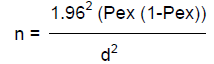ABSTRACT
A cross-sectional study was conducted in selected sites of Southern Nation, Nationalities and People Regional State (SNNPRS) of Konta, Ethiopia. The purposes of study were to determine the apparent density of tsetse flies and prevalence of bovine trypanosomosis. Ngu traps for entomological survey were deployed at grazing and watering points of animals in the village of Dolba (Kerara Peasants Association, PAS) and near Gojeb River. Assessment of tsetse indicated the presence of Glossina pallidipes with the apparent density 8.45% flies/trap/day. Other biting flies (tabanids) were also caught along with tsetse that transmits trypanosomes mechanically. For parasitological study, a total of 400 blood samples were collected from randomly selected animals and examined for the presence of trypanosomes and indicate overall prevalence of trypanosomosis in study cattle as 12%. The dominant trypanosome species were found Trypanosoma conglonse 29 (60.4%) followed by Trypanosoma vivax 14 (29.2%), Trypanosoma bruci 3 (6.25%) and mixed infection (T. congolonse and T. vivax) of 2 (4.2%). Based on these results it is concluded that trypanosomosis is a major constraint of livestock production in the study area.
Key words: Konta, Southern Nation, Nationalities and People Regional State (SNNPRS), Tsetse, Trypanosomosis, trypanosome, prevalence.
Trypanosomosis is serious constraints to livestock production and agricultural development in Ethiopia. A total of 14.8 million cattle, 6.12 million sheep and goats, 1 million camels and 1.23 million equine are at risk of contracting trypanosomes (MoA, 1995). Trypanosomosis is a wide spread and economically important disease in human and animals (Sumbria and Singla, 2016). It is caused by protozoan parasites belonging to the family trypanosomatidae genus Trypanosoma, which inhibits the blood plasma, various bodies’ fluids and tissue of the host (Singh and Singla, 2012). The species of Trypanosoma known to exist in Ethiopia, which are pathogenic to cattle and small ruminants, are: Trypanosoma vivax, Trypanosoma congolense and Trypanosoma bruci. They are distributed mainly in tsetse belt of the country (south west and southern parts) between 33Ëšand 38ËšE and 5Ëš to 12ËšN. T. vivax, also found in area outside of the belt,where it can possibly transmitted by mechanical vectors of biting flies like tabanus and stomoxy (Eneyew, 1997).
The most important trypanosome species affecting cattle are: T. congolense, T. vivax and T. brucei, which are generally termed as Nagana (Jordan, 1986; Langridge, 1976) and T. eqiperdum for equines (Dagnachew and Shafo, 1981). In 1962, the cattle survey in southern Ethiopia, by the livestock division, established that bovine trypanosomosis was a major cattle disease in the Omo valley. It was stated that the problem of trypanosomosis is the main cause of decline in the number of cattle and particularly draught oxen (Abebe and Jobere, 1996).
Trypanosomosis depends on the distribution of the vectors, the virulence of parasite and the response of the host (Langridge, 1976). Tsetse flies are the major and true cyclical vectors. They are blood feeding insects that belongs to phylum arthropoda, class insecta family muscidae sub family glossinidae and genus Glossina. Today five species of glossina have been identified in Ethiopia: Glossina submorsitans and Glossina palldipus (savanna groups), Glossina fuscipes and Glossina tacnoidus (Riverine groups) and Glossina longipennis (Langridge, 1976). In the area of tsetse transmitted trypanosomosis which affects cattle production, trypanocidal drugs, both prophylactic and curative drugs and other tsetse control methods such as insecticide application on the back of animals are the most widely used methods of trypanosomosis control.
Trypanosomosis is the most serious disease of cattle, which cause great socio-economic losses in SNNPR. The socio-economic impact of trypanosomosis is reflected on direct losses such as mortality, morbidity, reduction in milk and meat production, and still birth (Leak et al., 1993). Thus, trypanosomosis is one of the causes for food in security in the Omo-Ghibe belt as well as the tributaries of Gojeb Rivers, where wide grazing land which favors animal production is located. Therefore, the objectives of the current study were: To assess the apparent density and distribution of tsetse and other biting flies and prevalence of bovine trypanosomosis Konta special districts.
Study area
The present study was conducted in 5 selected peasant association (PAs) namely Biteti, Chida, Kecharoba, Kerara, and Mareka in Konta special woreda of SNNPRS which is located 498 km south of Addis Ababa. The area has humid, sub-humid climate with a moderately hot temperature and reliable annual average rain fall of 1200 mm. The annual temperature and altitude ranges from 12 to 24°C, 1062 (Gojeb river) to 1542 (Kerara PAs) m.a.s.l. respectively. Livestock species are cattle, sheep, goats, mule and donkey. The predominant species in the area is cattle which are estimated at 20,985 in the study area 5 PAs. Livestock management system is mixed farming system. The animals in the area mainly depend on communal grazing fields and watering points are at Gojeb River and its tributaries (Konta Woreda Agricultural Office).
Study population and design
Cross-sectional survey was conducted from January 2009 to March 2010 on indigenous cattle. They were kept under traditional extensive husbandry system with communal herding and watering in small and big rivers.
Sampling method and sample size determination
The sampling method applied in the present study was a simple random sampling and the sample size was approximated by using formula given in Thrusfield (1995) using 95% confidence interval and expected prevalence of 50%.
Where, n = sample size; Pexp = expected prevalence; d2 = desired absolute precision.
Accordingly, the estimated sample size was 384 animals; however, to increase the precision 16 cattle were added and a total of 400 cattle were sampled.
Entomological survey
Tsetse population and other flies were sampled using NGU traps deployed for 24 h at two sites and baited with acetone and 3 weeks old cow urine (Brightwell et al., 1992). All odors were placed on the ground about 15 cm up wind of the traps. The traps polls were greased to avoid the entry of insect predators like ants. Fly challenge has been taken as the product of the relative density of tsetse flies, their trypanosome infection rate and the proportion of feed that they have taken from domestic livestock. Site selection was done to include suitable tsetse habitats like savannah area, river valley, livestock grazing area and watering points and vicinity to assumed wild game reserve areas.
Parasitological survey
Blood samples were collected randomly from ear vein by using sterile blood lancet and capillary tubes. A capillary tube were filled with blood from animals to ¾ of their height and sealed at one end with crystal seal. The capillary tubes were loaded on the microhaematocrit centrifuge symmetrically and centrifuge at 1200 rpm for 5 min (Murray et al., 1977). Packed cell volume (PCV) was determined using haematocrit reader (Woo, 1969). After the PCV was read, capillary tubes were broken 1 mm below the buffy coat to include the RBC layer and the content were expressed on microscopic slide and mixed and cover with a 22 × 22 mm cover slip and examined under 40x objectives (Murray et al., 1977). From positive samples thin blood smears were made, fixed with methanol for 5 min and stained with geimsa solution for 30 min and examined using oil emersion under 100 x objectives to identify the species of trypanosomes.
Data management and analysis
Data collected from vector fly and trypanosomosis infection survey were entered into Microsoft excel spread sheet and analyzed using statically software program SPSS. Descriptive statics like percentage can be used to determine prevalence of trypanosomosis and chi- square (χ2) used to evaluate the associations with different risk factors. In the analysis, confidence level was held at 95% and p<0.05 was set for significance.
Hematological findings
Considering anemia as one of the major signs of a herd infecte
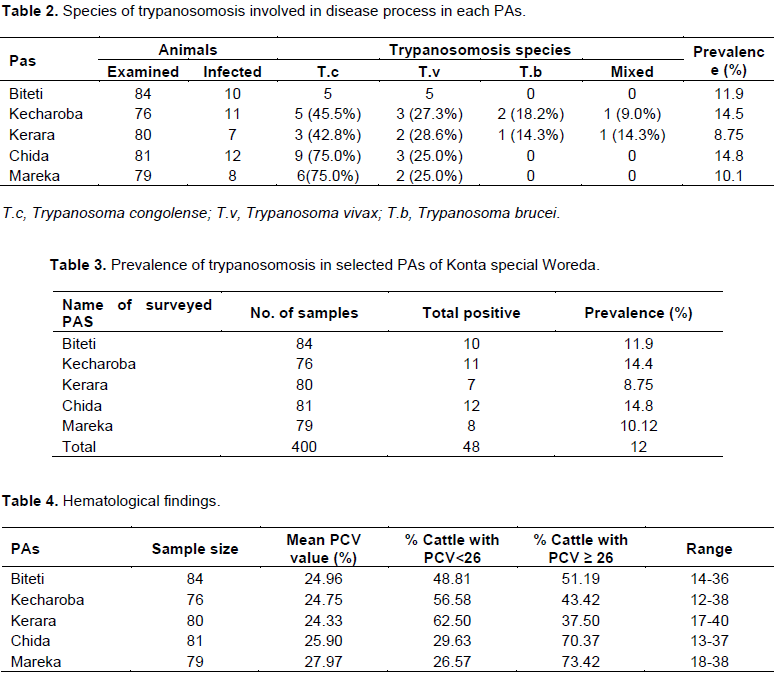
d with trypanosomosis, the anemic status of sampled animals was assessed by measuring the mean packed cell volume. The range of PCV value in parasitaemic was 12 to 30% and aparasitaemic 18 to 40%. Out of total 48 trypanosome positive cattle, 87.5% had PCV value was less than 26 and 12.5% of positive were found having normal PCV (Table 4).
Entomological survey
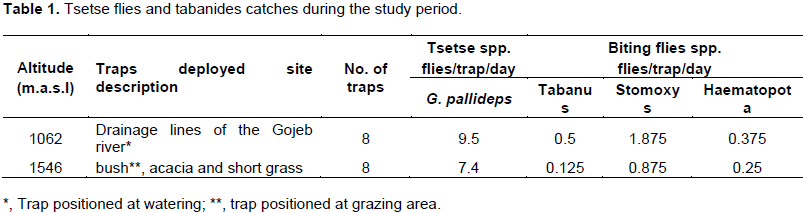
Tsetse flies found during the survey were only G. pallidipes. This species were registered at suspected fly habitat of kerara PAs and around Gojeb River which is watering points for farmers near and away from the river. Biting flies like tabanides also caught along with tsetse flies. The mean catch of G. pallidipes at kerara PAs was 7.4 flies/trap/day whereas around Gojeb River was 9.5 flies/trap/day and an overall apparent density was 8.45 flies /trap/day. A total of 167 flies were caught out of which G. pallidipes accounted 80.8% and tabanide accounted 13.2%. G. pallidipes were abundant at high gallery forest and valley flanks along the drainage lines of the river which is 59.3% whereas 43.7% flies were caught at Kerara PAs which tsetse are more suspected habitat were the vegetation dominated by thorny bush, short grass and dispersed acacia species (Table 1).
Parasitological survey
Overall trypanosome prevalence cattle in the 5 PAs were 12%. The dominant trypanosome species was T. congolense (60.8%) followed by T. bruci 3 (6.25%), T. vivax (29.17%) and mixed infection (T. bruci and T. vivax) 4.2% (Table 2). Almost similar prevalence of trypanosomosis was registered at the different study sites Table 3.
The disease trypanosomosis is the main important livestock constraint impeding the agricultural activity and livestock productivity. It was stated that trypanosomosis is the main cause of decline in the number of cattle and particularly draught oxen (Abebe and Jobre, 1996). The overall trypanosome prevalence in study area was 12% (Table 3). This result was comparable with works different scholars: Twelde (2001) who reports (15%) at Keto settlement area south western Ethiopia; Habtewold (1993 and 1995) at Humbo larena of Wolaita zone (9.3%) and at Konso wereda (11.5%). But, slightly lower than the finding of Afework (1998) at Pawe, North-west Ethiopia (17.2%), Abebe and Jobere (1996) for tsetse infested area of Ethiopia (17.67%). The study result also revealed that majority of infection (60.4%) were due to T. congolense a comparable results was reported by different researches who reported T. congolense in different parts of Ethiopia: Muturi (1999) at Merab Abaya, south west Ethiopia (66.1%), Afework et al., (2001) at Pawe, North west Ethiopia (60.9%) and Abebe and Jobre (1996) for tsetse infested area of Ethiopia (58.5%).
The results of present study showed an overall apparent density of G. pllidipes 8.45 flies/trap/day (Table 1) which was greater density than 1.9 and 1.0 in the late and dry season, respectively, reported by Masangi (1999) in the south rift valley of Ethiopia. This could suggest an absolute increase in the number of tsetse flies due to favorable environment has enough moisture, vegetation growth and suitable habitat or spread of flies from the rivers where they usually inhibit during day season to more open areas there by increasing relative density.
The present findings indicated that the tsetse fly distribution along the two altitudinal level (1062 m.a.s.l) was different from another (1546 m.a.s.l) irrespective of the areas indicating that the catch was decreasing with the increasing altitude (Table 1). This was in agreement with the previous work done in Ethiopia by Verysen et al., (1999) who found a significant high catch (over 93%) in altitude between 1100 and 1400 m.a.s.l level in the southern rift valley of Ethiopia.
Present finding reported 1.5% of the parasitaemic and 53.75% of the aparasitaemic animals PCV values was greater than or equal to 26% this result was in agree well with the result obtained by Rowland’s et al. (2001) at Ghibe valley in the south western Ethiopia, in which he indicated that as the proportion of samples detected parasitaemic increased, PCV value decreased.
CONCLUSION AND RECOMMENDATIONS
The results of the present study revealed that trypanosomosis is the most important problem for agricultural activity and animal production at study area of Konta special wereda in SNNPRS. One species tsetse flies G. pallidipes and biting flies such as tabanids were caught in the study area. Based on the conclusions made above the following recommendations are forwarded:
1. Designing and implementing of control strategies of trypanosomosis focusing on applying integrated approach (vector control and chemotherapy).
2. Further studies on the epidemiological aspects and development of drug resistance in pathogenic trypanosome are required.
3. Awareness creation about the disease and its transmission.
The authors have not declared any conflict of interests.
REFERENCES
|
Abebe G, Jobere Y (1996). Trypanomosis: A threat to cattle production in Ethiopia Revue de Médecine Vétérinaire 147(12):897-902.
|
|
|
|
Afework Y (1998). Field investigation on the appearance Drug resistant population of Trypanosomes in Metekel District, North-west Ethiopia. MSc thesis, Addis Ababa University and Freie University Berlin.
|
|
|
|
|
Afework Y, Clausen PH, Abebe G, Tilahun G, Dieter M (2001). Appearance of multiple drug resistant trypanosome population in village cattle of Metkel District. North West Ethiopia. Livestock community and environment. Proceedings of the 10th Copenhagen Denmark. pp. 1-2.
|
|
|
|
|
Brightwell R, Dransfield RD, Korku CA, Golder TK, Tarimo SA, Mugani D (1992). A new trap for Glossina Pallidipes. Trop. Pest Manag. 33:151-159.
Crossref
|
|
|
|
|
Dagnchew Z, shafo K (1981). An investigation of dourine in Arsi administrative region. Ethiope. Vet. Bull. 4:3-9.
|
|
|
|
|
Eneyew M (1997). Bovine Trypanosomiasis in south Gonder administrative Zone bordering Lake Tana, Ethiopia in the apparent absence of glossina J. Ethiop. Vet. Assoc. 1(1):19-34.
|
|
|
|
|
Habtewold T (1993). Bovine Trypanosomiasis in wolita: prevalence and assessment of drug efficacy. AAU, Faculty of Veterinary Medicine, Debre Zeit, DVM Thesis.
|
|
|
|
|
Habtewold T (1995). Community based tsetse and trypanosomosis control pilot programme using deltamethrin in Konso, Southern, Ethiopia. Proceeding of 11th Conference of the Ethiopia Veterinary Association, Addis Abeba, and Ethiopia. pp. 57-65.
|
|
|
|
|
Jordan AM (1986). The role of tsetse in Africa animal trypanosomomsis. In Livestock production in tsetse affected area of Africa. Proceedings of a meeting held in Nairobi, (ILCA/ILRAD). pp. 37-41.
|
|
|
|
|
Langridge W (1976). A tsetse and trypanosomosis survey of Ethiopia. Ministry of agricultural of Ethiopia. Addis Abeba, Ethiopia. pp. 96-98.
|
|
|
|
|
Leak SGA, Mulatu W, Authie E, d'Iletern GDM, Peregrine AS, Rowlands GJ, Trail JCM (1993). Epidemiology of bovine trypanosomosis in the Ghibe valley, South west Ethiopia. Acta Trop. 53:124-133.
Crossref
|
|
|
|
|
Ministry of Agriculture (MoA) (1995). Ethiopia Ruminant Livestock Development Strategy Addis Abeba, Ethiopia. pp. 112-113.
|
|
|
|
|
Msangi S (1999). Distribution, Density and Infection rates of tsetse flies in selected sites of Southern Rift valley of Ethiopia. MSc thesis, Addis Ababa University Faculty of Veterinary Medicine, Ethiopia.
|
|
|
|
|
Murray M, Murray PK, McIntyre WI (1977). An improved parasitological technique for the diagnosis of Africa trypanosomosis. Trans. R. Soc. Trop. Med. Hyg. 71:325-326.
Crossref
|
|
|
|
|
Muturi KS (1999). Epidemiology of bovine trypanosomosis in selected sites of the southern rift valley of Ethiopia. MSc Thesis, Addis Ababa University.
|
|
|
|
|
Rowlands GH, Leak SGA, Peregrine AS, Nagda SM, Mulatu W, d'Iteren GDM (2001). The incidence of new and the prevalence and persistence of recurrent trypanosome infection. Acta Trop. 79(2):149-163.
Crossref
|
|
|
|
|
Singh V, Singla LD (2012). Trypanosomosis in cattle and buffaloes from latent carrier status to clinical form of disease: Indian scenario. In: Integrated Research Approaches in Veterinary Parasitology, Shanker D, Tiwari J, Jaiswal AK and Sudan V (Eds), Bytes & Bytes Printers, Bareily. pp. 10-18.
|
|
|
|
|
Sumbria D, Singla LD (2016). Human atypical trypanosomosis in Indian subcontinent. Veterinaria 4(1):7-10.
|
|
|
|
|
Tewlde N (2001). Study on the occurrence of the drug resistant Trypanosomes in cattle in the farming tsetse control area (FITCA) project in western Ethiopia MSc thesis, Addis Ababa University and Freie Universitat, Berlin.
|
|
|
|
|
Thrusfield MV (1995). Veterinary Epidemology. 2nd edition. Blackwell Science, Oxford. P 83.
|
|
|
|
|
Vreysen MJ, Mebrate A, Menjeta M, Bancha B, Woldeyes G, Musie K, Bekele K, Aboset G (1999). The Distribution Relative abundance of tsetse flies in the southern rift valley of Ethiopia: International Scientific council for Trypanosomosis Research and control (ISCTRC). 120:202-213.
|
|
|
|
|
Woo PJK (1969). The hematological centrifugation technique for the detection of trypanosomes. Can. Vet. J. 200(47):921-923.
|
|