ABSTRACT
Nemafric-BL phytonematicide, produced from fermented fruit of wild watermelon (Cucumis africanus), had been consistent in suppressing root-knot (Meloidogyne species) nematode population densities. However, due to the biological nature of Nemafric-BL phytonematicide, quality could be compromised during increasing storage period. A study was, therefore, conducted to determine the response of cucurbitacin B concentration in Nemafric-BL phytonematicide over a six-month storage period. The product, in 50 ml hermetically-sealed plastic containers, was stored in a dark room at room temperature (±25°C), with 10 samples being analysed monthly for cucurbitacin B concentrations using Shimadzu High Performance Liquid Chromatography (HPLC). Cucurbitacin concentrations over increasing storage period exhibited density-dependent growth patterns, which were characterised by increases in cucurbitacin B during the initial period of storage and followed by gradual decreases. Relative to the initial storage time (T0), at the end of the storage period, cucurbitacin B concentration was still more than three-hundred times that at T0, suggesting that the product was still suitable for use in managing nematode numbers.
Key words: Cucumis africanus, effective microorganisms, product quality, phytonematicide, shelf-life.
Storage period of fermented products plays an important role in product quality (Rogers, 2010). Product quality (Q) is a function of its performance (P) and expectation (E), conceptualised as Q = P/E (Besterfield et al., 2003). Nemafric-BL phytonematicide had been consistent in suppressing nematode numbers in various crops (Mashela et al., 2015), with occasional incidents of stimulated plant growth (Mashela et al., 2015). The product is produced from fermented fruit of wild watermelon (Cucumis africanus) (Mashela et al., 2011). The product comprises cucurbitacin B (C32H46O8) active ingredient (Chen et al., 2005), which is both stable and insoluble in water (Jeffery, 1978).
Recently, Shadung et al. (2015) observed that when C. africanus fruit were dried at 52°C and stored over six months prior to manufacturing the product, the concentration of cucurbitacin B in phyto-inventories increased quadratically. The behaviour of cucurbitacin B in the stimulation phase of the density-dependent growth (DDG) patterns was explained on the basis of the thermo-stable enzyme-driven precursors (Chen et al., 2014), whereas in the inhibition phase the decreases were explained in terms of auto-oxidation. These behavioural-concentration changes, can invariably affect product quality. The objective of this study was, to determine the response of cucurbitacin B concentration in Nemafric-BL phytonematicide over a six-month storage period.
Study field and cultural practices
Hardened-off C. africanus seedlings were raised under irrigated-field conditions at the Green Technologies Research Centre (GTRC), University of Limpopo, South Africa (23°53'10"S, 29°44'15"E) in summer (October – December) 2014 and repeated in 2015. Seeds were prepared as described previously (Maila et al., 2016) and sown in seedling trays containing Hygromix-T (Hygrotech, Pretoria North, South Africa) growing medium. At two-leaf stage, seedlings were hardened-off for 5 days, selected for uniformity and at 4 weeks were transplated under field conditions. Plot size was 1 m x 1 m, each containing four equidistant-transplanted seedlings. Three days after transplanting, 3 g 2:3:2 (22) NPK fertiliser mixture/plant provided a total of 186 mg N, 126 mg K and 156 mg P per ml water, whereas 2 g 2:1:2 (43) fertiliser mixture provided 0.35 mg N, 0.32 mg K and 0.32 mg P, 0.9 mg Mg, 0.75 mg Fe, 0.075 mg Cu, 0.35 mg Zn, 1.0 mg B, 3.0 mg Mn and 0.07 mg Mo per ml water. Plants were irrigated weekly using overhead sprinklers to provide at least 20 mm water.
Experimental units
Fruit of C. africanus were harvested at 110 days after transplanting, cut into pieces and dried at 52°C for 72 h in an air-forced oven. Dried material was ground in a Wiley mill to pass through a 1 mm screen (Mashela, 2002). Approximately 40 g crude extract of C. africanus fruit, was placed in 20-l-plastic containers and 300 ml molasses, 100 g brown sugar, 300 ml effective microorganisms (EM) and 16 litre chlorine-free tapwater added and hermetically sealed (Nzanza and Mashela, 2012). The mixtures were fermented for 14 days at room temperature until pH declined to 3.7. Gases were allowed to escape from the container through a 5 mm-diameter tube with hermetically-glued end to a hole on the lid of the 20 L container, with an outlet end dangling in chlorine-free tapwater container in a litre bottle. After fermentation, 200 ml were pipetted into 300 ml plastic containers, which were hermetically sealed. Treatments, viz., 0, 1, 2, 3, 4 and 5 month storage time, were arranged in rando-mised complete block design, with five replications. Samples were stored in a dark room at room temperature (±25°C), with the initial storage period (T0) being the control.
Data collection
Prior to storage (T0) and then monthly, 1 ml subsamples were collected from 10 containers and centrifuged at 4500 rpm for 10 min before filtering through 0.22 µm-pore filter (Miller, Sigma).
Concentration of cucurbitacin B was quantified using the isocratic elution Shimadzu HPLC Prominence with CTO-20A diode array detector, with a wide pore reverse phase C18 (25 cm × 4.0 mm, 5 µm) discovery column (Sigma-Aldrich). A 2:3 (v/v) methanol and deionised water mobile phase at a flow rate of 1.0 ml/min in an oven at 35°C, was monitored at the 230 nm wavelength for 43 min. Standards (1 µg each) were dissolved in 1 ml methanol and then diluted to form 0.02, 0.04, 0.06, 0.08 and 1.0 µg/ml methanol dilutions. The retention times and peak areas of cucurbitacin B in subsamples was compared with those of pure (≈ 98%) cucurbitacin B standard (Wuhan ChemFaces Biochemical Co. Ltd., Wuhan: China).
Data analysis
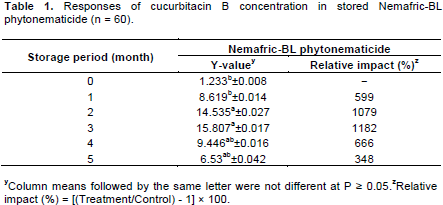
Cucurbitacin B data were subjected to analysis of variance procedure using SAS software (SAS Institute Inc., 2008). When treatment effects were significant at the probability level of 5%, the degrees of freedom and their associated sum of squares were partitioned to determine the percentage contribution of sources of variation to the total treatment variation (TTV) in the observed variable (Steyn et al., 2003). Mean separation was accomplished using Waller-Duncan multiple range test. Variables with significant (P ≤ 0.05) treatment means were further subjected to lines of the best fit using cucurbitacin responses (y-axis) versus increasing storage time (x-axis). The variables were modelled using the regression curve estimations in a quadratic equation: Y = b2x2 + b1x + a, where Y is the concentration of cucurbitacin B and x from the x = –b1/2b2 relation is the optimum storage time. Unless otherwise stated, only treatment means significant at the probability level of 5 % were discussed.
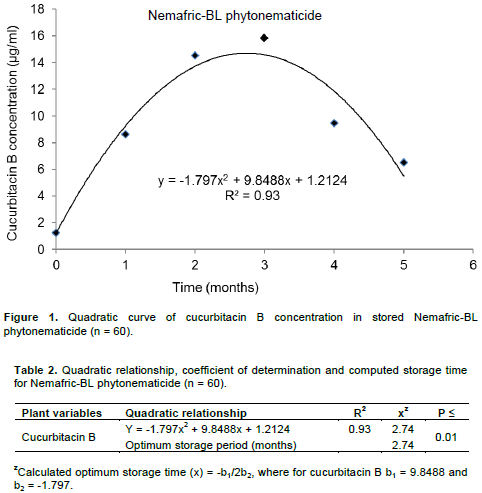
The interaction between the 2014 and 2015 growing seasons was not significant (P > 0.05) and therefore, data were polled (n = 60) and re-analysed. Storage period had highly significant (P ≤ 0.01) effects on concentration of cucurbitacin B Nemafric-BL phytonematicide. Treatments contributed 68% in TTV of cucurbitacin B. Relative to T0, storage increased concentration of cucurbitacin B Nemafric-BL phytonematicide (Table 1). Cucurbitacin B increased by between 599 and 1182% in the first three months and thereafter the rate of increase declined to 348% by the fifth month in Nemafric-BL phytonematicide. Concen-tration of cucurbitacin B versus storage period exhibited quadratic relationship (Figure 1). The model explained the observed relationship for cucurbitacin B by 93%. Using x = –b1/2b2 relation, concentration of cucurbitacin B in Nemafric-BL phytonematicide was optimised at 2.71 months (Table 2).
The quality of phytonematicides is dependent upon the concentration of active ingredient, which is directly associated with their performance. Active ingredients in phytonematicides, being secondary metabolites, are in a state of continuous change (Luckner, 1984) due to microbial degradation and/or auto-oxidation (Gunatilaka, 2006). Loss of product quality in commodities is a global concern (Straus, 2002; Drew and Myers, 1997), which had since necessitated the development of regulatory standards, collectively referred to as shelf-life (WHO, 2002). By definition, shelf-life is the length of time a product may be stored without becoming unsuitable for use (American Heritage® Dictionary of the English Language). In our study, the active ingredient in Nemafric-BL phytonematicide over increasing storage period exhibited strong DDG patterns, characterised by quadratic relations (Mashela et al., 2015). In a certain biofertiliser produced from EM-fermented plant materials, the chemical, physical and microbial characteristics exhibited DDG patterns over increasing storage period (Ngampimol and Kunathigan, 2008).
Gradual stimulation followed by gradual inhibition in concentration of cucurbitacin B over increasing storage period of Nemafric-BL phytonematicide within DDG patterns could be attributed to a series of both extrinsic and intrinsic factors. EM bioactivities are depended upon the availability of energy and carbon from the substrates; (Higa, 1991; Higa and Wididana, 1991). EM is widely used in fermenting plant materials to produce biofertilisers, biopesticides, phytonematicides and feeds (Pelinganga and Mashela, 2012; Pelinganga et al., 2012; Ngampimol and Kunathigan, 2008). Commercially available South African EM comprise photosynthetic bacteria, lactic acid bacteria, yeast, actinomycetes and fermenting fungi (Higa, 1991; Higa and Wididana, 1991; Higa and Parr, 1994). Upon depletion of readily available energy sources (sugar + molasses), EM degrade the plant crude extracts, thereby releasing active ingredients into solution (Margarita and Dengel, 2003). However various forces come into play as the concentration of the active ingredients increases. For example, during the early stages, EM increased B in solution of Nemafric-BL phyto-nematicide, through degradation of fruit crude extracts as sources of readily available energy and carbon. However, with progression time, once fruit crude extracts are depleted, EM starts to attack cucurbitacin B for the same sources, thereby reducing the concentration of cucurbitacin B. Maatooq et al. (1995) noted that the observed decrease in cucurbitacin E-glycoside concentration of bitter Hawkesbury watermelon (Citrullus vulgaris) was primarily ascribed to microbial activities. Additionally, auto-oxidation contributed to the reduction of active ingredients in most bioactive chemical compounds (Allen, 2013).
Reductions in cucurbitacins were also observed at low pH in bitter C. vulgaris solution extracts stored at varying temperatures over time (Martin et al., 2002). EM-produced products like the Nemafric-BL phytonematicide are naturally acidic, with fermentation directly reducing pH of the products (Merlin et al., 2013; Rizk et al., 2007). Miller and Blackwell (1986) reported that a persistent drop in pH could result in enzyme inactivation, which would eventually stop the fermentation process. However, pH did not appear to have played a role in the DDG patterns of cucurbitacin concentrations. At the end of the fermentation process, pH of the mixture was still approximately 3.7 (Mashela et al., 2015). One should appreciate that in addition to sources of energy and carbon for immobilisation in EM, the concentration of active chemical compounds from plant materials using the fermentation process could be affected by pH, temperature, light, presence plus form of precursors and the substrate components (Kumara and Rawal, 2008; Zain et al., 2009; Bhattacharyya and Jha, 2011; Gautam et al., 2011; Jain and Pundir, 2011; Sudarkodi et al., 2012).
In Nemafric-BL phytonematicide the concentration of the active chemical ingredient was optimised at 2.74 months, which is equivalent to ca. 71 days. This duration should not be viewed as being equivalent to shelf-life, because six months after T0, the concentration of cucurbitacin B was still more than three-hundred times to that at T0. The latter suggested that the product was still suitable for use as a phytonematicide.
The authors have not declared any conflict of interests.
Authors are grateful to the Land Bank Chair of Agriculture – University of Limpopo, the Flemish Interuniversity Council of Belgium, the Agricultural Research Council-Universities Collaboration Centre and the Technology Innovation Agency (TIA) for funding this study. All authors approved the manuscript.
REFERENCES
|
Allen LV (2013). An introduction to pharmacy. Pharmaceutical Press, London, UK, pp. 159-161.
|
|
|
|
American Heritage® Dictionary of the English Language (2011). Houghton Mifflin Harbcourt Publishing, Boston, USA.
|
|
|
|
|
Besterfield DH, Besterfield-Michna C, Besterfield GH, Besterfield-Sacre M (2003). Total quality management. Prentice Hall, New Jersey, USA.
|
|
|
|
|
Bhattacharyya PN, Jha DK (2011). Optimization of cultural conditions affecting growth and improved bioactive metabolite production by a subsurface Aspergillus strain TSF 146. Int. J. Appl. Biol. Pharmaceut. Technol. 2:133-143.
|
|
|
|
|
Chen C, Kuo TC, Yang M, Chien T, Chu M, Huang L, Chen C, Lo H, Jeng S, Chen LO (2014). Identification of cucurbitacins and assembly of a draft genome for Aquilaria agallocha. BMC Genom. 15:578.
Crossref
|
|
|
|
|
Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005). Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 22:386-399.
Crossref
|
|
|
|
|
Drew AK, Myers SP (1997). Safety issues in herbal medicines: Implications for the health professionals. Med. J. Aust. 166:538-541.
|
|
|
|
|
Gautam SP, Bundela PS, Pandey AK, Khan J, Awasthi MK, Sarsaiya S (2011). Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol. Res. Int. 1:1-8.
Crossref
|
|
|
|
|
Gunatilaka AAL (2006). Natural products from plant-associated microorganisms: Distribution, structural diversity and bioactivity and implications of their occurrence. J. Nat. Prod. 69:509-526.
Crossref
|
|
|
|
|
Higa T (1991). Effective microorganisms: A biotechnology for mankind. In: Parr JF, Hornick SB, Whitman CE (Eds.), Proceedings of the First International Conference on Kyusei Nature Farming. U.S. Department of Agriculture, Washington, D.C., USA. pp. 8-14.
|
|
|
|
|
Higa T, Parr JF (1994). Beneficial and effective microorganisms for a sustainable agriculture and environment. International Nature Farming Research Center, Atami, Japan.
|
|
|
|
|
Higa T, Wididana GN (1991). The concept and theories of effective microorganisms. pp. 118-124. In: Parr JF, Hornick SB, Whitman CE (Eds.), Proceedings of the First International Conference on Kyusei Nature Farming. U.S. Department of Agriculture, Washington, D.C., USA.
|
|
|
|
|
Jain P, Pundir RK (2011). Effect of fermentation medium, pH and temperature variations on antibacterial soil fungal metabolite production. J. Agric. Technol. 7:247-269.
|
|
|
|
|
Jeffery C (1978). Cucurbitaceae. In: Launert E (Ed.), Flora Zambesia Managing Committee, London, UK, pp. 414-419.
|
|
|
|
|
Kumara KLW, Rawal RD (2008). Influence of carbon, nitrogen, temperature and pH on the growth and sporulation of some Indian isolates of Colletotrichum gloeosporioides causing anthracnose disease of papaya (Carica papaya L). Trop. Agric. Res. Ext. 11:7-12.
|
|
|
|
|
Luckner M (1984). Secondary metabolites in microorganism, plants and animals. Springer-Verlag, Heidelbur, Berlin.
Crossref
|
|
|
|
|
Maatooq G, El-Sharkawy S, Aeiel MS, Rosazza JPN (1995). Microbial transformation of cucurbitacin E 2-O-β-D-glucopyranoside. J. Nat. Prod. 58:107-120.
Crossref
|
|
|
|
|
Maila MY, Mashela PW, Nzanza B (2016). Ex vitro elimination of seed dormancy and in vitro seedling performance of Cucumis africanus and Cucumis myriocarpus. Acta Agric. Scand. B Soil Plant Sci. pp. 1-6.
|
|
|
|
|
Margarita C, Dengel L (2003). Experiences with effective microorganisms in disease and pest control in farms and gardens in India. Accessed November 2, 2015. Available at: http://infrc.or.jp/english/KNF_Data_Base_Web/PDF%20KNF%20Conf%20Data/C7-5-354.pdf
|
|
|
|
|
Martin PAW, Blackburn M, Schroder RFW, Matsuo K, Li BW (2002). Stabilization of cucurbitacin E-glycoside, a feeding stimulant for diabroticite beetle, extracted from bitter Hawkesburg watermelon. J. Insect Sci. 2:19.
Crossref
|
|
|
|
|
Mashela PW (2002). Ground wild cucumber fruits suppress numbers of Meloidogyne incognita on tomato in microplots. Nematropica 32:13-19.
|
|
|
|
|
Mashela PW, De Waele D, Pofu KM (2011). Use of indigenous Cucumis technologies as alternative to synthetic nematicides in management of root-knot nematodes in low-input agricultural farming systems: A review. Sci. Res. Essays 33:6762-6768.
|
|
|
|
|
Mashela PW, Dube ZP, Pofu KM (2015). Managing the phytotoxicity and inconsistent nematode suppression in soil amended with phytonematicides. In: Meghvansi MK, Varma A (Eds.), Organic amendments and soil suppressiveness in plant disease management. Springer International Publishing, Switzerland.
Crossref
|
|
|
|
|
Merlin JM, Christhudas IVSN, Kumar PP, Agastian P (2013). Optimization of growth and bioactive metabolite production: Fusarium solani. Asian J. Pharm. Clin. Res. 6:98-103.
|
|
|
|
|
Miller JD, Blackwell BA (1986). Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLX 1503 in a stirred jar fermentor. Can. J. Bot. 64:1-5.
Crossref
|
|
|
|
|
Ngampimol H, Kunathigan V (2008). The study of shelf life for liquid biofertilizer from vegetable waste. AU JT 11(4):204-208.
|
|
|
|
|
Nzanza B, Mashela PW (2012). Control of whiteflies and aphids in tomato by fermented plant extracts of neem leaf and wild garlic. Afr. J. Biotechnol. 11:16077-16082.
|
|
|
|
|
Pelinganga OM, Mashela PW (2012). Mean dosage stimulation range of allelochemicals from crude extracts of Cucumis africanus fruit for improving growth of tomato plant and suppressing Meloidogyne incognita numbers. J. Agric. Sci. 12:8-12.
Crossref
|
|
|
|
|
Pelinganga OM, Mashela PW, Nzanza B, Mphosi MS (2012). Baseline information on using fermented crude extracts from Cucumis africanus fruit for suppression of Meloidogyne incognita and improving growth of tomato plant. Afr. J. Biotechnol. 11:11407-11413.
Crossref
|
|
|
|
|
Rizk M, Abdel-Rahman T, Metwally H (2007). Factors affecting growth and antifungal activity of some Streptomyces species against Candida albicans. J. Food Agric. Environ. 5:446-449.
|
|
|
|
|
Rogers LL (2010). Using sensory techniques for shelf-life assessment. In: Kilcast D (Ed.), Sensory analysis for food and beverages quality control: A practical guide 2010. Woodhead Publishing Limited, Cambridge, UK.
Crossref
|
|
|
|
|
SAS Institute Inc (2008). SAS/STAT® 9.2 qualification tool user's guide. SAS Institute Inc. Carry, (NC).
|
|
|
|
|
Shadung K, Mashela P, Mulaudzi V, Mphosi M, Ncube I (2015). Optimum harvest time of Cucumis africanus fruit using concentration of cucurbitacin B as a maturity standard. J. Agric. Sci. 7:181-186.
Crossref
|
|
|
|
|
Steyn AGW, Smit CF, Du Toit SHC, Strasheim C (2003). Modern statistics in practice. Van Schaik, Pretoria.
|
|
|
|
|
Straus SE (2002). Herbal medicines-what's in the bottle? N. Engl. J. Med. 347(25):1997-1998.
Crossref
|
|
|
|
|
Sudarkodi C, Subha K, Kanimozhi K, Panneerselvam A (2012). Optimization and production of itaconic acid using Aspergillus flavus. Adv. Appl. Sci. Res. 3:1126-1131.
|
|
|
|
|
World Health Organisation (WHO) (2002). Quality control methods for medicinal plant materials. Geneva, AT.T.B.S. Publisher and Distributor, Delhi.
|
|
|
|
|
Zain ME, Razak AA, El-Sheikh HH, Soliman HG, Khalil AM (2009). Influence of growth medium on diagnostic characters of Aspergillus and Penicillium species. Afr. J. Microbiol. Res. 3:280-286.
|
|

