ABSTRACT
An examination of raw milk for the isolation of coliforms as members of mastitis-causing organisms was conducted on 300 cows from four Local Government Areas in Kaduna State, northern Nigeria. A 10.3% prevalence was recorded for coliform organisms using the Microgen GN-ID A+B Kit (Medica-TecTM), these were; Enterobacter spp., Citrobacter spp., Klebsiella spp., Serratia marcescens, Proteus spp. and Pantoea agglomerans (similar to Enterobacter aerogenes). Antibiotic sensitivity test using commonly available antibiotics showed that all isolates were sensitive to amoxicillin and ciprofloxacin, but resistant to tetracycline and erythromycin. Age, parity number, stage of lactation, management system, hygiene of milking process, and presence of lesion on udder/teat were found to be significantly associated (p<0.05) with the prevalence of mastitis in cows. The lowest prevalence (24%; 48 of 200) was recorded in cows within 3 to 4 years of age while, the highest (60.6%; 20 of 33) was in cows aged above 5 years. Stage of lactation was significant with the prevalence of mastitis being the highest (45.5%; 30 of 66) during the initial stage of lactation (0 to 5 month). It was concluded that, the relatively high prevalence of coliforms in bovine mastitis in dairy herds could significantly reduce milk production and cause economic losses. Good hygiene in milking process, milking clinically infected cows last, culling chronic mastitis cases, treating clinically infected cows and dry period therapy could reduce the prevalence of coliform mastitis in Kaduna State, Nigeria.
Key words: Cow, milk, mastitis, coliforms, antibiotic sensitivity.
Almost any microbe that can opportunistically invade tissues and cause infection can cause mastitis. However, most infections are caused by various species of streptococci, staphylococci, and gram-negative rods, especially lactose-fermenting organisms of enteric origin, Commonly termed coliforms .Escherichiacoli, Enterobacter aerogenes, Klebsiella pneumoniae and Serratia marcescens are four common coliform bacteria that cause mastitis (Radostits et al., 2007; Junaidu et al., 2011). Coliform bacteria are normal inhabitants of soil, digestive tract and manure. They accumulate and multiply in contaminated bedding. Coliform numbers of 1,000,000 or more per gram of bedding increase the likelihood of an udder infection and clinical mastitis (Podder et al., 2014). Klebsiella pneumoniae is common in sawdust bedding, especially rough-cut sawdust that contains bark or soil (Kagkli et al., 2006). Coliforms invade the udder through the teat sphincter when teat ends come in contact with coliform bacteria. Once coliform bacteria enter the mammary gland, they either multiply rapidly or remain dormant. However, the immune response of the cow is highly successful in destroying these bacteria. Many inflammatory and systemic changes seen in severe coliform mastitis result from the effects of release of lipopolysaccharide (LPS) endotoxin (a component of the bacteria cell wall) and subsequent activation of cytokine and arachidonic acid–derived mediators of inflammation and the acute phase response. By the time therapy is initiated, maximal release of LPS has likely occurred (Burns et al., 2000; Smith and Hogan, 2001). Coliform bacteria are responsible for a great number of acute clinical mastitis cases in dairy cows. Severely affected cows may show signs of high fever, udder inflammation (swelling), depressed appetite, dehydration (sunken eyes), diarrhea, decreased production and abnormal milk. Coliform bacteria are also capable of producing subclinical infections that persist for longer periods of time (Radostits et al., 2007). Studies have shown that coliform mastitis apart from resulting to agalactiae can cause substantial losses to producers (NMC, 2000). This disease costs the US dairy industry about 1.7 to 2 billion USD each year (Jones and Bailey, 2010). Consequently, due to economic losses resulting from infection, the relatively high incidence of infection as compared to other clinical mastitis pathogens, and the occasional severe nature of infection, coliform mastitis continue to be a major problem confronting dairy producers (FAO, 2008; Junaidu et al., 2011).
Mastitis caused by coliforms results in a higher incidence of cow death or agalactia-related culling (30 to 40%) than mastitis caused by other pathogens (2%). Prognosis for cases of Klebsiella infection should be particularly guarded, because these cows are twice more likely to be culled or die than those infected by other coliforms (Jones and Bailey, 2010). Serratia mastitis may arise from contamination of milk hoses, teat dips, water supply, or other equipment used in the milking process. The organism is resistant to disinfectants (Kagkli et al., 2006). The bedding used to house cattle is the primary source of environmental pathogens, but contaminated teat dips, intramammary infusions, water used for udder preparation before milking, water ponds or mud holes, skin lesions, teat trauma, and flies have all been incriminated as sources of infection (Matofari et al., 2003; Kivaria and Noordhuizen, 2007). A study carried out in Sokoto State, Nigeria reported E. coli (9.78%), Klebsiella spp. (4.35%), Proteus spp. (8.69%) and Enterobacter spp. (1.09%) (Junaidu et al., 2011). A cross-sectional study carried out in Gondar town, Ethiopia had 54 different bacterial species identified but, E. coli (29.6%), Pseudomonas aeruginosa (18.5%), and Klebsiella pneumoniae (16.7%), were the most commonly identified gram-negative staining bacterial pathogens (Garedew et al., 2012). Majority of coliform isolates from a study conducted using the raw milk consumed in Khartoum, Sudan were E. coli 32%, Enterobacter spp. 29.2%, Klebsiella spp. 19.4%, Serratia spp. 11.1% and Citrobacter 1.0% (Salman and Hamad, 2011).
Study design
A cross-sectional study was carried out on 26 farms in Kaduna State targeting peri-urban farms that provide milk to the community. A total of 300 cows were sampled. Selection of farms was based on; different management conditions, willingness of the farmers/pastoralists to participate in the study, and accessibility of the location, so that samples collected could be immediately transferred to the laboratory for further analysis. All cows in-milking were sampled in every farm visited and it was ascertained that the cows did not receive any treatment before sampling.
Clinical examination of udder
Individual cows were properly restrained, identified and clinically examined. Clinical findings like abnormalities of udder secretions, abnormalities of udder size, consistency, and temperature of mammary gland were examined by visual inspection and palpation. Pain reaction upon palpation, changes in the milk (blood tinged milk, watery secretions, clots, pus), and change in consistency of udder were considered as indications of the presence of clinical mastitis.
Sample collection
The teats were disinfected using a disposable paper towel immersed in 70% ethyl alcohol. California Mastitis Test (CMT) was performed on the first stream of milk before volumes of about 10ml (composite) were aseptically collected into labelled sterile universal bottles. The samples were kept in a cool box containing ice packs and transported to the laboratory immediately.
Media cultivation and identification
A 10 fold serial dilution was done using 3 tubes containing 9mls of sterile physiological saline (PSS). The 10-2 and 10-3dilutions were used to inoculate MacConkey and Nutrient Agar respectively, by surface plating. One tenth of a milliliter of the diluted sample was added to the culture media and evenly spread out with an ‘L’ shaped sterile glass rod. Plated culture media were aerobically incubated at 37°C for 24 h. Individual pinkish colonies on MacConkey agar were picked and sub cultured onto freshly prepared MacConkey agar plate and incubated at 37°C for 24 h to purify the coliform isolate. Representative colonies were stored in slant bottles containing freshly prepared nutrient agar and kept in the refrigerator (4°C) until required for further work (David, 2011).
Biochemical tests for coliforms
Conventional biochemical tests (TSI, SIM, Citrate, Urea and MRVP) were done to preliminarily identify the bacteria isolates before using a commercial kit for Enterobacteriaceae (Medica-TecTM Microgen GN-ID A+B Kit) to confirm the identities of the isolates according to the manufacturers’ instructions.
Antibiotic sensitivity testing
Antibiotic sensitivity was carried out on the coliform isolates using commercially prepared antibiotics (Oxoid). The antibiotics employed were: gentamicin, amoxicillin, chloramphenicol, streptomycin, ciprofloxacin, tetracycline and erythromycin. Colonies of the isolate of interest was inoculated into peptone water, the inoculum was standardized to 0.5 McFarland and incubated for 4 hours. A sterile cotton swab was soaked into the peptone water culture and excess fluid expressed from the swab by rolling it on the side of the culture bottle. The swab was used to streak the entire surface of the Mueller-Hinton agar plate. Then antibiotic discs were placed on the surface of the plate 15 mm apart. The plates were then incubated at 37°C for 24 h. The zone of inhibition was measured in millimeters (mm) and compared against a reference standard which contains measurement ranges and their equivalent qualitative categories of susceptible/sensitive (CLSI, 2013).
Administration of questionnaires
Two sets of structured questionnaire were developed and administered through discussion with the Zonal Veterinary Assistants and Livestock Extension Specialists, in order to capture information on herd/farm and individual cow. A total of 26 questionnaires on herd information and 300 questionnaires on individual cow information were distributed. The questionnaire was composed in English and translated to the Fulani herdsmen in Hausa language. Each questionnaire took about 10 to 15 minutes to be administered.
Data analyses
The Statistical Package for Social Science (SPSS) version 20 was used to analyze the data. Each cow-in-milking was a statistical unit. Outcome of coliform examination and sensitivity to antibiotics were compiled in tabular forms. Data obtained from questionnaires were analyzed to determine the prevalence and distribution of mastitis in cows of the study area. Significance of risk factors on the prevalence of mastitis in cows was calculated using chi-square (χ2) technique to test the association between affected cows and risk factors like age, parity and stage of lactation. In addition, logistic regression analysis was used to calculate odds ratio (with 95% confidence interval and P < 0.05 regarded as significant) to measure the degree of association between risk factors and the disease in cows. Results of different isolates were compared.
Thirty-one (10.3%) milk samples were positive for coliforms in this study (Table 1) and are in close agreement with that of Abdurahman (2006) in eastern Ethiopia and Kalla et al. (2008) in Kano State, Nigeria. However, higher prevalence of coliform mastitis were reported by Sena et al. (2001) in India, Woubit et al. (2001) in Southwestern Ethiopia and in Jordan by Hawari and Hassawi (2008). The highest prevalence of coliform mastitis so far were documented by Junaidu et al. (2011) who reported a 52% for Sokoto State, Nigeria and Karimurbo et al. (2005) who reported 66% in Tanzania. The differences in sample size, environmental condition, breed and feeding regimen are possibly the major causes for this discrepancy. The isolated genera of coliform bacteria in this study were Klebsiella, Pantoea, Enterobacter, Serratia, Citrobacter and Proteus (Table 1). This is in close agreement with Matofari et al. (2003), Abdurrahman (2006), Kalla et al. (2008), Giannino et al. (2009), Abera et al. (2010) and Garedew et al. (2012) Who all found Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter, Serratia and Proteus as major mastitogens. Although E. coli was not isolated from this study, the other coliform bacteria are generally found in high concentrations in organic matter, such as bedding and manure (environment). Therefore from an epidemiologic standpoint, the primary source of infection for most pathogens in this study is environmental. Klebsiella spp. accounted for 51.6% of the isolates in this study; Podder et al. (2014) asserted that Klebsiella pneumoniae is well adapted to survive in the udder and usually establishes a mild subclinical infection of long duration, from which it is shed in milk, facilitating transmission to healthy animals, mainly during milking procedures. This might be due to the fact that there are no established mastitis control practices that are employed by the farmers, but instead mastitis control relies heavily on drug use.
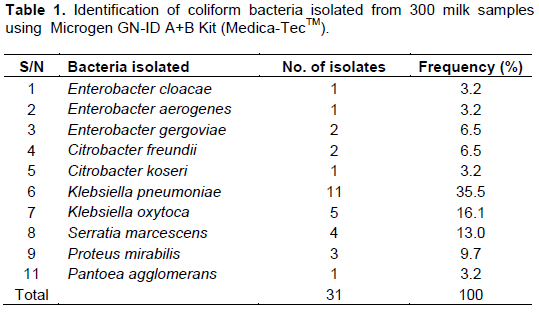
Moreover, the unhygienic housing and milking practices observed among the dairy herds increase both exposure and infection pressure to cows, with the subsequent high infection levels. The present study showed that there was high prevalence of coliform mastitis and, Klebsiella spp. were the dominant coliform isolates in the sampled area. Studies have shown Klebsiella spp, Enterobacter spp, Citrobacter spp. and Serratia spp. to be the most frequently isolated microrganisms in connection with nosocomial infections (Radostits et al., 2007; Podder et al., 2014). Recently, all species and all serotypes of Klebsiella, all species of Proteus, Citrobacter, Enterobacter, Pantoea and Serratia are listed by the United States Public Health Service, Department of Health and Human Services bioterrorism list of dangerous biological agents that have the potential to pose a severe threat to public health and safety, to animal health, to plant health, or to animal and plant products (Bynum, 2011). All of these organisms were isolated from cow milk samples and are therefore of public health importance.
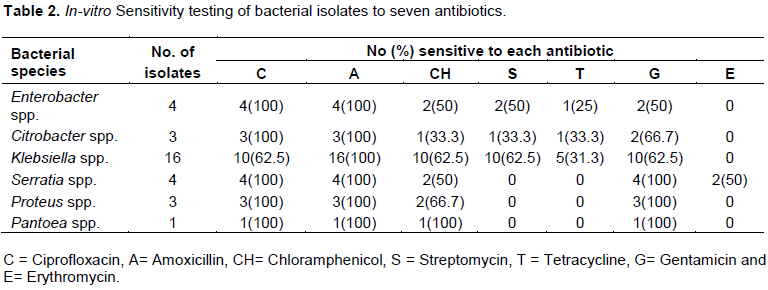
Antibacterial susceptibility testing of coliform isolates showed that, all coliform isolates were sensitive to amoxicillin followed by decreasing susceptibilities to ciprofloxacin, gentamicin, streptomycin, tetracycline and erythromycin (Table 2). The pattern of susceptibility and resistance exhibited in the present study may be due to prolonged and indiscriminate usage and prescription of particular antibiotics which often leads to possible resistance development in animals (Kwaga, 2012; Sharma, 2014). On table 3, questionnaires data analysis showed that 89(29.7%) milk samples were CMT positive of these; 13(4.3%) were contaminated with only yeasts, 7(2.3%) with only coliforms while, 24(8%) with yeasts and coliforms. More than 60% of cows aged above 5 years were affected with mastitis (Table 3) this is in agreement with Sena et al. (2001) who reported that animals aged more than 5 years were more susceptible to mastitis. After the age of 7 years, immunity of the animals is affected making them vulnerable to diseases.
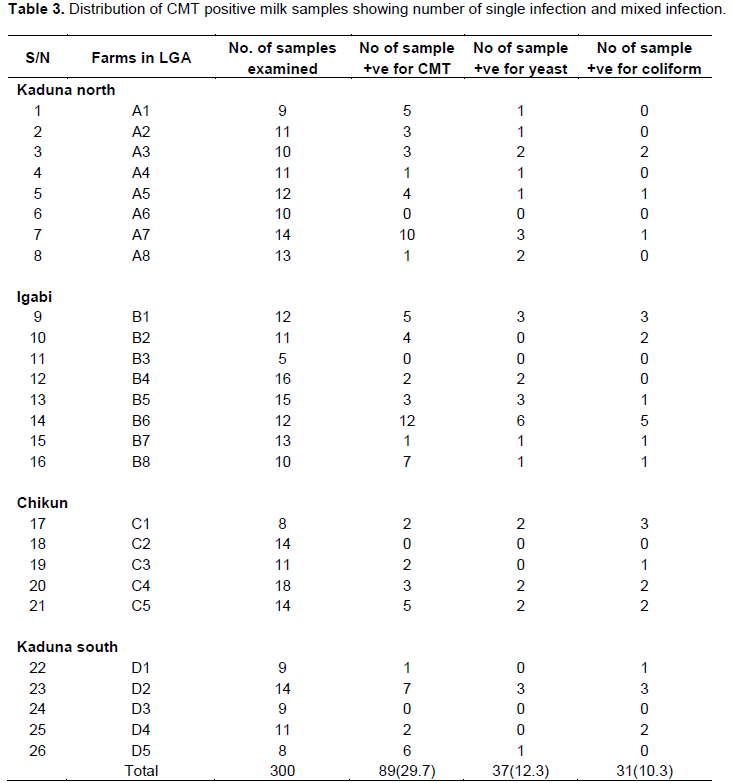
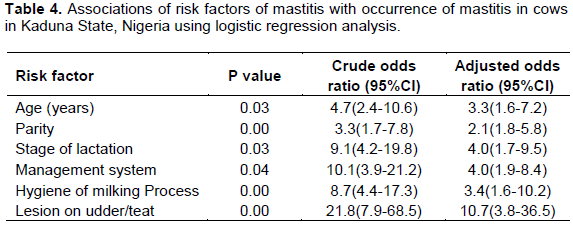
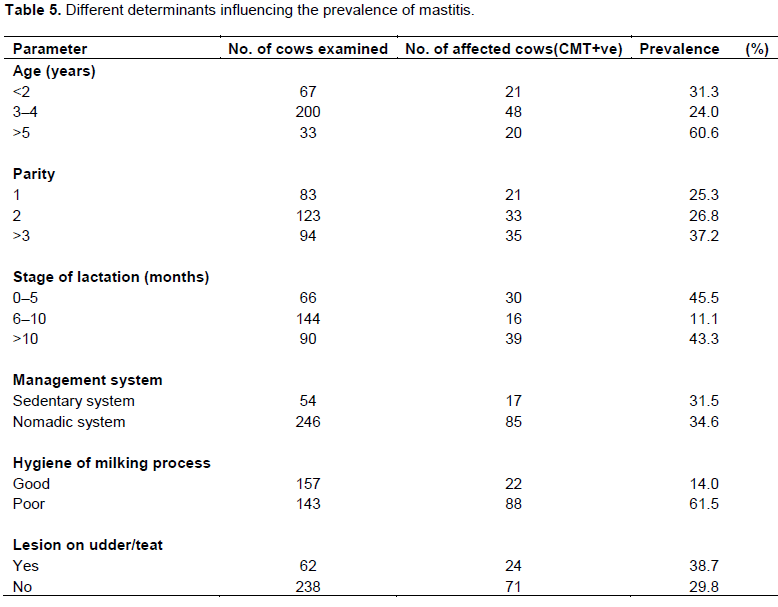
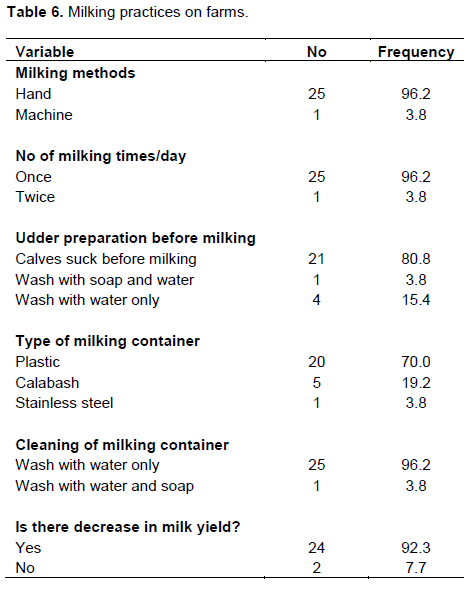
The study determined the increasing prevalence of mastitis with an increase in parity number which agrees with findings by Abdurahman (2006). Radostits et al. (2005) stated that high yielding animals are more susceptible to mastitis than low-yielding ones. Also observed were cows with lesions on their teats and/or udder had a 10.7 time chances of developing mastitis than cows that had no teats lesions (Table 4). This is similar to the findings of Mulei (1999), who found in the Kiambu district of Kenya that mammary gland quarters with teat lesions were 7.2 times more likely to have microorganisms isolated from them than those without any teat lesions. Also, cows managed by nomadic system were 4 times likely to develop mastitis than cows in the sedentary system. In addition, animals with poor hygiene of milking process had a 3.4 times chance of developing mastitis. The nomadic system of cattle rearing and poor hygiene of milking process were also identified as risk factors for occurrence of coliform mastitis in another study in Ethiopia (Abdurahman, 2006). This might be due to absence of udder washing, milking cows that had been suckled by calves without washing the udder/teats, milking of cows with milkers who had cuts and chaps on their hands, and using of common udder cloths, which could be fomites for spread especially for contagious mastitis (Tables 5 and 6). On Table 7, twenty- one (80.8%) farmers consumed boiled or pasteurized milk while 5(19.2%) of the farmers consumed raw milk.
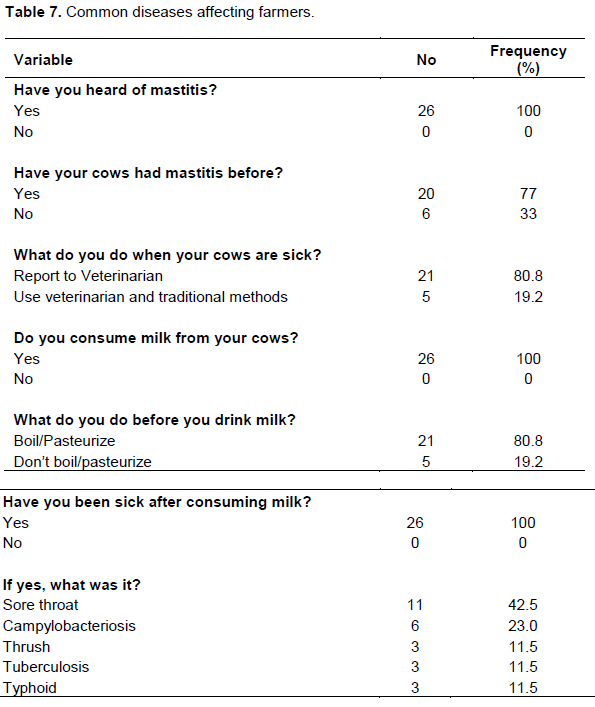
Although, milk consumption in Africa is fairly low compared to the rest of the world, in tribes where milk consumption is popular, such as the Fulani and Maasai, milk is typically consumed unpasteurized (Mulei, 1999; Ocholi et al., 2004). Those favoring the consumption of raw milk believe that raw milk and associated products are healthier and taste better but, under such conditions, public health hazards definitely exist as seen from the type of diseases farmers suffered from such as; sore throat (42.5%), campylobacteriosis (23%), thrush (11.5%), tuberculosis (11.5%) and typhoid (11.5%). Agencies such as theCenters for Disease Control and Prevention (CDC),Food and Drug Administration (FDA) and other regulatory agencies around the world note that pathagens from raw milk, including potentially agents of tuberculosis, diphtheria,typhoid, and streptococcal infections, make it unsafe to consume (FDA, 2009). Similarly, a recent review authored by the Belgian Federal Agency for the Safety of the Food Chain and experts from Belgian universities and institutions concluded that "raw milk poses a realistic health threat due to a possible contamination with human pathogens. It is therefore strongly recommended that milk should be heated before consumption. With the exception of an altered organoleptic (flavor) profile, heating (particularly ultra-high temperature and similar treatments) will not substantially change the nutritional value of raw milk or other benefits associated with raw milk consumption" (Claeys et al., 2013).
CONCLUSION AND RECOMMENDATIONS
The diversity of organisms isolated from milk of cows in this study might have been influenced by the type of management system employed or, the milk samples may have been contaminated by infected persons during milking process or the environment. Good hygiene in milking process, milking clinically infected cows last, culling chronic mastitis carriers, treating clinically infected cows and dry period therapy could reduce the prevalence of coliform mastitis in Kaduna State, Nigeria.
Veterinarians, livestock extension workers and farmers should implement in-vitro susceptibility testing prior to the use of antibiotics for treatment of intra-mammary infections in cows. Also, efforts must be made to encourage dairy farmers to apply safe substitutes such as probiotics and bio active natural compounds for prophylactic and therapeutic use.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdurahman OAS (2006). Udder health and milk quality among camels in the Errer valley of eastern Ethiopia, Livestock Res. Rural Dev.18:1-9.
|
|
|
|
Abera M, Abdi O, Abunna F, Megersa B (2010). Udder health problems and major bacterial causes of camel mastitis in Jijiga, Eastern Ethiopia: implication for impacting food security. Trop. Anim. Health Prod. 42:341-347.
Crossref
|
|
|
|
|
Burns C, Wolfgang D, Jayarao B (2000). A survey of milking procedures and management practices on dairy herds in Pennsylvania. National Mastitis Council 9th Annual Meeting, Atlanta, USA. pp.152-153.
|
|
|
|
|
Bynum J (2011). Coliforms: Dangerous Biological Bioterrorism Agents. The Watchers pp. 1-81.
|
|
|
|
|
Claeys WL, Sabine C, Georges D, Jan D, Koen D, Katelijne D, Lieven D, André H, Hein I, Pierre T, Yvan V, Lieve H (2013). "Raw or heated cow milk consumption: Review of risks and benefits". Food Control. 31(1):251-262.
Crossref
|
|
|
|
|
Clinical and Laboratory Standards Institute (CLSI) (2013). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals.4th edition. Wayne, Pennsylvania, USA. P 33.
|
|
|
|
|
David RC (2011). Staining and Interpretation of Smears. Laboratory Studies in Applied Microbiology. Rice University, USA. pp. 74-78.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2008). Milk hygiene in milking, milk production hygiene and udder health. FAO Animal Production and Health Papers -78.FAO Corporate Document Repository (CDR). pp. 1-7.
|
|
|
|
|
Garedew L, Berhanu A, Mengesha D, Tsegay G (2012). Identification of gram-negative bacteria from critical control points of raw and pasteurized cow milk consumed at Gondar town and its suburbs, Ethiopia. BMC Public Health pp.12-950.
Crossref
|
|
|
|
|
Giannino ML, Marzotto M, Dellaglio F, Feligini M (2009). Study of microbial diversity in raw milk and fresh curd used for Fontina cheese production by culture-independent methods. Int. J. Food Microbiol.130:188-195.
Crossref
|
|
|
|
|
Hawari AD, Hassawi DS (2008). Mastitis in one-humped she camels (Camelus dromedarius) in Jordan. J. Biol. Sci. 8:958-961.
Crossref
|
|
|
|
|
Jones GM, Bailey TL (2010). "Understanding the Basics of Mastitis". Virginia Coop. Ext. Retrieved 4 February 2010.
|
|
|
|
|
Junaidu AU, Salihu MD, Tambuwala FM, Magaji AA, Jaafaru S (2011). Prevalence of mastitis in lactating cows in some selected commercial dairy farms in Sokoto Metropolis. Pelagia Adv. Appl. Sci. Res. 2(2):290-294.
|
|
|
|
|
Kagkli DMM, Vancanneyt P, Vandamme CH, Cogan TM (2006). Contamination of milk by enterococci and coliforms from bovine faeces. J. Appl. Microbiol. 13:64-70.
|
|
|
|
|
Kalla DJU, Butswat ISR, Mbap ST, Abdussamad AM, Ahmed MS, Okonkwo I (2008). Microbiological examination of Camel (Camelus dromedarius) milk and sensitivity of milk micro-flora to commonly-Available antibiotics in Kano, Nigeria. Savan. J. Agric. 3:1-8.
|
|
|
|
|
Karimuribo ED, Kusiluka LJ, Mdegela RH, Kapaja AM, Sindato C, Kambarage DM (2005). Tanzania J. Vet. Sci. 6(3):213-221.
|
|
|
|
|
Kivaria FM, Noordhuizen JPTM (2007). A retrospective study of the aetiology and temporal distribution of bovine clinical mastitis in smallholder herds in the Dares Salaam region of Tanzania. Vet. J. 173:617-622.
Crossref
|
|
|
|
|
Kwaga JKP (2012). Veterinary intervention on the global challenge of antimicrobial resistance. Paper presented at the World Veterinary Day Celebration, 28th April, 2012. NVMA, Kaduna.
|
|
|
|
|
Matofari JW, Mario Y, Mwatha EW, Okemo PO (2003). Microorganisms associated with sub-clinical Mastitis in the Kenyan Camel (Camelus dromedarius). J. Trop. Microbiol. Biotechnol. 2(1):11-16.
|
|
|
|
|
Mulei M (1999). Teat lesions and their relationship to intramammary infections on small-scale dairy farms in Kiambu district in Kenya: research communication. J. South African Vet. Assoc. 70(4):156-157.
Crossref
|
|
|
|
|
National Mastitis Council (NMC) (2000). Recommended Mastitis Control Program. National mastitis Council Madison, Wisconsin, USA. pp. 45-60.
|
|
|
|
|
Ocholi RA, Kwaga JKP, Ajogi I, Bale JOO (2004). Phenotypic characterization of Brucella strains isolated from livestock in Nigeria. Vet. Microbiol. 103:47-53.
Crossref
|
|
|
|
|
Podder MP, Rogers L, Daley PK, Keefe GP, Whitney HG, Tahlan K (2014). Klebsiella species Associated with Bovine Mastitis in Newfoundland. PLoS ONE 9(9):106518.
Crossref
|
|
|
|
|
Radostitis OM, Blood DC, Gay CC (2005). In: Veterinary Medicine, A text book of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. ELBS. pp. 241-248.
|
|
|
|
|
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007). Diseases caused by fungi. Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th edition. Saunders Elsevier Ltd. Philadelphia, USA. pp. 842-860.
|
|
|
|
|
Salman AMA, Hamad IM (2011). Enumeration and identification of coliform bacteria from raw milk in Khartoum State, Sudan. J. Cell Anim. Biol. 5(7):121-128.
|
|
|
|
|
Sena DS, Mal G, Kumar R, Sahani MS (2001). A preliminary study of prevalence of mastitis in camel. J. Appl. Anim. Res. 20:27-31.
Crossref
|
|
|
|
|
Sharma M, Dogra BB, Misra R, Gandham N, Sardar M, Jadhav S (2014). Multidrug resistant Pantoea agglomerans in a patient with septic arthritis-a rare report from India. Int. J. Microbiol. Res. 4:263-265.
Crossref
|
|
|
|
|
Smith KL, Hogan JS (2001). The world of mastitis. Proceedings of 2nd International symposium on mastitis and milk quality, September 13-15, Vancouver, British Columbia, Canada. pp. 1-12.
|
|
|
|
|
Woubit S, Bayleyegn M, Bonnet P, Jean-Baptiste S (2001). Camel (Camelus dromedarius) mastitis in Borena lowland pastoral area, southwestern Ethiopia, Revue d'Elevage et de Medecine Veterinaire des Pays Tropicaux. 54:207-212
|
|