSC was 0.73, and the standard error deviation (SED) value was greater than 2 (Table 1).
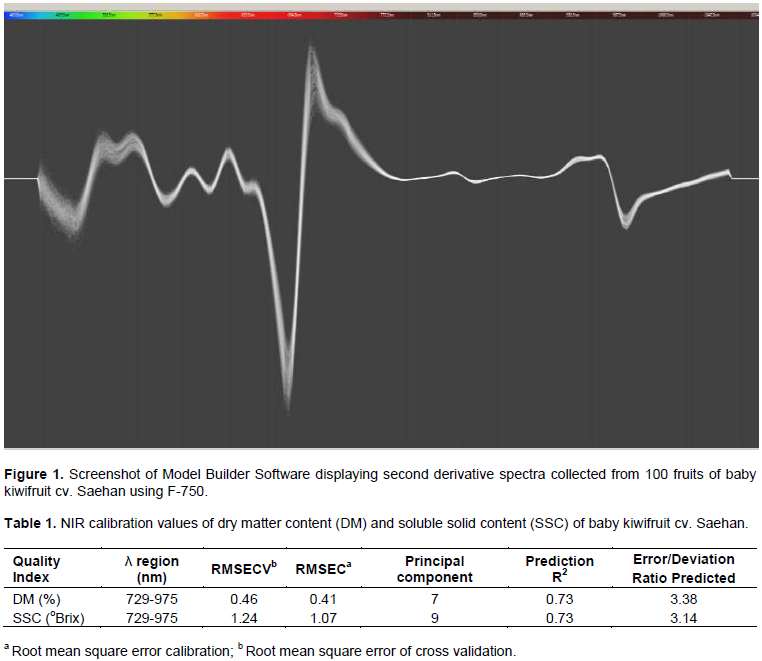
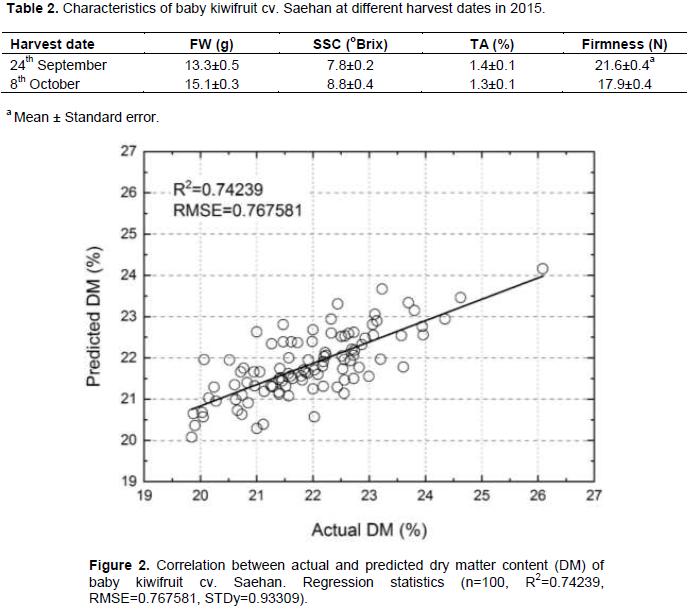
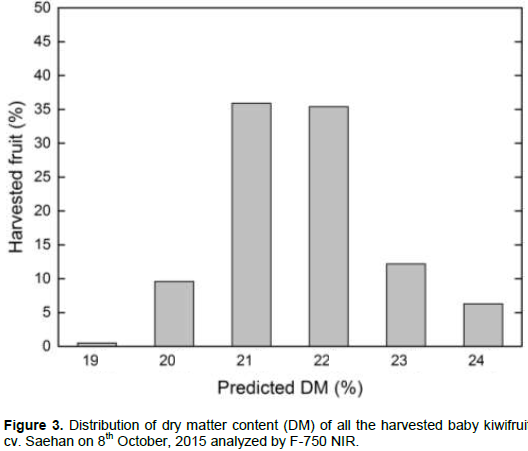
The baby kiwifruit cv. Seahan is known to be harvested at the beginning of October encompassing a full bloom date (108 DAFB). The change of fruit weight (FW) and maturity was observed for 2 weeks (Table 2). The SSC was increased by 1° Brix and the firmness was decreased by 3.7 N at the week before actual harvesting date. The correlation between the actual value and predicted model of DM were r = 0.74 (Figure 2). The predicted DM variation among the 384 harvested fruits is shown in Figure 3. Among 384 fruits, 71% fruits were shown 21 - 22% predicted DM, while 10% fruits were shown less than 21% predicted DM and 18% fruits were shown more than 23% predicted DM. Therefore, to prevent the accumulation of non-commodity acceptable fruits with unacceptable levels of SSC, it is necessary to study the uniformity of fruit quality in a sample through a nondestructive approach, such as NIR-spectroscopy.
The estimated SSC values in fruits for predicated DM were recorded as, 9.1° Brix for 20% DM, 9.6° Brix for 21% DM, 11.1° Brix for 22% DM, and 11.2° Brix for 23% DM (Figure 4). This infers that higher DM content at harvest correlates to higher SSC. The correlation between the predicted DM content and the actual SSC, using SSC model was r = 0.65 (Figure 5). The correlation between the predicted value of SSC and the actual value of SSC was r = 0.48, which was lower than the actual SSC model (Figure 6).
The use of NIR spectrometry to determine SSC, DM, and fruit firmness has been reported previously in kiwifruit (Schaare and Fraser, 2000; Clark et al., 2004). Park et al. (2003) found that the F-750 can be successfully used to predict SSC through spectra ranging from 800 - 1,100 nm in apple varieties, ‘Gala’ and ‘Red Delicious’ with a high accuracy (r = 0.97 and 0.96, respectively). Further, Angra et al. (2009) evaluated °Brix values of apple fruits with the wavelength that ranged from 800 - 1,600 nm. Similarly, Pissard et al. (2013) built models with NIR to predict °Brix in 150 apple genotypes. The present results obtained from F-750 and all other reports depicted that NIR is an accurate and reliable method to obtain physiochemical measurements particularly, SSC, °Brix, and starch content.
Effect of calcium chitosan treatment on physiochemical parameters
The preharvest application of Ca-chitosan on baby kiwifruit influences the metabolism and respiration of the preharvest fruits and may inhibit the maturation. Ca-chitosan treated baby kiwifruit cv. Saehan reported 9.5° Brix, which was significantly higher compared to untreated fruits with 8.4° Brix. The titrable acidity was not significantly different among Ca-chitosan treated and untreated fruits at p=0.05 level (Table 3). The Ca-chitosan treated fruits of baby kiwifruit cv. Saehan showed lower fruit weights (12.8 g) compared to the untreated fruits (14.9 g) (Table 3). The firmness of Ca-
chitosan treated fruits and untreated fruits were 21.9 N and 21.1 N, respectively (Table 3). The actual DM content of untreated fruits was 21.4% and it was 22.3% in Ca-chitosan treated fruits (Table 3). Also, the predicted DM content was significantly high in Ca-chitosan treated fruits (22.7%) compared to untreated fruits (22.0%).
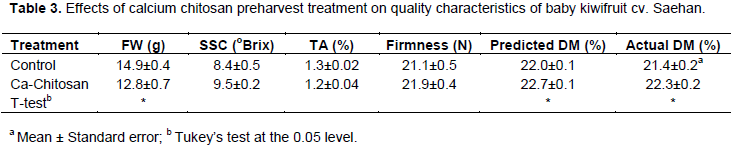
Both pre-harvest and postharvest applications of Ca have been widely used as a preservative and firming agent in fruits and vegetables and are known to increase fruit firmness and delay fruit decay (Chardonnet et al., 2003; Saftner et al., 2003; Madrid et al., 2004). Furthermore, Ca-chitosan has been reported to have fungicidal properties which work against fruit pathogen development (Bautista-Banos et al., 2003). The apparent relationship between the combined treatment of Ca and chitosan and increases in SSC content of kiwifruit are likely due to a decrease in the rate of ripening. This assertion agrees with reports obtained for strawberries (Eryani-Raqeeb et al., 2009; Petriccione et al., 2015). The SSC content in Ca-chitosan applied mango and banana was gradually increased with slow maturation, ripening, respiration and metabolism rates (Kittur et al., 2001; Li and Yu, 2001).
In peaches and golden kiwifruit, pre-harvest Ca-chitosan spray increased the internal CO2 of fruit, probably resulting in the delay of ripening and ethylene activity (unpublished data). Hence, it is essential to have further studies on Ca-chitosan applications for insightful understanding of their effects on fruit maturation and ripening.
Changes in physiochemical parameters during storage
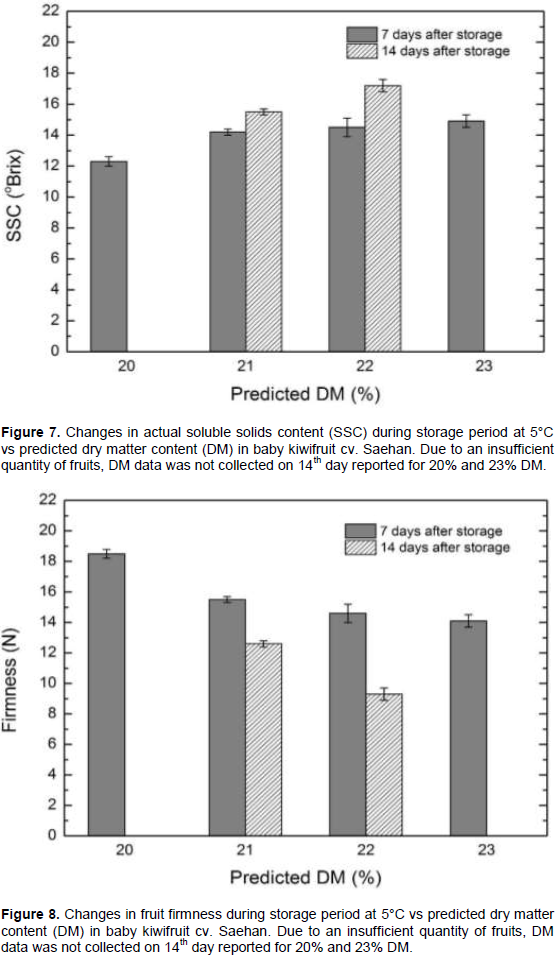
The SSC of baby kiwifruit cv. Saehan, 7 days after storage was measured as °Brix and compared to the DM at harvest. Kiwifruit at 12.3° Brix had a 20% predicted DM at harvest. Additionally, at 14.2° Brix, 14.5° Brix, and 14.9° Brix showed predicted DM as 21, 22 and 23%, respectively (Figure 7). On the 14th day of kiwifruit storage, 15.5° Brix was aligned with 21% predicted DM, which was 43% higher than value observed at harvest. At 22% predicted DM, 17.2° Brix was observed, which was 49% higher than the day of harvest. As shown by Liu et al. (2010), the fruit with a higher DM at time of harvest correspond with a higher °Brix value after ripening. The firmness of baby kiwifruit cv. Saehan at the time of harvest was 17.9 N. After 7 days of storage at 5°C, the predicted DM and firmness (N) correlated as follows: 20% predicted DM with 18.5 N, 21% predicted DM with 15.5 N, 22% predicted DM with 14.6 N, and 23% predicted DM with 14.1 N, showing a reduction in firmness. On the 14th day of storage at 5°C, the DM and firmness correlated as follows: 21% DM with 12.6 N firmness, which was 30% lower than the harvest date; and 22% DM with 9.3 N firmness, which was 48% lower than the harvest date (Figure 8). According to Fisk et al. (2006), harvest maturity (6.0, 8.7, and 15.1 average SSC) affects the fruit quality after ripening and the storability of baby kiwifruit cultivar ‘Ananasnaya’. They suggested that ‘Ananasnaya’ baby kiwifruit should be harvested at SSC > 8% and stored at low temperatures to achieve fruits with high consumer preference. Baby kiwifruit cv. Saehan is suggested to be harvested at over 21% DM content with a higher SSC; hence DM can be used as a maturity index for non-destructive quality prediction of baby kiwifruits based on NIR-spectroscopy.
Based on present findings it can be concluded that, the NIR-spectroscopy is an effective and efficient method to measure DM and SSC to determine the fruit harvest maturity hence, date of harvest and storability of quality baby kiwifruits. In addition, preharvest Ca-chitosan application can be implemented as a better strategy to improve the postharvest quality and shelf life of baby kiwifruits. The quality of NIR-spectroscopy method is cultivar specific with special emphasis on the different fruit quality indices. Moreover, the DM of unripe baby kiwifruits at harvest is very important factor affecting eating quality (SSC) of ripe baby kiwifruits. Ripe baby kiwifruits would have excellent eating quality with high SSC if they contained sufficient amounts of DM at harvest. By the NIR-predicted DM of unripe baby kiwifruits, the SSC of ripe baby kiwifruits could be precisely predicted at time of harvest. Hence, the development of a NIR-spectroscopy technique to predict eating quality of ripe baby kiwifruits from its quality at harvest or unripe stage is very important from the marketing point of view.
The authors have not declared any conflict of interests.
This research was supported by a grant from National Institute of Forest Science, Republic of Korea.
REFERENCES
|
Angra SK, Dimri AK, Kapur P (2009). Nondestructive brix evaluation of apples of different origin using near infrared NIR filter based reflectance spectroscopy. Instrumentation Science and Technology 37(2):241-253.
Crossref
|
|
|
|
Bautista-Banos S, Hernandez-Lopez M, Bosquez-Molina E, Wilson CL (2003). Effects of chitosan and plant extracts on growth of colletotrichum gloeosporioides anthracnose levels and quality of papaya fruit. Crop Protection 22(9):1087-1092.
Crossref
|
|
|
|
|
Chardonnet CO, Charron CS, Sams CE, Conway WS (2003). Chemical changes in the cortical tissue and cell walls of calcium infiltrated 'Golden Delicious' apples during storage. Postharvest Biology and Technology 28(1):97-111.
Crossref
|
|
|
|
|
Clark CJ, McGlone VA, De Silva HN, Manning MA, Burdon J, Mowatt AD (2004). Prediction of storage disorders of kiwifruit (Actinidia chinensis) based on visible-NIR spectral characteristics at harvest. Postharvest Biology and Technology 32(2):147-158.
Crossref
|
|
|
|
|
Cozzolino D, Liu L, Cynkar WU, Dambergs RG, Janik L, Colby CB, Gishen M (2007). Effect of temperature variation on the visible and near infrared spectra of wine and the consequences on the partial least square calibrations developed to measure chemical composition. Analytica Chimica Acta 588(2):224-230.
Crossref
|
|
|
|
|
Drevinskas T, Naujokaityte G, Maruska A, Kaya M, Sargin I, Daubaras R, Cesoniene L (2017). Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydrate Polymers 173:269-275.
Crossref
|
|
|
|
|
Eryani-Raqeeb AA, Mahmud TMM, Omar SRS, Eryani ARA (2009). Effect of calcium and chitosan treatments on controlling anthracnose and postharvest quality of papaya (Carica papaya L.). International Journal of Agricultural Science 4(2):53-68.
Crossref
|
|
|
|
|
Fisk CL, Strik BC, Zhao Y (2006). Iodine staining does not indicate harvest maturity of 'Ananasnaya' hardy kiwifruit berries. HortTechnology 16(4):655-658.
|
|
|
|
|
Kaya M, Cesoniene L, Daubaras R, Leskauskaite D, Zabulione D (2016). Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. International Journal of Biological Macromolecules 85:355-360.
Crossref
|
|
|
|
|
Kazemi M, Aran M, Zamani S (2011). Effect of calcium chloride and salicylic acid treatments on quality characteristics of kiwifruit (Actinidia deliciosa cv. Hayward) during storage. American Journal of Plant Physiology 6(3):183-189.
Crossref
|
|
|
|
|
Kittur FS, Saroja N, Habibunnisa, Tharanathan RN (2001). Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. European Food Research and Technology 213(4-5):306-311.
Crossref
|
|
|
|
|
Latocha P (2017). The nutritional and health benefits of kiwiberry (Actinidia arguta)-a review. Plant Foods for Human Nutrition 72(4):325-334.
Crossref
|
|
|
|
|
Lee JS, Kim SC, Seong KC, Kim CH, Um YC, Lee SK (2012). Quality prediction of kiwifruit based on near infrared spectroscopy. Korean Journal of Horticultural Science and Technology 30(6):709-717.
Crossref
|
|
|
|
|
Li H, Yu T (2001). Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. Journal of the Science of Food and Agriculture 81(2):269-274.
Crossref
|
|
|
|
|
Liu Y, Sun X, Ouyang A (2010). Nondestructive measurement of soluble solid content of navel orange fruit by visible-NIR spectrometric technique with PLSR and PCA-BPNN. LWT- Food Science and Technology 43(4):602-607.
Crossref
|
|
|
|
|
Madrid R, Valverde M, Alcolea V, Romojaro F (2004). Influence of calcium nutrition on water soaking disorder during ripening of Cantaloupe melon. Scientia Horticulturae 101(1-2):69-79.
Crossref
|
|
|
|
|
Maniwara P, Nakano K, Boonyakiat D, Ohashi S, Hiroi M, Tohyama T (2014). The use of visible and near infrared spectroscopy for evaluating passion fruit postharvest quality. Journal of Food Engineering 143:33-43.
Crossref
|
|
|
|
|
Park B, Abbott JA, Lee KJ, Choi CH, Choi KH (2003). Near-infrared diffuse reflectance for quantitative and qualitative measurement of soluble solids and firmness of delicious and gala apples. Transactions of the American Society of Agricultural Engineers 46(6):1721-1731.
Crossref
|
|
|
|
|
Peinado AC, van den Berg F, Blanco M, Bro R (2006). Temperature-induces variation for NIR tensor-based calibration. Chemometrics and Intelligent Laboratory Systems 83(1):75-82.
Crossref
|
|
|
|
|
Petriccione M, Mastrobuoni F, Pasquariello MS, Zampella L, Nobis E, Capriolo G, Scortichini M (2015). Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 4(4):501-523.
Crossref
|
|
|
|
|
Pissard A, Pierna JAF, Baeten V, Sinnaeve G, Lognay G, Mouteau A, Dupont P, Rondia A, Lateur M (2013). Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using infrared spectroscopy. Journal of the Science of Food and Agriculture 93(2):238-244.
Crossref
|
|
|
|
|
Saftner RA, Bai J, Abbott JA, Lee YS (2003). Sanitary dips with calcium propionate, calcium chloride, or a calcium amino acid chelate maintain quality and shelf stability of fresh cut honeydew chunks. Postharvest Biology and Technology 29(3):257-269.
Crossref
|
|
|
|
|
Schaare PN, Fraser DG (2000). Comparison of reflectance, interactance and transmission modes of visible-near infrared spectroscopy for measuring internal properties of kiwifruit (Actinidia chinensis). Postharvest Biology and Technology 20(2):175-184.
Crossref
|
|
|
|
|
Shiri MA, Ghasemnezhad M, Moghadam JF, Ebrahimi R (2016). Effect of CaCl2 sprays at different fruit development stages on postharvest keeping quality of "Hayward" kiwifruit. Journal of Food Processing and Preservation 40:624-635
Crossref
|
|
|
|
|
Wang H, Peng J, Xie C, Bao Y, He Y (2015). Fruit quality evaluation using spectroscopy technology: A review. Sensors 15(5):11889-11927.
Crossref
|
|