An epidemiological study was conducted to establish the prevalence of the Bovine Leukemia Virus (BLV) in Colombia and to describe risk and protecting factors associated with this infection disease. The study was performed with an observational descriptive cross-sectional process in twelve Colombian regions, by collecting blood samples from 8150 bovines in 390 cattle farms between February and September 2014. The seroprevalence obtained by enzyme-linked immunosorbent assay (ELISA) tests was 42.7% in animals and 67.7% in farms. The highest seroprevalence was found in Villavicencio with 91% in animals. The infection with blood parasites and another virus was attributed to be among the main risk factors associated to BLV. The use of individual needles during veterinary procedures was found to be the main source of protection against the virus. Climate data and ecological groups were recorded at sampling sites in order to elaborate geo-referencing maps by using analyzes of viral distribution around the country. Results obtained showed that there is a probability of an increase on the incidence of this pathology as well as a predictive issue associated with places and climate variables. It was found that developing epidemiological analyzes aiming to report and monitor the presence of this disease and its risk factors is the only alternative to generate prevention and control strategies.
Bovine leukemia is a neoplastic disease of cattle and is classified into enzootic and sporadic bovine leukosis. Enzootic bovine leucosis (EBL) is caused by bovine leukemia virus (BLV), which belongs to the genus delta retrovirus in the Retroviridae family. This disease is asymptomatic in 70% of infected cattle, it produces a persistent lymphocytosis in 30% and leukemia in 5% (
Bartlett et al., 2014;
Frie and Coussens, 2015).
Bovine leukemia virus does not exist as free viral particles in peripheral blood; rather than that, proviral
DNA integrates into the genomic DNA of lymphocytes in blood and milk, leading to lifelong infection despite the presence of antibodies.
Therefore, detection of host anti-BLV antibodies may indicate the presence of the infection source (
Kobayashi et al., 2014).
Animals with a positive diagnosis of the virus are considered carriers of the virus, even though the majority of these animals do not present lymphocytosis or lymphoma as common symptoms. BLV is present in the lymphocytes circulating in peripheral blood of infected cattle; horizontal and vertical transmission of the virus often occurs by direct contact with infected blood and with secretions containing lymphocytes from infected animals. Horizontal routes of transmission include breeding status, hematophagous insects, plastic instruments used for dehorning and rectal examinations, previously used needles, physical contact and age. On the other hand, vertical routes of transmission include ingestion of colostrum and in uterus infection (
Murakami et al., 2011;
Ohno et al., 2015;
Åževik et al., 2015).
Epidemiological studies are used around the world as a main strategy to determine the behavior of the disease and to have a better understanding on animal health. BLV spreading worldwide; indeed, EBL is listed by the World Organization for Animal Health (OIE) as a disease of high importance to international trade (
Ohno et al., 2015). However, BLV has been detected at various frequencies on farms worldwide. For instance, it was estimated that in the United States 89.0% of dairy herds were infected with BLV in 1996 and 83.9% in 2007 (
Kobayashi et al., 2014).
On the other hand, a seroprevalence around 40% was reported in studies conducted in Colombia in 2010 (
Hernández-Herrera et al., 2011), and is possible that BLV presence is rising among the population due to the lack of information, the absence of a specific vaccine against it and the poor preventive strategies adopted in the country to decrease its prevalence.
The rise in BLV presence in cattle prompts a reduction on animal productivity, which generates economic losses; annual economic losses to the US dairy industry associated with BLV are estimated to be $285 million for producers and $240 million for consumers; in the United States (
Bartlett et al., 2014;
Åževik et al., 2015). In Colombia, there are not studies related to the economic impact of this disease.
This epidemiological study used an observational descriptive cross-sectional process and was developed considering the impact of BLV on animal health and productivity.
The main objective of this study was to establish the prevalence of the bovine leukemia virus in Colombian cattle and to determine the risk and protective factors associated with the disease. This study presents the updated prevalence of the virus in Colombian cattle as well as the tools to prevent and control its prevalence.
Type of study and sampling
An epidemiological study using an observational descriptive cross-sectional method (
Nekouei et al., 2015)was performed in seven different regions around Colombia. They were: the municipalities of El Rosal (8 farms), Madrid (5 farms), Puerto Salgar (14 farms) and Subachoque (53 farms) for a total of 80 studied farms in the Department of Cundinamarca. Additionally, 65 farms located in the municipality of Sotaquira, Department of Boyaca, 29 farms at the municipality of San Pedro de Los Milagros in the Department of Antioquia, 151 farms in the municipality of the Guachucal in the Department of Nariño, 29 farms from the city of Villavicencio in the Department of Meta, 1, 8 and 19 farms from the municipalities of La Gloria, Rio de Oro and 8 farms Aguachica in the Department of Cesar and 28 farms from the city of Monteria in the Department of Cordoba were included. A total of 8150 animals in 380 farms from 12 municipalities and 7 departments agreed to participate.
The size of the animal sample was selected based on an estimation of the prevalence of the disease, methodology that is commonly used for large populations (
Nekouei et al., 2015). The total amount of samples was 1000 per region; this number was obtained considering an average of 15000 animals, a 50% of prevalence, a confidence level of 95% and an error estimation of 3%, resulting in a 6.6% sample fraction.
Blood samples from the coccygeal vein of each animal were taken between February and September, 2014. Blood without anticoagulant was centrifuged and the serum was frozen and stored at -4°C until processing. A structured epidemiological survey was performed in order to check risk factors (Martin and Meek 1997; Thrusfield, 2005).
BLV, parasites, bacteria and other virus detection
Samples were analyzed with Serelisa BLV Ab monoblocking kit for the detection of antibodies to the viral surface glycoprotein, gp 51 which is located in the envelope of the virus and it is involved in the fusion and attachment process of the virus to the cell (Synbiotics®, serelisa BLV Abmonoblocking). The kit is endorsed by the World Organization for Animal Health (OIE) which offers prescribed tests for international trade.
The positivity was determined according to the kit instructions, where the limits are negative for PC values lower than 30 and positive for PC values greater than 50. Values between 30 and 50 are considered suspicious (gray zone). For farms, a positive result was considered where at least one seropositive animal was found (
Åževik et al., 2015). For parasites, Mac Master test was done to count the number of eggs per gram of feces. Parasites Dictyocaulus, Muellerius, and Protostrongylus were found using Baermann test and cultures in agar and biochemistry tests were performed for bacterial identification. For viruses, different to BLV as well as for some other bacteria, a serological test was applied.
Application and information survey
The application of surveys was done by interviewing the owners, tenants, and stewards of the farms included in the study. Knowledgeable people in the area were hired as interviewers; assistants and trained technical staff for governmental institutions were previously trained in their areas of influence to avoid bias.
The survey applied was one of a structured type, consisting of 70 questions. The independent variables were used to group the risk factors associated with BLV infection: abortions at different stages of pregnancy, handling and care on the farm, infectious diseases as well as their causative agents and demographic characteristics such as age, parity, and place.
Methodology
The frequency measurement obtained was the prevalence (
OIE, 2008)only due to the lack of monitoring of the study population. This prevalence crossed with determinants that were established by formulating questions during surveys.
The analysis of the information collected in the field consisted of categorizing variables in a question form to be evaluated through listings, frequency and statistical tables. Listing information was stripped and a frequency analysis was developed over the refined database and obtaining absolute results and relative frequencies. The analysis indicated the number of cases followed by the cumulative percentage. The average, sum and standard deviation were presented if fields consisted of numerical values (Dean et al., 1991; Londoño 1996 ).
The ratio of animals and farms affected by BLV were exposed to a factor and compared with results of the same proportion not exposed to the factor. The results obtained were analytically processed in order to determine the association between clusters and to compare with referenced values from other publications. The variables from all categories were analyzed using the chi-square test (Martinez et al., 1997). Prevalence ratio (PR) was used to estimate the risk and the significance of the association between BLV, specific symptoms and a hypothetical causal factor (Dean et al., 1991). The PR was interpreted similarly to the relative risk (RR) which measures the association regardless of the used sampling method. The odds ratio (OR) was later used in the multivariate analysis (
Martin et al., 1993).
A stratified analysis methodology was assessed to obtain free effect association measurements that can lead to confusing variables (Londoño, 1996; Martin et al., 1997). The methodology was also executed to study the interaction between variables, which were acting together on the observed effect (presence of BLV).
The OR was subsequently used in a multivariate analysis (Martin et al., 1997). The associated variables that showed significant numbers were also identified and multifactorial relationships were determined. The BLV in this analysis is explained by multiple factors or independent variables by using the basics of multivariate analysis through logistics regression methodology and also using the free software (Epi-Info 7 ) (Londoño 1996).
The quantitative variables consisted of verifying the homogeneity of variances in groups using the Bartlett test. After confirming the homogeneity of variances, the next step consisted of performing the traditional parametric analysis of variance (Fisher f Test). The Student t test was also used to compare adjacent averages.
The Kruskal-Wallis test was used in the case that there was no homogeneity between variances, and the media of each group were compared. The Mann-Whitney/Wilcoxon test was later used to assess the difference between adjacent media (Dean et al., 1992; Florez et al., 1994; Martinez et al., 1997).
The Ecological Niche models described by Carpenter et al. (1993) were used to predict the distribution of the BLV with the type of climate, and BIOCLIM (Bushby, 1991;
Nix, 1986)or DOMAIN (
Mekata et al., 2015)approaches were applied. The climate data was first taken at study Departments, geo-referencing maps were later elaborated including all 390 farms. Finally, the tools to predict the distribution of BLV were applied.
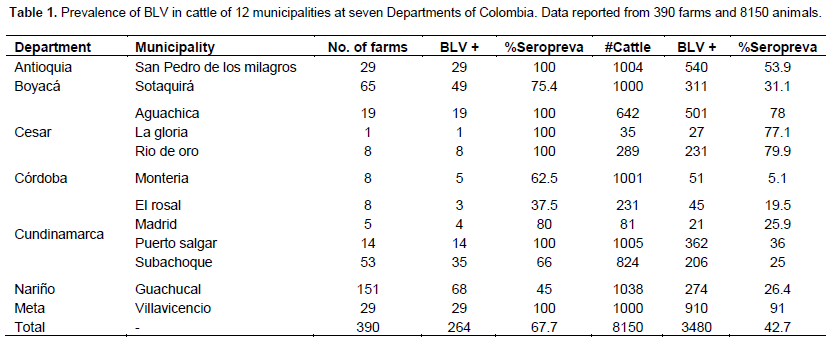
The assessment of the prevalence of BLV was conducted using samples of animals and farms. The seroprevalence in 12 surveyed regions was found to be 42.7% in cattle and 67.6% in farms. Table 1 shows the prevalence found in each Department and Municipalities. Villavicencio was found to be the place with higher seroprevalence in animals with 91.5%. El Rosal in Cundinamarca was found to have the lower seroprevalence with 19.5%. San Pedro de LosMilagros, as well as Aguachica and La Gloria, showed that 100% of their farms had, at least, one infected animal. The municipality of El Rosal was found to be the zone with the lowest percentage of infected farms with a 37%.
The analysis of risk factors was more focused on animals rather than on farms. The hypothesis testing was conducted to determine risk and protective factors related to the presence of BLV. The Chi2 test was applied in order to obtain a PR. A risk factor was considered for PR values higher than 1 with an Low confidence Limit (LCL) greater than 1 and a p lower than 0.05. A protective factor was determined for PR values lower than 1 with a Upper Confidence Limit (UCL) and p lower than 1 and 0.05, respectively. PR value equal to 1 indicated that the factor was not associated.
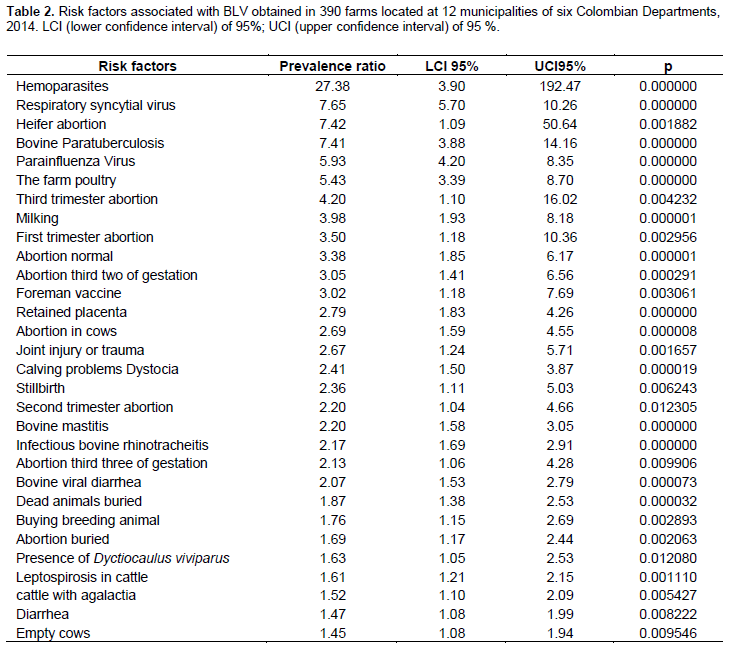
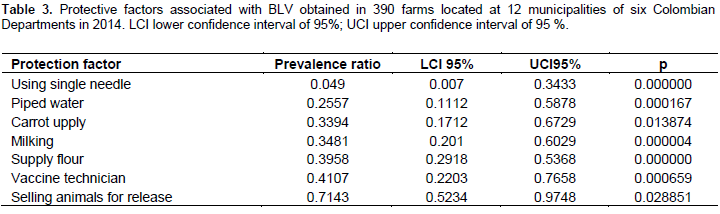
Among all data collected from surveys, seventy factors were included as a potential risk or protective factors. Thirty out of the seventy were found to be risk factors, where the presence of blood parasites was found to be the major factor with a PR of 27.4 (p=0.00000). Table 2 displays the presence of non-lactating cows on the farm that showed the lower risk of acquiring BLV with a PR of 1.45% (p=0.009). On the other hand, seven protective factors were found, where the use of individual needles was the most effective with an RP of 0.049 (p=0.000000). The lowest protective factor was found to be the purchasing of dairy animals with a PR of 0.71 (p=0.028) (Table 3).
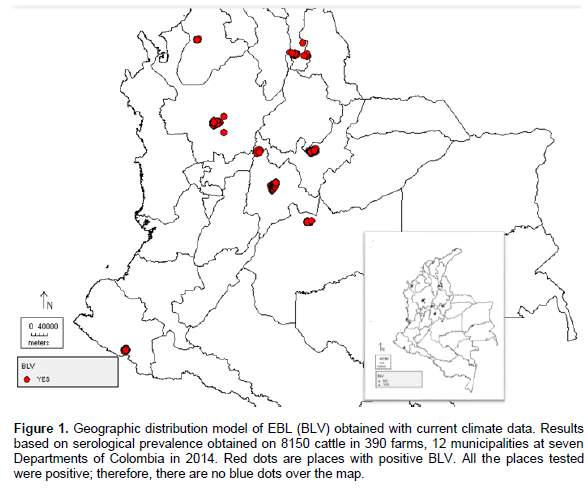
Figure 1 show the geographical distribution of BLV obtained on 8150 animals in 390 farms at seven Departments. The red and yellow dots are showing positive and negative results, respectively.
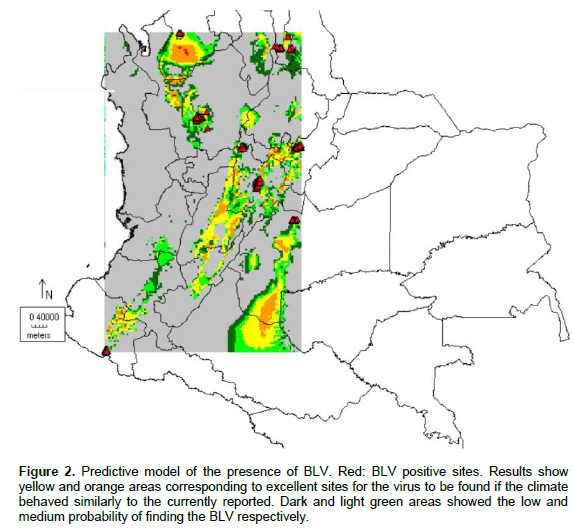
The Ecological Niche model was applied on a geo-referenced map of farms to predict the places that were most likely to acquire BLV. The data was estimated from different climate models and analysis modes provided by the Intergovernmental Panel on Climate Change Data Distribution Center (1999). The original data was interpolated into a grid of ten minutes. Figure 1 shows the positive and negative results in red and blue dots, respectively. Figure 2 indicates the places with the highest risk of developing BLV. Results show yellow and orange areas corresponding to excellent sites for the virus to be found if the climate behaved similarly to the currently reported. The dark green areas indicate sites of low probability of finding the BLV.
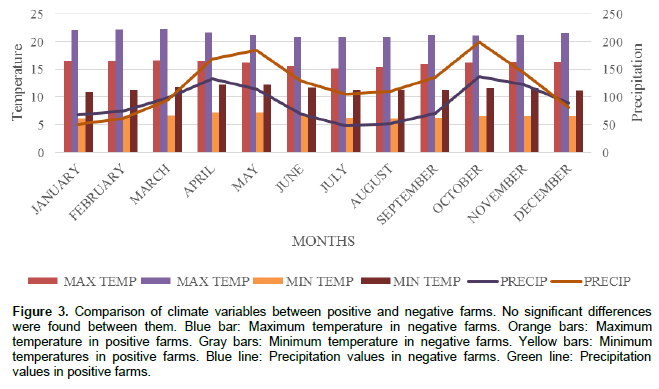
Figure 3 shows the relationship between climate factors such as precipitation, temperature, and presence or absence of BLV. The comparison of climate variables be-tween positive and negative farms was conducted according to the methodology described for quantitative variables. The statistical analysis applied showed significant differences between positive and negative farms as shown in Figure 3 (p<0.05). High rainfall intensity was reported in negative farms as compared with low rainfall intensity reported for positive farms during December, January, February and March. The rainfall results changed during the rest of the year indicating high rainfall intensity in positive farms as compared with low rainfall intensity in negative farms. Maximum and minimum temperatures were obtained in March and January with 22 and 9°C for positive farms and also in March and January with temperatures of 16 and 6.1°C for negative farms. Therefore, maximum and minimum temperatures were higher in positive farms as compared with negative farms.
Unfortunately, the EBL has not been included yet in the group of diseases categorized as mandatory notifiable in Colombia. Other countries such as Turkey have diminished the infection due to the notification of the disease and getting seroprevalences lower than 2% (
Åževik et al., 2015). Colombia does not have a well-established tool for prevention yet. It has not been a matter of major concern among producers or specialists in public health because it has not shown a major impact on animal health. The only defined impact is the cost that involves the low weight of infected animals and the low productivity of seropositive cattle who manage to move to leukocytosis stage, immunodeficiency or eventually leukemia.
There is an important issue to be considered here. Most of the farms are very small, with an average number of cows of not more than 35 each. There are some exceptions, like the municipalities of Monteria and Puerto Salgar, which have 125 and 72 animals on average, respectively. On the other end, we have Guachucal in Nariño, with only 7 cows on average. It supposes that small farms are handled carefully, then they have low risk to be infected. In bigger farms the risk increases because the care is not as cautious as it should be. It has been described that in larger herds, there might be an increased chance of animal exposure to the virus (
Nekouei et al., 2015).
The first data to be analyzed is the high BLV prevalence of both, animals (42.7%) and farms (67.7%). The same analysis performed in Colombia in 2007 showed results between 34 and 50% per animal and farms, respectively, which means that the values increased and that the virus is present endemically with a considerable seroprevalence.
The seroprevalence is being compared with data from different countries such as Canada, which reported 45% for farms and around 10% for animals in 1980 and increased to 78% in 2015. Argentina and Japan reported 80% for animals in 1999 (
Gutiérrez et al., 2015;
Hernández-Herrera et al., 2011)and also United States presented 39% for animals and 83% for farms in 2007 (
Bartlett et al., 2014;
Frie and Coussens, 2015). Also, between 1997 and 2007, the prevalence of cattle had an average of 85% in the United States. These data show that there is a high and variable seroprevalence around the Americas and it is even higher when it is measured per farms rather than per animals. The referenced information apparently indicates that there are no tools to control the disease due to the lack of a real political will and a poor understanding of how this retrovirus can affect human population (
Rodríguez et al., 2011).
Considering a lack of control and reduction strategies, an alternative solution is to avoid factors that cause the risk associated with the infection. The most relevant monitoring conditions are repeated infections because BLV-positive animals have some level of immuno-suppression. It means that they can easily get infected with a large number of etiology infection pathogens such as blood parasitic infections, viral diseases, bacterial diseases and lung parasites and pathogens as well as those associated with mastitis and abortions (
Ohno et al., 2015;
Rodríguez et al., 2011)
Therefore, considering these conditions as risk factors, the most important element will be to have a special monitoring of the animal with repeated infections as well as to prevent healthy animals from sharing the space, utensils or medical practices with them.
Risk factors obtained in this study are considered similar with those already described by several authors, such as the use of needles (one per animal), insemi-nation or palpation gloves, the practice of insemination instead of using bulls to mount the female cattle, vector control for flies and birds, feeding with negative or heat-treated colostrum for BLV, selection and separation of se-ropositive animals from herd and the proper management and control of material used for practices such as vaccination, dehorning, insemination, etc (
Bartlett et al., 2014;
Hernández-Herrera et al., 2011). The use of pen for cows is perhaps the least risk factor reported by other authors. In Colombia, the pen is used for multiple activities such as surgeries, dehorning, insemination, delivery and management of sick animals making its use a risk factor for dissemination of the virus.
Two conditions are found as risk and protective factors; they consist of the person who applies vaccines and milk the animals on the farm. The vaccination executed by experts is considered as a protective factor, while the vaccination performed by any other worker on the farm is considered as a risk. Additionally, the mechanical milking became a risk factor compared to the manual milking which is considered a protective factor. These variables may be related to hygienic and public health standards and GMP (Handling and manufacturing best practices). Hand milking is a more hygienic practice while in the mechanical milking the virus can remain on the machine and potentially infect the next cow.
Several authors in the literature have reported some other alternatives to decrease the prevalence of BLV. They are summarized in the present study as the following: The use of single-use needles during vaccination or therapeutic protocols, the use of obstetric removable sleeves when used in one animal and other, the use of disposable materials (cleaning, disinfection or sterilization of reusable materials and surgical instruments at least) in procedures such as dehorning, tattooing, implantation, cauterization, castration or tagging the animal’s ear, the use of electronic or gas combustion devices instead of surgery equipment during dehorning, the elimination of insects, particularly in densely populated agricultural areas (milking areas, stalls and barns), in order to minimize possible transmission between cattle through arthropod vectors, direct mounting artificial insemination and embryo transfer with BLV-free donors and bulls (Rodríguez et al., 2011). Supplying foods such as carrots and flour is also considered a protective factor because they are supplies that reinforce the diet at a time when food decreases and improve the nutritional level of animals.
Among the other protective factors, the drinking water is also included. In Colombia, it is usual to give potable water to the cows. The same water used for human consumption. This water is free of pathogens and any kind of substances that can cause disease. Selling animals for release is a protective factor because they are usually led to the slaughterhouses to be sold as meat. In this case, the virus cycle is interrupted and it stops its infection to more animals.
Having high seroprevalence of BLV in animals and farms, a distribution of the virus in cattle, losses to livestock in our country and the possibility of a zoonotic infection, indicate the importance of developing more studies to monitor the virus. They should also generate preventive policies that will reduce the virus presence to prevent future problems that could have humans infected with BLV.
Climatic factors such as precipitation and temperature constitute determining factors in the occurrence of the disease. Results have shown significant differences between positive and negative farms (p < 0.05). A high rainfall is reported in negative farms and a lower rainfall in positive farms during December, January, February and March. The rainfall results changed during the rest of the year indicating high rainfall in positive farms as compared with low rainfall in negative farms promoting the positivity. The maximum and minimum temperatures were higher in positive farms as compared with negative farms throughout the year.
These results become an important decision-making tool for prevention and control. They allow having an adequate resource distribution to generate tool intervention programs. While the results are similar to those reported by other authors around the word, avoiding the risk and implementing protective factors are initiatives linked to the cultural process. For example, the use of poultry in Colombia is implemented not only to isolate animals but also with iatrogenic objective, for this reason good hand hygiene standards must be implemented in order to reduce the risk that means using contaminated pens.
Nevertheless, some reports already showed the presence of antibodies and viral genome segments in humans, specifically in women with breast cancer (Buehring et al., 2003; Gutiérrez et al., 2015; Anonymous, 2007;
Ochoa-Cruz et al., 2006;
Schwartz et al., 1997). The information suggests the presence of the retrovirus in humans; however, the authors have not referenced the statement as a zoonotic disease. Despite our conclusion, it is important to continue developing epidemiological analyzes tending to report and monitor the presence of this disease and its risk factors; this is the only alternative to generate prevention and control strategies.
The results of the research show seroprevalence of 42.7% in animals and 67.7% in farms. The infection with blood parasites and another virus was attributed to be among the main risk factors associated to BLV. The use of individual needles during veterinary procedures was found to be the main source of protection against the virus. EBL should be included in the group of diseases categorized as mandatory notifiable in Colombia.
The authors have not declared any conflict of interests.
The authors would like to thank Dra. Katia Benavidez from Universidad de Nariño, ProyGuachucal; Dra. Clemencia Fandiño and coworkers from Universidad del Tolima, Proy Puerto Salgar; Dr. Agustín Góngora and coworkers from Universidad de los Llanos, Proy Villavicencio; Dr Darío Cárdenas and coworkers from Universidad Cooperativa de Colombia, Proy Villavicencio; Dr. Martin Pulido and coworkers from UPTC, Proy Sotaquirá; Dr. Salim Mathar and coworkers from Universidad de Córdoba, Proyecto Montería; and Dra. Marta Olivera and coworkers from Universidad de Antioquia, Proy San Pedro de los Milagros.