ABSTRACT
Pathogenicity studies were conducted using two bacteria (Proteus mirabilis and Pseudomonas aeruginosa) that were previously recovered from oral and cloacal swabs of red-headed rock agamas (Agama agama) living in poultry farms at Oyo State, Nigeria. Both bacteria were inoculated in two experiments having 60 broilers each. In each experiment, three groups of 15 broilers were given separately graded doses of P. mirabilis or P. aeruginosa. A group of 15 non-inoculated broilers were used in each experiment as negative controls. Clinico-haematological findings, and bacterial examination of cloacal swabs of the broilers were evaluated weekly for 5 weeks post-inoculation (PI). At necropsy, some tissues were harvested for bacteriological and histopathological examinations. Apart from the significant increase (p<0.05) recorded in the total white blood cell and heterophilic counts at week 3 and 4 PI in birds with high and medium doses of Proteus, all haematological values remain within reference intervals in Psedomonas groups. P. mirabilis and P. aeruginosa were isolated from cloacal swabs in all inoculated birds at the 3-4 weeks PI. The Proteus groups (25.0%; 15/60) showed moderate lung and liver congestion, and a few greyish white nodules on the myocardium while extensive myocardiac fiber degeneration, necrosis and loss of striation with numerous inflammatory cells were also observed. This is the first documented report of P. mirabilis induced nodular myocarditis in chickens. These findings also suggested that P. mirabilis could be pathogenic in adult broilers with possible oral transmission of this microorganism from lizards to poultry, thus, could represent a threat to human health.
Key words: Broiler chickens, Agama agama, Proteus mirabilis, Pseudomonas aeruginosa, Oyo State.
Proteus mirabilis and Pseudomonas aeruginosa are the bacterial species often isolated from commercial chickens necropsied at the Department of Veterinary Pathology, University of Ibadan, Nigeria (Happi et al., unpublished observation). However, there is no documentation of the pathogenicity of these bacterial species in adult chickens. A previous study on bacterial isolation from oral and cloacal swabs of red-headed rock agamas (RHRA) (Agama agama) co-habiting with poultry in local poultry farms in Oyo State (Nigeria) revealed numerous species bacteria among which P. mirabilis and P. aeruginosa were the most common isolated (Ajayi et al., 2015). Lizards and rats are commonly found in and around poultry pens in Oyo State (Funmilayo, 1982; Ogunleye et al., 2013). They both commonly share poultry feed and water and while doing so may defecate in them in poultry houses. Studies on reptiles in connection with emerging infectious diseases and zoonosis have been previously reported (Chiodini and Sundberg, 1981; HPSC, 2013; Johnson-Delaney, 1996).
Previous reports of frequent isolation of
Salmonella spp
. from free-ranging and captive reptiles have also been documented (
Oboegbulem and Isegbohimhen, 1985; Kourany et al., 1990;
Van der Walt et al., 1997; Mitchell and Shane, 2000; Geue and Loschner, 2002). Ogunleye et al
. (2013) characterized
Salmonella enterica serotype
Pullorum isolated from the intestine of lizard captured in poultry pen in the South-western Nigeria. The pathogen was able to produce varying degree of pathological lesions in experimentally infected pullet chicks. Other bacterial infections such as campylobacteriosis and leptospirosis, and parasitic infections such as trichinellosis have also been associated with reptile keeping (HPSC, 2013). In Nigeria, RHRA are commonly seen in poultry houses having unlimited access to poultry feed, water and utensils (Ogunleye et al., 2013). There were no previous report of RHRA as source of transmission of some poultry diseases through poultry feed and or water contamination.
In our previous study, 20 bacteria species were isolated from oral and cloacal swabs from RHRA captured around poultry farms in Oyo State (Ajayi et al., 2015) among which Klebsiella pneumoniae, P. mirabilis and P. aeruginosa were the most common bacteria identified. These three species of bacteria were also usually isolated from samples of necropsied chickens sent for diagnosis to the Department of Veterinary Pathology, University of Ibadan, Nigeria (Happi et al., unpublished observation). These frequently isolated bacteria are known to be pathogenic in human with proteus ranking as the cause of uncomplicated urinary cystitis, pyelonephritis and prostitis (Gaynes and Edwards, 2005). Pseudomonas is generally considered to be an opportunist that produces respiratory infections, sinusitis, keratitis and keratoconjunctivitis, or septicaemia and its sequelae when introduced into tissues of susceptible or young birds (Saif, 2008).
The clinical signs and lesions vary depending on whether infections are localized or systemic. These may include anorexia, stunting, lameness, in-coordination, ataxia, swelling of head, wattles, and sinuses, swelling of hock joints or foot pads, respiratory distress, diarrhea and conjunctivitis with lesions of subcutaneous edema and fibrin, occasionally with hemorrhages, exudate in affected joints, inflammation of serous membranes, pneumonia, swelling and necrotic foci in liver, spleen, kidney and brain (Saif, 2008). Proteus, another sporadic bacteria occasionally cause embryonic death, yolk sac infections, and mortality in young chickens, turkeys, and ducks as reported in Saif (2008). Septicaemia due to Proteus spp. has occurred in quails and broilers suspected of having immunologic deficiency (Saif, 2008).
However, reports of Proteus spp. occasionally producing arthritis, salpingitis, airsacculitis, septicaemia, and granulomatous inflammation of salt glands in waterfowl have been found (Saif, 2008). Several authors also reported the presence of P. mirabilis in poultry meat and in layers in Asia (Kim et al., 2005; Wong et al., 2013; Dadheech et al., 2015). In Africa and in Nigeria in particular, reports of Pseudomonas or Proteus as the causative agent of poultry disease are almost non-existent or very scanty. Despite reports in Asia and particular India (Dadheech et al., 2015), these microorganisms, however, have no established pathology in adult broiler chickens. In this study, we sought to investigate the clinical and pathological changes induced by P. mirabilis and P. aeruginosa isolated from oral and cloacal swabs of RHRA in broiler chickens.
[A1]Not in reference list. However a reference with two authors is present.
Response 1 : Thank you. It has been corrected
[A2]Check the year as per the reference in the list.
Response 2: The year has been corrected in the text
Experimental birds
One hundred and twenty unsexed Arbor Acres broiler chicks were used for this study. The chicks were initially raised in a deep litter brooder house and later transferred to experimental cages at the age of two weeks. Both brooder house and cages were managed under strict hygienic conditions throughout the period of study. The broilers were fed with antibiotic-free broiler starter feed for the first 4 weeks of age and broiler finisher feed from the 5th week of age, and chlorinated water ad-libitum. The birds were vaccinated against Newcastle disease, coccidia, infectious bursal disease, fowl pox, Salmonella spp. and fowl cholera following the manufacturer recommendations. At the age of 6 weeks, the 120 chickens were bacteriologically screened for P. mirabilis and P. aeruginosa using cloacal swabs.
The birds were also examined for some other notable bacterial diseases in the locality of Oyo State such as fowl typhoid, fowl cholera, infectious coryza according to previously standardized bacteriological methods (Ewing, 1986; Barrow and Feltham, 2004). Prior to bacterial inoculation, 2 ml of blood samples were collected from each bird in lithium-heparinized bottle by venipuncture for haematological evaluation according to previously standardized methods (
Schalm et al., 1979). Subsequently, the 120 broiler growers were divided into 2 experimental groups of 60 chickens each inoculated with graded doses designated as none (control), low, medium and high for
P. mirabilis or
P.
aeruginosa. Each experimental group was subdivided into 4 subgroups of 15 birds each and designated as PR0, PR1, PR2 and PR3 for the
P. mirabilis groups and PS0, PS1, PS2 and PS3 for
P. aeruginosa groups.
Bacteria inoculation
Viable count of P. mirabilis and P. aeruginosa isolated from RHRA was done 24 h after incubation at 37°C in broth culture using the plate count methods (Miles et al., 1938) for the determination of infective dose. Broiler chickens of the P. mirabilis inoculated groups were orally administered with 0.5 ml of 8 h broth containing low (105) for PR1, medium (PR2) (107) and high (PR3) (109) colony-forming unit (CFU)/ml of P. mirabilis. Those in the P. aeruginosa inoculated groups were orally given 0.5 ml of 8 h broth containing low (PS1) (102), medium (105) (PS2) and high (108) (PS3) CFU/ml of P. aeruginosa. Both non-inoculated control groups (PR0 and PS0) of broiler chickens were given 0.5 ml of plain sterile nutrient broth. All broiler chickens were examined daily for clinical signs and mortality for a period of 5 weeks PI. Two milliliters of blood samples were obtained weekly from each bird in each group for haematological analyses.
Bacteriology
Cloacal swabs of both inoculated and non-inoculated birds were collected weekly for
P. mirabilis and
P. aeruginosa isolation using standard methods (Ewing, 1986; Barrow and
Feltham, 2004). Briefly, swab samples were inoculated on blood, Mac Conkey, Deoxycholate Citrate and nutrient agars separately. These were incubated at 37°C for 24 h. Inoculated plates were examined for bacteria growth and all the discrete colonies of the various bacteria growth on the laboratory media were subjected to further morphological and biochemical screening using standard bacteriological methods (Ewing, 1986; Barrow and Feltham, 2004; Garcia and Isenberg, 2007).
Necropsy, gross pathology and histopathological examinations
At the end of the 5
th week PI, all birds were euthanized in batches in chloroform chambers, followed by necropsy examinations. Fresh organs such as lungs, liver, kidney and heart were aseptically collected for bacteriological culture using standard methods (
Ewing, 1986; Barrow and
Feltham , 2004). Tissue samples of liver, kidney, lungs spleen, bursa of Fabricius, (small and large) intestine and brain were also collected, fixed in 10% neutral buffered formalin for 24 h. Later all tissue samples were processed for histopathology according to standardized procedures, and were stained with Haematoxylin and Eosin (H&E) (Lee, 1969) and examined.
Statistical analysis
Statistical Package for Social Sciences (SPSS. 17) was used for statistical analysis. Haematological data of the inoculated and non-inoculated P. mirabilis and P. aeruginosa groups were studied by using analysis of variance (ANOVA) for comparison among various groups. The correlation analysis were also carried out using Pearson Correlation analysis to establish any relationship between the bacterial pathogens inoculated and the lesions scored as well as the association between the gross and/or microscopic lesions and the inoculums dose of the bacterial pathogens isolated. Data were presented as mean ± standard deviation (SD) at p<0.05 considered significant.
Ethical approval
Institutional ethical approval was obtained from the Animal Care and Use Research Ethic Committee (UI ACUREC) with the identification-ACUREC/APP/2015/036, while international guidelines for animal use under experimental conditions were also followed (Naderi et al., 2012).
[A1]Not seen in the reference list
Response 3: The reference has been added in the reference list
[A2]Check spellings. Not cited in reference list.
Response 4: The spelling is correct and the spelling mistake has been corrected in the reference list.
[A3]Not in reference list check the year.
[A4]Check spellings. Not cited in reference list.
Response 6: It was a typo error and has been corrected to (Ewing, 1986)
Clinical and haematological findings
All inoculated and non-inoculated birds showed no observable clinical signs and no mortality during the 5 weeks period of the study. Broiler chickens inoculated with P. aeruginosa showed no significant difference (p>0.05) in the erythrocyte, platelet and leucocyte values among the dosage groups compared to the non-inoculated controls for the 5 weeks of the study period (Tables 1 and 2). There was no significant difference in the erythrocyte and platelet counts among the P. mirabilis inoculated groups and compared to the non-inoculated control group. Although the leucogram findings in all inoculated and non-inoculated control groups remained within the reference interval for broiler chickens, there was a significant increase (p<0.05) in the total white blood cell and heterophilic values among the medium and high dosage groups of P. mirabilis inoculated broiler chickens at the 3rd and 4th week PI (Table 3).
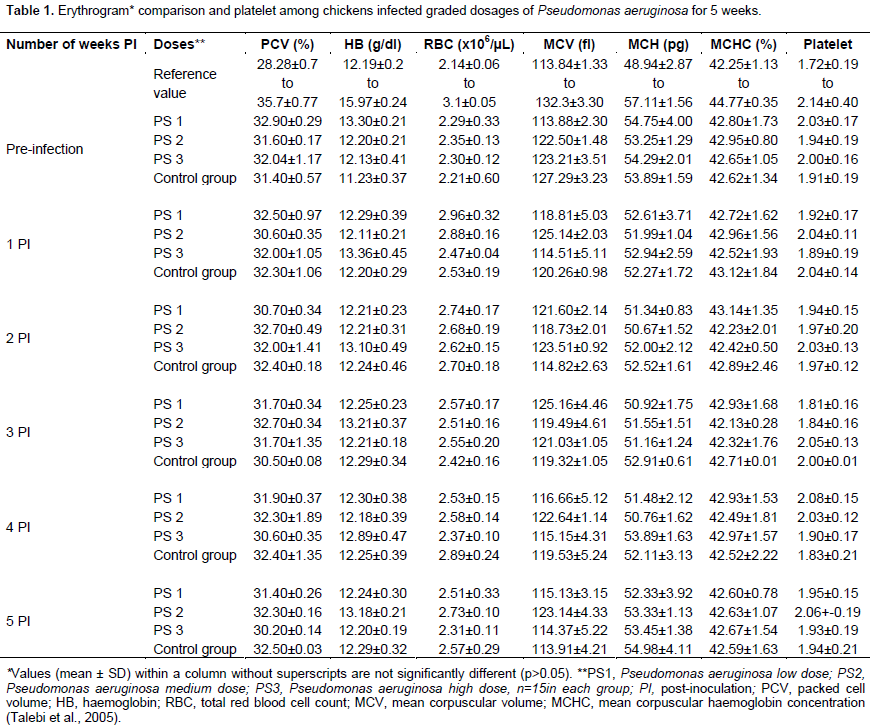
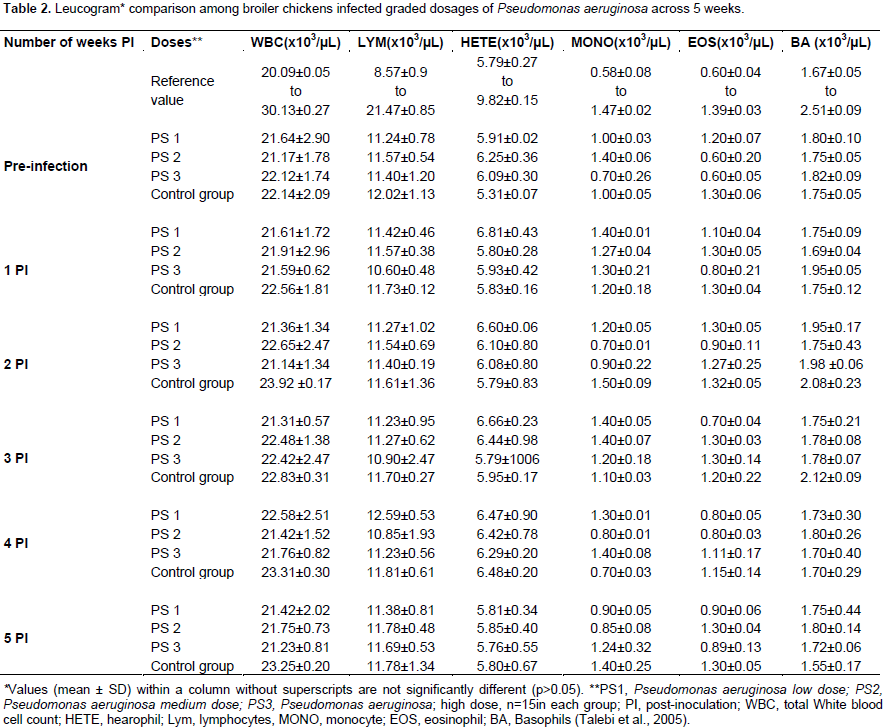
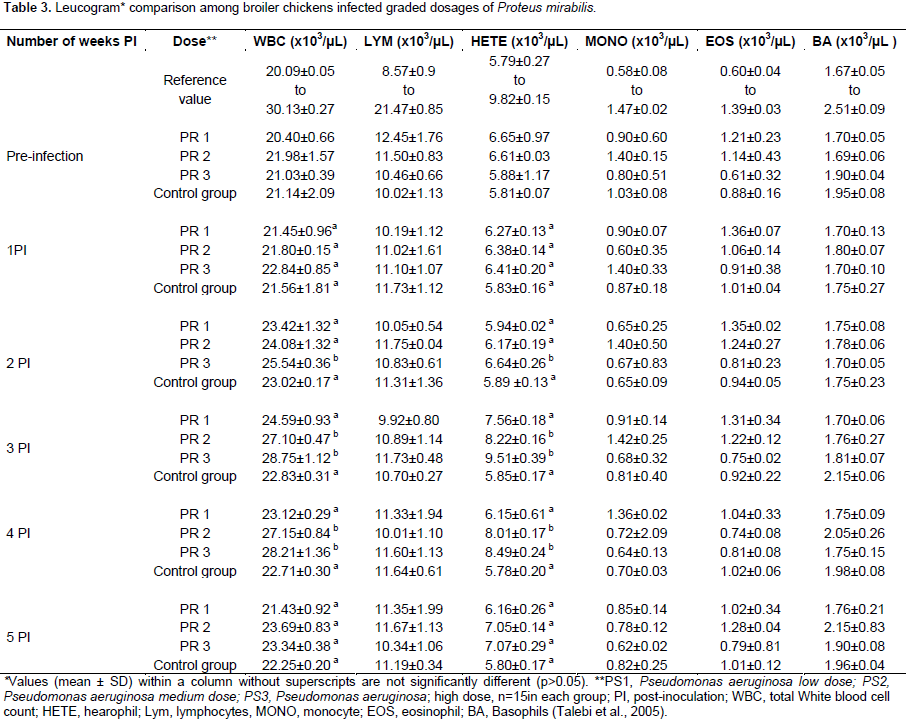
Pattern of bacteria among inoculated groups
Cloacal swab samples of inoculated broiler chickens did not yield P. mirabilis and P. aeruginosa in the 1st and 2nd week PI. The P. aeruginosa inoculated groups started shedding this bacteria species in the 3rd and 4th week PI (13.33% in high dose), after which P. aeruginosa was not isolated in the inoculated groups (Table 4). Cloacal swabs from broiler chickens in all the P.mirabilis inoculated subgroups yielded bacteria from the 3rd week PI, and they continuously shed the named bacterium throughout the remaining period of the study. Forty percent (6/15) of the broiler chickens inoculated with high dose of P. mirabilis showed the microorganism in their cloaca compared to 33.33 (5/15) and 26.67% (4/15) in the medium and low doses groups, respectively. However, the difference between these three sub-groups of the P. mirabilis inoculated chickens was not significant (p>0.05) (Table 4). The broiler chickens of the P. mirabilis and P. aeruginosa non-inoculated control groups did not shed both microorganims throughout the course of the period of study.
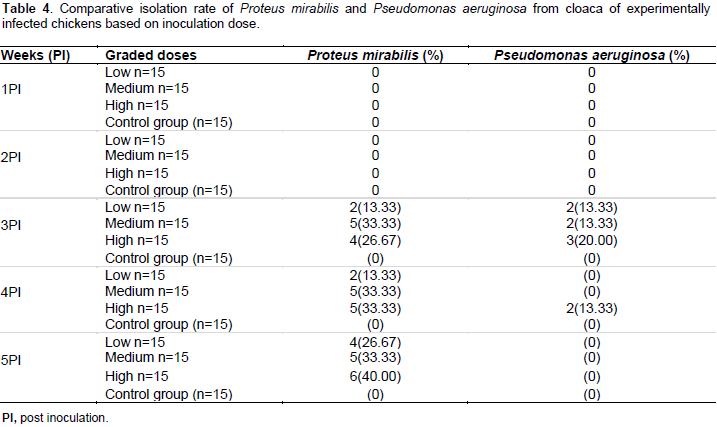
Gross pathological and tissue bacteriological findings
There was no gross lesion in both
P. aeruginosa inoculated and non-inoculated groups of broiler chickens. However, 13.33 (2/15), 33.33 (5/15) and 53.33% (8/15) of the low (PR1), medium (PR2) and high dose (PR3) respectively, showed gross lesions in the
P. mirabilis inoculated broiler chicken groups. Such lesions were identified as moderate and diffuse pulmonary and hepatic congestion together with hepatomegaly. In addition, 13.33 (2/15) and 53.33% (8/15) of the broiler chickens which belonged to the medium and the high dose groups, respectively, showed single to multiple greyish white, soft, discrete, nodules of about 0.4 cm in diameter affecting
the myocardium (Figure 1). No other gross lesions were seen in the
P. mirabilis inoculated broiler chickens. All broiler chickens of the non-inoculated groups showed no evidence of gross lesions. Bacteriological examination of tissues (lungs, liver and heart) did not yield any of the tested bacteria at necropsy.
Histopathology
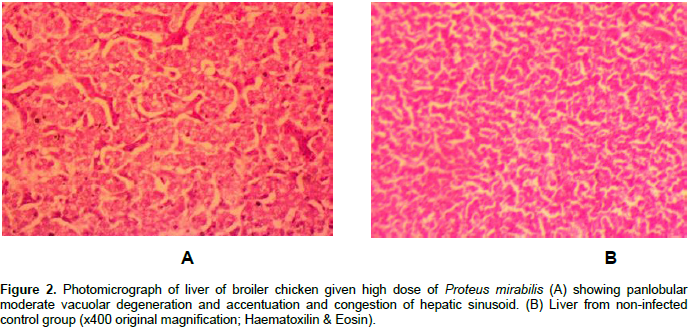
Only P. mirabilis inoculated broiler chickens showed histopathological lesions. Almost all the recorded microscopic lesions were found in the high, medium and low dose P. mirabilis inoculated groups. However, a higher number of the broiler chickens inoculated with the high dose of P. mirabilis showed a severe grade of histopathological lesions. Moreover, 60% of those inoculated broiler chickens showed moderate congestion of portal blood vessels, panlobular vacuolar degeneration of hepatocytes and centrilobular atrophy of hepatic cords with sinusoidal dilatation (Figure 2). Forty percent of all the P. mirabillis inoculated broiler chickens showed marked vascular congestion of the epicardium. The grossly identified nodular lesions on the myocardium of several broiler chickens of the medium and high dose P. mirabilis subgroups had multiple foci of marked necrosis of the myocardiac fibers with inflammatory cell infiltration mainly composed of macrophages, lymphocytes and few heterophils (Figure 3A and B).
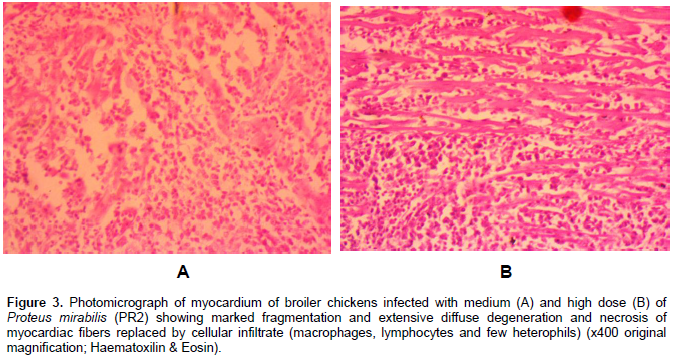
Similar inflammatory cell infiltrations were found in the adipose tissue of the epicardium of the named inoculated broiler chickens. Eighty percent of the high dose P. mirabilis inoculated subgroup showed lungs with marked congestion of vessels, moderately thickened mucosa of mesobronchi and haemorrhages with cellular debris in the atria. Also, 33.33% (5/15) of the high dose P. mirabilis inoculated subgroup showed mild to moderate degree of tubular epithelial cell degeneration, necrosis with moderate heterophilic and mild mononuclear cell infiltration of interstitium in kidneys as well as moderate villi atrophy with moderate inflammatory cell infiltration by macrophages and lymphocytes in the lamina propria and within crypts of the duodenum. However, no visible microsocpic lesions were observed in non-inoculated control group.
This study sought to assess the clinical and pathological changes induced by
P. mirabilis and
P. aeruginosa isolated from oral and cloacal swabs of RHRA in broiler chickens. The absence of clinical signs and mortality recorded in
P. aeruginosa infected broilers between 6 and 11 weeks in this study were in agreement with the results previously reported by Mohamed (2004) who recorded zero clinical sign and mortality in 9 week-old broiler chickens. Todar (2007), Janda and Abboh (2010) also reported that
P. aeruginosa is an opportunistic pathogen that rarely cause disease in older chickens. Walker et al. (2002), however, suggested that the organism can invade fertile eggs causing death of embryos and virulent strains can cause diarrhea, dehydration, dyspnea, septicaemia and death to newly hatched chicks.
Devriese et al. (1975) also described upper respiratory tract infection to
P. aeruginosa with heavy losses in broilers less than 5 weeks of age. Fekadu (2010) and Abadi et al. (2013), equally described
P. aeruginosa as the causes of Yolk sac infection and omphalitis with high fatality only in chicks of less than 11 days old.
Our study concluded that the P. mirabilis isolate orally inoculated in broiler chickens from 6 week-old did not produce any discernable clinical signs which agreed with the previously reported findings described by Jiang et al. (1996). There were no differences of the haematological profiles between the P. aeruginosa inoculated and non-inoculated broiler chickens. However, the P. mirabilis isolate caused a significant increase in the total leucocyte and heterophilic counts in the medium and high dose P. mirabilis inoculated broiler chicken subgroups. The increase in the total leucocyte and heterophilic counts while it is suggestive of an inflammatory response due to bacterial infection also indicates a clinical manifestation of a disease, which was not detectable by physical evaluation of the affected birds. Thus, this finding could suggest that the use of haematological examination is necessary for disease monitoring for early detection of inflammatory conditions of chickens during the health monitory of an experimental study or routine check-up and may offer a valuable help as diagnostic and/or research tool.
The fact that P. mirabilis and P. aeruginosa inoculated groups did not shed pathogen in the first 2 weeks PI may suggest a period of incubation. In addition, only the subgroups inoculated with the higher dose P. aeruginosa shed the bacterial pathogen between the 3rd and the 4th week, after which no pathogen was further shed till the end of the study. This finding suggested that there was probably an infection, which was aborted and resolved thereafter, and healthy birds above 6 weeks of age are probably tolerant to the P. aeruginosa infection. Our results also showed that the P. aeruginosa inoculated broiler chickens were able to ward off the bacterial pathogen within a shorter period. On the contrary, broiler chickens of the P. mirabilis groups continually shed the bacterial pathogen throughout the period of the study.
In addition, the rate at which the bacteria were shed from the cloaca of inoculated subgroups was highly dependent on the dose of the bacterial pathogens inoculated. Thus, given the zoonotic potential of P. mirabillis, it poses a threat to poultry farm workers, consumers during processing and consumption of poultry meat, other chickens and to the environment. Due to the fact that there were no clinical signs or mortality throughout the period of the study and both groups shed the inoculated bacteria, it may suggest that broiler chickens may act as reservoirs of these bacteria, and possibly disseminate them to the environment risking susceptible animals and humans. P. aeruginosa did not produce pathological lesions in infected broiler chickens 6 weeks and above. Dinev et al. (2013) reported pathologies such as pododermatitis, periarthritis and arthritis in 5 weeks old chickens parenterally inoculated with P. aeruginosa.
However, absence of clinicopathological findings, mortality and lesions in P. aeruginosa infected birds can be ascribed to the age (> 6 weeks) or oral route of inoculation and/or health status of experimental chickens. The lack of pathological findings in birds infected with P. aeruginosa in this study could also be due to genetic variations in strains of bacterium, thus resulting in milder infections in birds. Overall, our findings complement some of the already known facts that P. aeruginosa rarely cause disease in adult healthy chickens (Kebede 2010; Mohamed, 2004). An overall 25% (15/60) of the P. mirabilis infected birds showed significant gross and microscopic pathological lesions recorded in some vital organs. The main pathological findings in the P. mirabilis inoculated birds comprised congestion of lungs and liver with hepatomegaly. The congestion of organs corroborates the work of Sah et al. (1983) where organs such as liver and lungs were reportedly congested in Japanese quail chicks infected with P. mirabilis.
In one other study, Jiang et al. (1996) recorded osteomyelitis as main lesion in less than 5 weeks chickens parenterally infected with P. mirabilis in an experiment. A very unique and interesting finding in this study was the gross and histopathological findings of multiple nodular myocarditis in proteus-inoculated chickens. This is the first documentation of this observation in proteus infected broilers chickens Our study shows that the pathological findings were dose-dependent, considering the highest dose of the P. mirabilis causing the above named gross lesions. The nodular lesions found in the heart of infected broilers need to be differentiated from the heart nodules caused by other poultry diseases such as Salmonella enterica ser. Gallinarum-Pullorum septicaemia, chronic respiratory disease, Marek’s disease and lymphoid leucosis.
Bacteriological examination of organs (lung, kidney, liver and heart) of chickens infected P. aeruginosa and P. mirabilis did not yield any of the tested bacteria at necropsy. In a study conducted by Sah et al. (1983), P. mirabilis was however recovered from the heart blood and lung of infected quail chicks, where it was associated with septicaemia. It may suggest that the selected organs for the isolation of both bacteria in this study could have been correct, but other factors might negatively influenced the isolation of these organisms. Furthermore, isolation of proteus from the heart may thus depend and the severity of the condition or the window of time in which there is clinical manifestation of the condition.
Overall, our study corroborated previous results in which reptiles constitute a potential health and zoonotic risk as reservoir of several pathogens. The study also suggests the potential public health hazard that these lizards pose to human population, particularly the attendants and their dependents and their animals. Broiler chickens older than 6 weeks of age may not be susceptible to P. aerugonosa. A major unexpected finding from this study was the presence of nodular myocarditis in broiler chickens infected with P. mirabilis. This is the first documented report of P. mirabilis induced nodular myocarditis in chickens. This infection in broiler chickens may thus result in significant economic loses under field conditions.
The authors have not declared any conflict of interest
REFERENCES
|
Abadi A, Ali MA, Ashenafi S, Shahid N, Haileleul N (2013). Yolk sac infection (omphalitis) in Kombolcha Poultry farm, Ethiopia. Am-Euras J. Sci. Res. 8(1):10-14.
|
|
|
|
Ajayi JO, Ogunleye AO, Happi AN, Okunlade AO (2015).Bacteria isolated from the oral and cloaca swabs of lizards co-habitating with poultry in some poultry farms in Ibadan, Oyo State, Nigeria. Afr. J. Biomed. Res.18:211- 215.
|
|
|
|
|
Barrow GH, Feltham RKA (2004). Cowan and Steel's manual for identification of medical bacteria, 3rd Edn. Cambridge, UK, CambridgeUniversity Press. P 331.
|
|
|
|
|
Chiodini RJ, Sundberg JP (1981). Salmonellosis in reptiles: a review. Am. J Epidemiol. 113:449-499.
Crossref
|
|
|
|
|
Dadheech T, Vyas R, Rastogi V (2015). Prevalence, bacteriology and pathogenesis of Proteus species in sick layer chickens in Ajmer region of Rajasthan. Int. J. Inst. Pharm. Life Sci. 5(2):272-281.
|
|
|
|
|
Devriese NJ, Viaene NJ, Demedts G (1975). Pseudomonas aeruginosa infection on a broiler farm. Avian Pathol.4(3):233-237.
Crossref
|
|
|
|
|
Dinev I, Dinev S, Beev G (2013). Clinical and morphological studies on spontaneous cases of Pseudomonas aeruginosa infection in birds. Pak. Vet. J. 33(3):398-400.
|
|
|
|
|
Ewing WH (1986). Edwards and Ewing's Identification of Enterobacteriaceae, 4th edn. New York, NY. Elsevier Science. pp. 181-340.
|
|
|
|
|
Fekadu K (2010). Pseudomonas infection in chickens. J. Vet. Med. Anim. Health 2(4):55-88.
|
|
|
|
|
Funmilayo O (1982). Commensal rats: A threat to poultry production in Nigeria. Proceedings of the tenth vertebrate pest conference. Available at:
View
|
|
|
|
|
Garcia LS, Isenberg HD (2007). Clinical microbiology procedures handbook. Vol. 1, 3rd edn Update SMA Press. American Society for Microbiology 1752 N. St. NW. Washington, DC 20036-290.
|
|
|
|
|
Gaynes R, Edwards JR (2005). Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41: 848-854.
Crossref
|
|
|
|
|
Geue L, Loschner U (2002).Salmonella enterica in reptiles of German and Austrian origin. Vet. Microbiol.84:79-91.
Crossref
|
|
|
|
|
Health Protection Surveillance Centre [HPSC] (2013). Reptiles and the risk of Infectious Diseases. Available at:
View
|
|
|
|
|
Janda JM, Abboh SL (2010). The genus Aeromonas: Taxonomy, pathogenicity and infection. Clin. Microbiol. Rev. 23(1):35-73.
Crossref
|
|
|
|
|
Jiang Y, Long T, Cheng X, Wang S, Qin C, Song W (1996). Comprehensive report on the research of Proteus mirabilis infection in chicken in China. Chin. J. Vet. Sci. 16(1):67-70
|
|
|
|
|
Johnson-Delaney CA (1996): Reptile zoonoses and threat to public health. In reptile medicine and surgery edn. Mader, D.R. Philadelphia, PA : WB saunders company. pp 20-33.
|
|
|
|
|
Kebede F(2010). Pseudomonas infection in chickens. J. Vet. Med. Anim. Health. 2:55-58.
|
|
|
|
|
Kim SH, Wei CI, An H (2005). Molecular characterization of multidrug-resistant Proteus mirabilis isolates from retail meat products. J. Food Protect. 68:1408-1413.
Crossref
|
|
|
|
|
Kourany M, Myers CW, Schneider CR (1990). Panamanian amphibian and reptiles as carriers of Salmonella. Am. J. Trop. Med. Hyg. 19:632-1253.
Crossref
|
|
|
|
|
Lee GL (1969). Manual of histologic method of armed forces institute of Pathology, 3rd edn. Mc Craw-Hill book Company, New York. pp 28-40.
|
|
|
|
|
Miles AA, Misra SS (1938): The estimation of bactericidal power of the blood. J. Hyg. 38(6):732-749.
Crossref
|
|
|
|
|
Mitchell MA, Shane SM (2000). Preliminary findings of Salmonella spp. in captive green iguanas (Iguanaiguana) and their environment. Prev. Vet. Med.45:297-304.
Crossref
|
|
|
|
|
Mohamed HAAEH (2004). Some studies on Pseudomonas species in chicken embryos and broilers in Assiut Governorate. Ass. Univ. Bull. Environ. Res. 7(1):23-30.
|
|
|
|
|
Mohamed HAAEN (2004). Some studies on Pseudomonas species in chicken embryos in Assiut Governorate. Ass. Univ. Bull. Environ. Res. 7:23-30.
|
|
|
|
|
Naderi MM, Sarvari A, Milanifar A, Boroujeni SB, Akhondi MM (2012). Regulations and ethical considerations in animal experiments: international laws and Islamic perspectives. Avicenna J. Med. Biotechnol. 4(3):114-120.
|
|
|
|
|
Oboegbulem SI, Isegbohimhen AU (1985). Wall Geckos (Geckonidae) as reservoirs of Salmonellae in Nigeria, problem for epidemiology and public health. Int. J. Zoonoses 12(3):228-232.
|
|
|
|
|
Ogunleye AO, Ajuwape ATP, Alaka OO, Adetosoye AI (2013). Characterization of a Salmonella enterica serotype pullorum isolated from a lizard co-habitating with poultry. Afr. J. Microbiol. Res. 7(14):1215-1221.
Crossref
|
|
|
|
|
Sah RL, Mall MP, Mohany GC (1983). Septicaemic Proteus infection in Japanese quail chicks. Avian Dis. 27(1):296-300.
Crossref
|
|
|
|
|
Saif YM (2008). Diseases of Poultry.12 th Edn. Pp 959-961. Other Bacterial Diseases. Blackwell Publishing Professional. 2121 State Avenue, Ames, Iowa 50014, USA.
|
|
|
|
|
Schalm JN, Jain NC, Carol, EY (1979). Veterinary Haematology, 3rd Ed. Lea and Febiger, Philadelphia. pp. 15-81.
|
|
|
|
|
Todar K (2007). Pathogenic Escherichia coli. On-line text book of Bacteriology. University of Wisconcinmadison, Department of Bacteriology. Available at:
View
|
|
|
|
|
Van der Walt ML, Huchzermeyer FW, Steyn HC (1997). Salmonella isolated from crocodiles and other reptiles during the period 1985-1994 in South Africa. Available at:
View
|
|
|
|
|
Walker SE, Sander JE, Cline JL Helton JS (2002). Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. 46(4):1045-1050.
Crossref
|
|
|
|
|
Wong MH, Wan HY, Chen S (2013). Characterization of multidrug-resistant Proteus mirabilis isolated from chicken carcasses. Foodborne Pathog. Dis.10:177-181.
Crossref
|
|