Full Length Research Paper
ABSTRACT
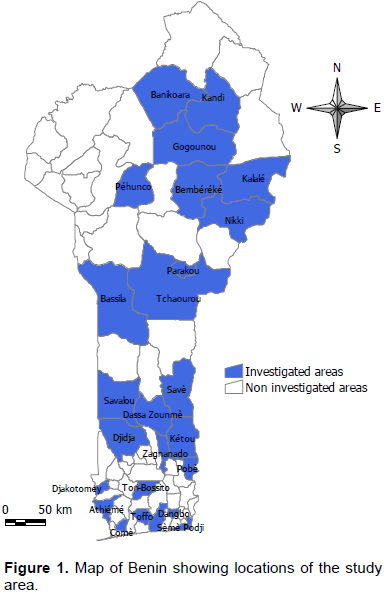
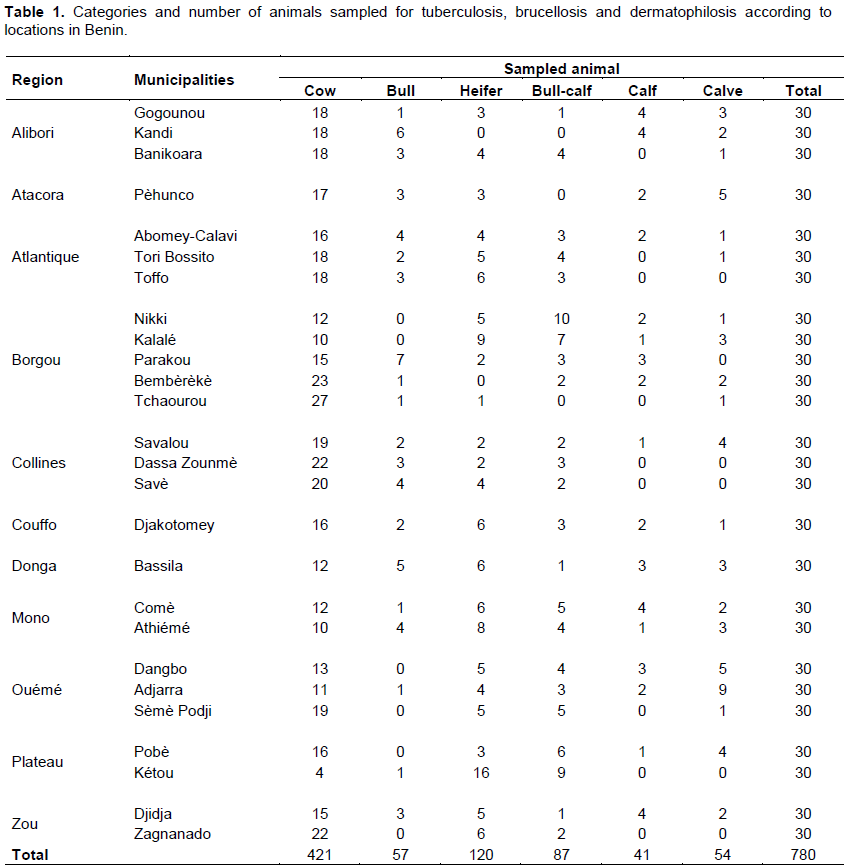
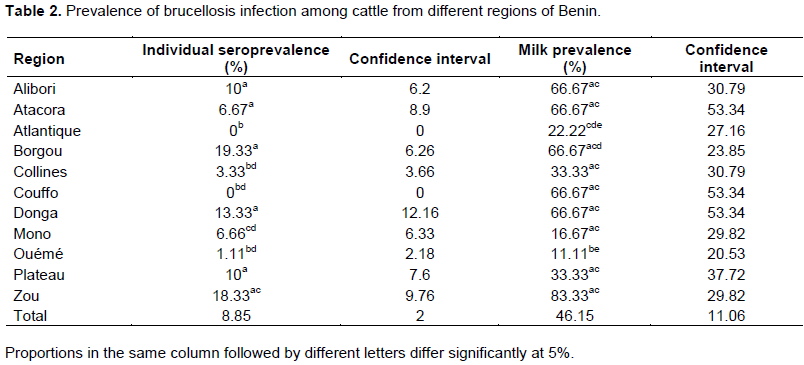
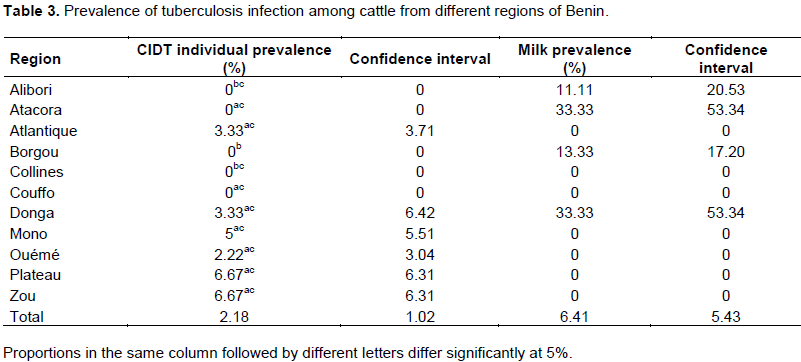
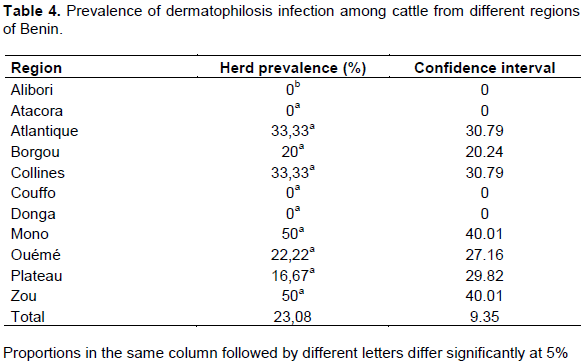
In order to determine the prevalence of bovine brucellosis, tuberculosis and dermatophilosis, a study was carried out in main dairy areas of Benin from April to September 2015. For brucellosis, 780 sera and 78 milk samples were analyzed by indirect enzyme-linked immunosorbent assay (iELISA). For tuberculosis, 780 cattle underwent a comparative intradermal tuberculin test and 78 milk samples were used for Ziehl-Neelsen’s staining. About dermatophilosis, 78 samples of scabs were collected for Giemsa’s staining. For brucellosis, the overall individual animal seroprevalence was 8.85%. The regions of Borgou with 19.33% and Atlantique with 0% prevalence showed significant differences (p <0.05) with the other regions. For tuberculosis, the overall individual animal prevalence was 2.18%. The regions of Borgou and Alibori, with 0% prevalence each, showed significant differences (p <0.05) with most other regions. Taking into account the individual animal prevalence, Zou (brucellosis 18.33%, tuberculosis 6.67%) and Plateau (brucellosis 10%, tuberculosis 6.67%) were the areas at risk for these two diseases. For dermatophilosis the overall herd prevalence was 23.08%. There was significant difference (p<0.05) between Alibori and Mono but also between Alibori and Zou. It is urgent, therefore, to put in place an adapted control strategy taking into account these geographical realities.
Key words: Brucellosis, tuberculosis, dermatophilosis, prevalence, cattle, Benin
INTRODUCTION
MATERIALS AND METHODS
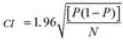
RESULTS
DISCUSSION
CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Abbas B, Agab H (2002). A review of camel brucellosis. Prev. Vet. Med. 55(1):47-56. |
|
|
Acha PN, Szyfres B (1989). Zoonoses and communicable diseases common to humans and animals. Paris: O.I.E. P 1063. |
|
|
Adehan R, Koutinhouin B, Baba-Moussa LS, Aigbe L, Agbadje PM, Youssao AKI (2005). Prevalence of bovine brucellosis in Benin's State farms from 2000 to 2003. R.A.S.P.A. 3:3-4. |
|
|
Adugna KE, Agga GE, Zewde G (2013).Sero epidemiological survey of bovine brucellosis in cattle under a traditional production system in western Ethiopia. Rev. Sci. Technol. 32(3):765-773. |
|
|
Akakpo JA, Bonarel P, d'Almeida JF (1984). Epidemiology of bovine brucellosis in tropical Africa.Serological survey in Benin's Republic. Rev. Méd. Vét. 37 :133-137. |
|
|
Ali-Emmanuel N, Moudachirou M, Akakpo AJ, Quetin-Leclercq J (2002). In vitro antibacterial activities of Cassia alata, Lantana camara, Mitracarpus scaber, Dermatophilus congolensis isolated in Benin. Rev. Elev. Méd. Vét. Pays Trop. 55(3):183-187. |
|
|
Asante-Poku A, Aning KG, Boi-Kikimoto B, YeboahManu D (2014). Prevalence of bovine tuberculosis in a dairy cattle farm and a research farm in Ghana. Onderstepoort J. Vet. Res. 81(2):1-6. |
|
|
Asgedom H, Damena D, Duguma R (2016). Seroprevalence of bovine brucellosis and associated risk factors in and around Alage district, Ethiopia. Springerplus. 5:851. |
|
|
Awad WS, Nadra-Elwgoud, Abdou MI, El-Sayed AA (2008).Diagnosis and Treatment of Bovine, Ovine and Equine Dermatophilosis. J. Appl. Sci. Res. 4(4):367-374. |
|
|
Berhe G, Belihu K, Asfaw Y (2007). Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. Int. J. Appl. Res. Vet. Med. 5:65-71. |
|
|
Boukary AR, Thys E, Abatih E, Gamatie D, Ango I (2011). Bovine Tuberculosis Prevalence Survey on Cattle in the Rural Livestock System of Torodi (Niger). PLoS One 6(9):e24629. |
|
|
Boussini H, Traoré A, Tamboura HH, Bessin R, Boly H, Ouédraogo A (2012). Prevalence of tuberculosis and brucellosis in intra-urban and peri-urban dairy cattle in Ouagadougou's city in Burkina Faso. Rev. Sci. Tech. Off. int. Epizoot. 31(3):943-951. |
|
|
Cadmus SIB, Alabi PI, Adesokan HK, Dale EJ, Stack JA, (2013). Serological investigation of bovine brucellosis in three cattle production systems in Yewa Division, south-western Nigeria. J. South African Vet. Assoc. 84(1):00-00. |
|
|
Chatikobo P, Kusina NT, Hamudikuwanda H, Nyoni O (2004).A monitoring study on the prevalence of dermatophilosis and parafilariosis in cattle in a smallholder semi-arid farming area in Zimbabwe. Trop. Anim. Health. Prod. 36(3):207-215. |
|
|
Cutler SJ, Whatmore AM, Commander NJ (2005). Brucellosis: new aspects of an old disease. J. Appl. Microbiol. 98:1270-1281. |
|
|
Dejene B, Ayalew B, Tewodros F, Mersha C (2012). Occurance of Bovine Dermatophilosis in Ambo town West Shoa Administrative Zone, Ethiopia. American-Eurasian J. Sci. Res. 7(4):172-175. |
|
|
Dinka H, Chala R (2009). Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa Zone, Oromia Regional State, Ethiopia. American-Eurasian J. Agric. Environ. Sci. 6(5):508-512 |
|
|
Direction of Animal Production (DAP) (2012). Statistical Yearbook of 2011. Benin. P 31. |
|
|
Direction of Animal Production (DAP) (2013).Statistical Yearbook of 2012. Benin. P 31. |
|
|
Direction of Animal Production (DAP) (2014).Statistical Yearbook of 2013. Benin. P 28. |
|
|
Direction of Animal Production (DAP) (2015).Statistical Yearbook of 2014. Benin. P 25. |
|
|
Direction of Animal Production (DAP) (2016).Statistical Yearbook of 2015. Benin. P 12. |
|
|
Dossou J, Atchouké GD, Dabadé DS, Azokpota P, Montcho JK (2016). Nutritional and health quality comparative evaluation of milk from different breeds of cows in some Benin's breeding areas. Eur. Sci. J. 12(3). |
|
|
Farougou S, Agbadjè P, Kpodékon M, Adoligbé C, Akakpo JA (2006). Prevalence of bovine tuberculosis in the state farms of Samiondji and Betecoucou of Benin. R.A.S.P.A. 4(1-2). |
|
|
Hamid M, Musa MS (2009).The treatment of bovine dermatophilosis and its effects on some haematological and blood chemical parameters. Rev. Sci. Tech. Off. int. Epizoot. 28:1111-1118. |
|
|
Kassaye E, Moser I, Woldemeskel M (2003). Epidemiological study on clinical bovine dermatophilosis in northern Ethiopia.Dtsch. Tierarztl.Wochenschr.110(10):422-425. |
|
|
Katale BZ, Mbugi EV, Karimuribo ED, Keyyu JD, Kendall S, Kibiki GS, Faussett PG, Michel AL, Kazwala RR, Helden PV, Matee MI (2013). Prevalence and risk factors for infection of bovine tuberculosis in indigenous cattle in the Serengeti ecosystem, Tanzania. BMC Vet. Res. 9(1):267. |
|
|
Koutinhouin B, Youssao AKI, Houéhou AE, Agbadjè PM (2003). Prevalence of bovine brucellosis in the traditional breeding farms supervised by the Project for Livestock Development (PDE) in Benin. Rev. Méd. Vét. 154(4):271-276. |
|
|
Kusina NT, Chatikobo P, Hamudikuwanda H, Nyoni O (2004). A monitoring study on the prevalence of dermatophilosis and parafilariosis in cattle in a smallholder semi-arid farming area in Zimbabwe. Trop. Anim. Health. Prod. 36:207-215. |
|
|
Makita K, Fèvre EM, Waiswa C, Eisler MC, Thrusfield M, Welburn SC (2011). Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Vet. Res. 7:60. |
|
|
Mariam SH (2009). Interaction between lactic acid bacteria and M. bovis in Ethiopian fermented milk. Appl. Environ. Microbiol. 75:1790-1792. |
|
|
Matope G, Bhebhe E, Muma JB, Lund A, Skjerve E (2010). Herd-level factors for Brucella seropositivity in cattle reared in smallholder dairy farms of Zimbabwe. Prev. Vet. Med. 94(3-4):213-221. |
|
|
McDermott JJ, Arimi SM (2002). Brucellosis in Sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 20:111-134 |
|
|
Megersa B, Biff D, Niguse F, Rufael T, Asmare K, Skjerve E (2011b). Cattle brucellosis in traditional livestock husbandry practice in Southern and Eastern Ethiopia, and its zoonotic implication.Acta. Vet. Scand. 53:24. |
|
|
Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ (1994). The tuberculin test. Vet. Microbiol. 40(1-2):111-124. |
|
|
Morrow AN, Arnott JL, Heron ID, Koney EBM, Walker AR (1993).The effect of tick control on the prevalence of dermatophilosis on indigenous cattle in Ghana. Rev. Elev. Med. Vet. Pays Trop. 46:317-322. |
|
|
Mostowy S, Inwald J, Gordon S, Martin C, Warren R, Kremer K, Cousins D, Behr MA (2005). Revisiting the evolution of Mycobacterium bovis. J. Bacteriol. 187:6386-6395. |
|
|
Muma JB, Syakalima M, Munyeme M, Zulu VC, Simuunza M, Kurata M (2013). Bovine Tuberculosis and Brucellosis in Traditionally Managed Livestock in Selected Districts of Southern Province of Zambia. Vet. Med. Int. 2013:730367. |
|
|
Okeke LA, Cadmus S, Okeke IO, Muhammad M, Awoloh O, Dairo D, Waziri EN, Olayinka A, Nguku PM, Fawole O (2014). Prevalence and risk factors of Mycobacterium tuberculosis complex infection in slaughtered cattle at Jos South Abattoir, Plateau State, Nigeria. Pan Afr. Med. J. 18(1):7. |
|
|
Omer K, Holstand G, Skjerve E, Woldehiwet Z, MacMillan AP (2000).Prevalence of antiodies to Brucella spp in catte, sheep, goats, horses and camels in the State of Eritrea; influence of husbandry system. Epidemiol. Infect. 125:447-453. |
|
|
Ragassa G, Mekonnen D, Yamuah L, Tilahun H, Guta T, Gebreyohannes A, Aseff A, Abdoel TH, Smits HL (2009). Human brucellosis in Traditional pastoral communities in Ethiopia. Int. J. Trop. Med. 4:59-64 |
|
|
Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, Tanner M, Zinsstag J (2003). Brucellosis and Q-fever sero prevalence of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 61:279-293 |
|
|
Scott DW (1988). Large animal dermatology.Edition Sanders.Philapdelhia. pp.136-146. |
|
|
Singh AP, Singla LD and Singh A (2000) A study on the effects of macroclimatic factors on the seasonal population dynamics of Boophilus micropus (Canes, 1888) infesting the cross-bred cattle of Ludhiana district. Int. J. Anim. Sci. 15(1):29-31. |
|
|
Swai ES, Schoonman L (2010).The Use of Rose Bengal Plate Test to Asses Cattle Exposure to Brucella Infection in Traditional and Smallholder Dairy Production Systems of Tanga Region of Tanzania. Vet. Med. Int. 2010:837950 |
|
|
Thoen CO, Bloom BR (1995). Pathogenesis of Mycobacterium bovis In: Thoen CO, Steele JH, editors. Mycobacterium bovis Infection in Animals and Humans. First ed. Ames, Iowa: Iowa State University Press. pp: 3-14. |
|
|
Traoré A, Tamboura HH, Bayala B, Rouamba DW, Yaméogo N, Sanou M (2004). Overall prevalence of major pathologies affecting cow milk's production in intra urban breeding system in Hamdallaye (Ouagadougou).Biotechnol. Agron. Soc. Environ. 8 (1):3-8. |
|
|
Tschopp R, Abera B, Sourou SY, Guerne-Bleich E, Aseffa A, Wubete A, Zinsstag J, Young D (2013). Bovine tuberculosis and brucellosis prevalence in cattle from selected milk cooperatives in Arsi zone, Oromia region, Ethiopia. BMC Vet. Res. 9:163. |
|
|
Walker RL (1999). "Brucella," in Veterinary Microbiology, C. H. Dwight and C. Z. Yuang, Eds. Blackwell Science, Cambridge, Mass, USA. pp. 196-203. |
|
|
Wareth G, Melzer F, Elschner MC, Neubauer H, Roesler U (2014).Detection of Brucella melitensis in bovine milk and milk products from apparently healthy animals in Egypt by real-time PCR. J. Infect. Dev. Ctries. 8(10):1339-1343. |
|
|
Wilson ML (1986). Reduced Abundance of adult Ixodesdammini (Acari: Ixodidae) following destruction of Vegetation. J. Econ. Entomol. 79(3):693-696. |
|
|
Wold Health Organization (WHO) (2004).Report of the WHO/FAO/OIE Joint Consultation on Emerging Zoonotic Diseases, Geneva, Switzerland. P 72. |
|
|
World Organization for Animal Health (OIE) (2009).Bovine brucellosis and bovine tuberculosis.In OIE Terrestrial Manual. Chapter 2.4.3.Paris: France. |
|
|
World Organization for Animal Health (OIE) (2010).Manual of the Diagnostic Tests and Vaccines for Terrestrial Animals.vol. 1.Office International Des Epizooties.5th edition. Paris: France. |
|
|
Yeruham I, Elad D, Perl S (2000). Economic aspects of outbreaks of dermatophilosis in first calving cows in nine herds of dairy cattle in Israel. Vet. Rec. 146(24):695-698. |
|
|
Zinsstag J, Schelling E, Roth F, Bonfoh B, Savigny D, Tanner M (2007). Human benefits of animal interventions for zoonosis control. Emerg. Infect. Dis. 13:527-532. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0