ABSTRACT
Comparison was made for sperm storage capacity and total protein concentration in the testes of postpubertal West African Dwarf (WAD) and Sokoto Red (SR) bucks, this was determined using 12 postpubertal bucks. The average weight of the breeds was 7.50 ± 0.35 kg for the WAD and 11.00 ± 0.71 kg for the SR bucks. All the animals were slaughtered and their testes excised. Portions of the reproductive tracts were homogenised and biochemical assessment was carried out using standard procedure and data obtained were subjected to t-test. While gonadal sperm reserves were similar (p>0.05) between the breeds, all extragonadal regions of the reproductive tract differed significantly (p<0.05) between the breeds. With respect to total protein, significant differences (p<0.05) were recorded in testis, corpus epididymis and ductus deferens with clear similarities (p>0.05) in the caput and cauda epididymal regions for both breeds. Meanwhile, very high and positive relationship were also observe between caudal sperm motility and corpus epididymis with the values, r= 0.84; ***p<0.001 and r= 0.78; ***p<0.001 in the WAD and SR bucks, respectively. The relationship in the testis and the caudal epididymis for both breeds were high and positively correlate with values, r= 0.73; **p<0.01 in the WAD and r=0.71; **p<0.01 in the SR bucks. The significantly high extragonadal sperm reserves and protein activity in the testes of this breeds confirmed its good attribute for a selection programme in the native tropical environment.
Key words: West African Dwarf, Sokoto Red, testes, genotype, bucks.
The system of goat production in Nigeria is basically traditional with animals are left to scavenge for feed, while commercial production is in the hands of a few farmers whose production has not met up with the rising demand for animal protein in our native environment. However, goats have considerable advantage over other
classes of livestock in Nigeria. Besides its ability to thrive under extreme conditions of natural location and its flexible integration into the most diverse socioeconomic conditions, it is small in size and commonly reared among women and children, especially in the rural areas (Ngere, 1983; Gallaha and Pitman, 2001). Apart from milk and dung, goat meat in form of ‘isi-ewu’ is used to garnish delicacies in the eastern part of Nigeria and it is an animal recommended for sacrificial offerings in the annual festival of thanksgiving by the Royal Benin Kingdom of South-South Nigeria. The West African dwarf goats are mostly common in Nigeria; it is of a superior and most popular genotype and it has for long been known to be more prolific owing to the number of kid born per doe per year (Gall et al., 1992). The Sokoto red goats on the other hand is of great economic importance in that, the popularity of the high “Moroccan Leather” has been of great benefit to Europe since the medieval period (Bitto and Osayande, 2013). The study on the epididymal sperm reserve of goats came to light when Corteel (1973) reported on goats of 140 and 168 days of age. Jindal and Panda (1980) gave similar report on epididymal sperm reserves in adult billies a decade after. In the ram, there are wide variations in the extragonadal sperm reserves (ESR) as reported by different authors. Researchers have also reported values of 27.13 x 109 (Dot and Skinner, 1967) from same breed. The correlation between testicular weight and epididymal sperm numbers as reported by Ugwuegbu et al. (1985) in the Maradi buck found that mean epididymal sperm was highly and positively correlated with testicular sperm count. Bitto (1989) reported that extragonadal sperm reserves for the pubertal WAD bucks were similarly unaffected by season. However in the early 1990s and the millennium, several authors have reported on the gonadal and extragonadal sperm reserves in the pubertal WAD buck (Inegedu et al., 2005), adult Sokoto red bucks (Bitto et al., 2008). Information on the WAD and Maradi buck direct comparison is lacking, which leaves a lot of question on the sexual activity that takes place at the post pubertal phases in these two breeds with economic importance in our native environment. Evaluation of biochemical characteristics enhances the assessment of the functional state of the epididymis in which the spermatozoa are stored awaiting ejaculation during natural mating or artificial insemination. Malfunction of both epididymis and other assessor glands affect the fertilizing capacity of the spermatozoa adversely (White, 1973). More recently, Ewuola et al. (2014) reported a significant decline in the total protein concentration in the testis of WAD bucks fed varied levels of aflatoxin, although, the improvement and commercial production of the West African Dwarf and Sokoto Red goats is still scanty at the post pubertal stage and there is still a dearth of knowledge on the reproductive physiology on these breeds of goats. However, this work was carried out to evaluate the gonadal, extragonadal sperm reserves and protein concentrations in the testes of post pubertal WAD and RS bucks in their native environment.
Experimental site
The experiment was conducted in the Animal Physiology and Bioclimatology Laboratory of the Teaching and Research Farm, University of Ibadan, South West, Nigeria. Ibadan is on Latitude 7 20' N and longitude 3 50' E.
Animals and management
Twelve post pubertal bucks of 7 to 8 months, with an average weight of 7.5± 0.61 kg for the WAD and 11.00±1.22 kg for the RS bucks were utilized for this study. The animals were housed in two separate pens and diets supplemented with composite dried cassava peels were fed to meet the nutrient requirement of goats. Animals were allowed to freely graze on forage and ample drinking water was provided ad libitum.
Data collection and statistical analysis
At eight weeks of observatory feeding, animals were all sacrificed by restraint and exsanguination; the reproductive tracts were decapitated and frozen at -20°C. The testes, epididymis and the ductus deferens was carefully separated, trimmed off adhering fat and weighed individually. The epididymis was divided into the caput, corpus and cauda regions on the basis of external morphology. Estimate of the testicular and epididymal sperm reserves was determined by the homogenization technique of Amann and Almquist (1962) and Amann (1970) and as also reported by Egbunike (1980). Five grams weight of the testes, epididymis (caput, corpus and cauda) and ductus deferens was homogenized in 100 ml of 0.154 m NaCl in clean beakers with a pair of scissors for 5 min after which each of the homogenates was filtered through two layers of loosely netted bandage into clean glass test tubes. The protein concentration of testicular spermatozoa from the testis, caput, corpus and caudal epididymis and ductus deferens were evaluated using the biuret method of Weichselbaum (1946). Daily sperm production (DSP) was determined by dividing the values obtained from the testicular homogenates by a time divisor 3.56. The DSP/g were evaluated by dividing the corresponding DSP value by weight of testicular tissue obtained (Amann, 1970). Data obtained was subjected to analysis of variance ANOVA, using t-test between the regions of the testes of both breeds (SAS, 2013).
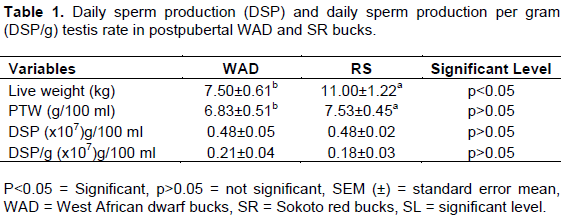
The comparative differences in paired testis weight (PTW), daily sperm production (DSP) and daily sperm production per gram testis (DSP/g) is as indicated in Table 1. DSP did not differ significantly (p>0.05) with values 0.48±0.05 (x108) g/100 ml and 0.48±0.05a g/100 ml for the WAD and SR bucks, respectively. Although, PTW and DSP/g testis showed significance (p<0.05) with values, 6.73±0.39 kg g/100 ml to 7.63±0.05 kg and 0.21±0.04(×108) g/100 ml to 0.18±0.03(×108) g/100 ml for the post pubertal WAD and SR, respectively. Differences between the post pubertal WAD and SR bucks in sperm storage capacity as indicated in Table 2 showed similarities (p>0.05) in the testis region with values of 1.71±0.18(×108) g/100 ml in the WAD and 1.71±0.15(×108) g/100 ml in the SR bucks, with percentage contributions of 29.80 and 31.05%, respectively. Estimate of the extragonadal sperm reserves ranged from the caput to the cauda epididymis with values of 0.49±0.07(x108) g/100 ml to 3.06±0.26(x108) g/100 ml in the WAD and 0.38±0.03(x108) g/100 ml to 2.68±0.29(x108) g/100 ml in the SR bucks. However, significant differences (p<0.05) were observed for the caput, corpus and cauda epididymis with clear similarities (p>0.05) in the ductus regions for both breeds. The cauda regions recorded the highest level of contribution with the values of 53.31 and 49.20% in the WAD and SR bucks, respectively. Total protein concentrations in the reproductive tract in both breeds as indicated in Table 3, followed the order: testis, corpus epididymis, cauda epididymis, caput epididymis and ductus deferens in the WAD and ductus deferens, cauda epididymis, testis, caput epididymis and corpus epididymis in the SR bucks. There were significant differences (p<0.05) in the testis, corpus epididymis and ductus deferens, while the cauda and caput regions did not differ significantly (p>0.05). Estimated values ranged from 0.41±0.15 g/100 ml in the ductus deferens and 1.81±0.85 g/100 ml in the testis in the WAD, while the SR bucks had values ranging from 0.31±0.25 g/100 ml in the corpus epididymis to 1.05±0.24 g/100 ml in the ductus deferential region. Protein concentration in the reproductive tract, both breeds exhibited significant differences (p<0.05) in the testis, corpus epididymis and cauda epididymis with values ranging from 0.41±0.15 to 1.81±0.85 in the WAD and 0.31±0.25 to 1.05±0.24 in the SR bucks. Table 4 shows the correlation matrix between cauda epididymal sperm motility, mass activity and protein concentrations in sperm cells and fluids in post pubertal WAD and SR bucks. Both breeds displayed a high and positive correlation between the testis (r= 0.97; ***p<0.001) and corpus epididymis (r= 0.97; ***p<0.001). A very high and positive correlation were also observed between caudal sperm motility and corpus epididymis with the values (r= 0.84; ***p<0.001 and r= 0.78; ***p<0.001) in the WAD and SR bucks, respectively. Meanwhile, the WAD bucks displayed a low and negative correlation between mass activity and corpus epididymis (r= -0.36; p<0.05), with low and positive correlation (r= 0.46; p<0.05) between caudal epididymal sperm motility and caudal epididymis in the SR bucks. Comparing the relationship in the testis and the caudal epididymis, both breeds displayed a high and positive correlation with values r= 0.73; **p<0.01 in the WAD and r=0.71; **p<0.01 in the SR bucks.
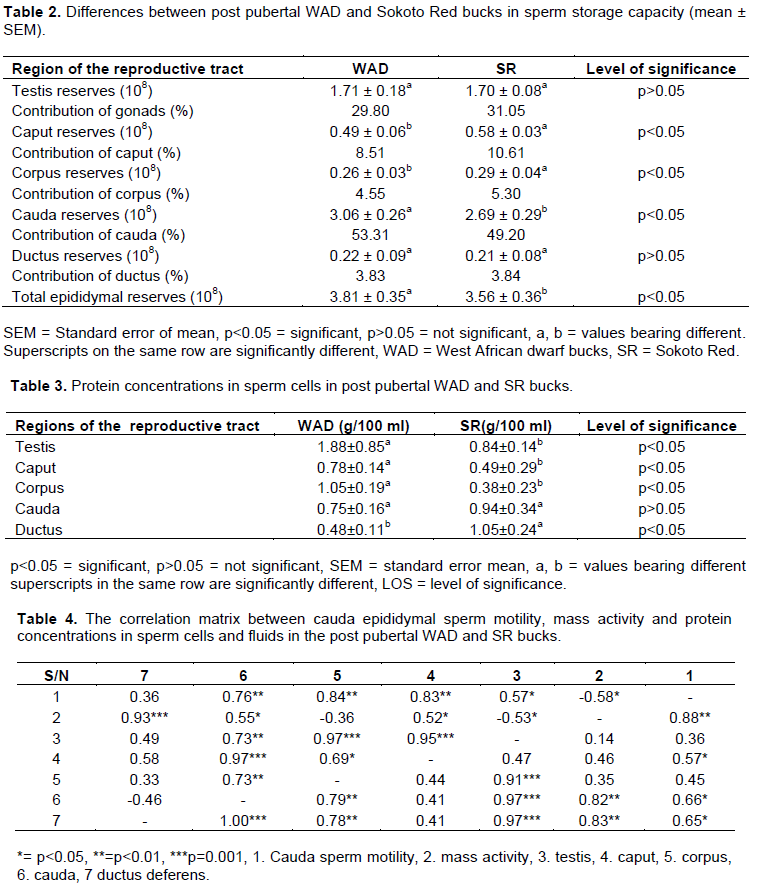
The relative contributions of gonadal and epididymal sections in the present study (at the post pubertal phase) followed a similar pattern. The values of sperm reserves obtained are generally lower than values earlier reported in pubertal WAD bucks, values were in line with earlier reports of Inegedu et al. (2005) in the WAD bucks and Carew and Egbunike (1980), Ugwuegbu et al. (1985) in the RS buck. The higher sperm reserves in the cauda epididymal regions confirm well established fact that the cauda epididymis is the store house of spermatozoa in several animal species. The higher sperm storage capacity in the post pubertal WAD and RS bucks confirms earlier reports of Oyeyemi and Ubiogoro (2005) in large boars and Sharma and Gupta (1978) in buffalo bulls. This could have taken into account sperm transit time which is determined by number of factors including frequency of ejaculation and the demand placed on testicular spermatozoa post collection. Gonadal sperm reserve values were also lower for that obtained by Ewuola et al. (2014) and this disparity could be as a result of factors due to nutrition, age and genetics in this study. The values obtained in protein concentrations in the testes of both breeds fell within the range, although values were lower than that obtained more recently (Ewuola et al., 2014) in WAD bucks. The significant differences (p<0.05) observed for both genotypes in the caput and corpus epididymis could be due to breed effect, in that adjustment occur during the ripening of spermatozoa in order to cater for buffering capacity (Wildeus and Enwistle, 1982; Weisgold and Almquist, 1979); so far no documented report on the protein concentrations in these regions in the SR bucks. Protein levels in the testis were highest in the WAD with the SR bucks recording the highest in the ductus differential regions. The significant differences (p<0.05) observed for both breeds in the corpus epididymis could be due to breed effect (Wildeus and Enwistle, 1982; Weisgold and Almquist, 1979). Report on the protein concentration in these regions in the SR buck has not been documented. It is worthy to note that the values obtained in the post pubertal WAD were in the order: testis, corpus epididymis, cauda epididymis, caput epididymis and ductus deferens and that of the SR bucks were also in the order: ductus deferens, cauda epididymis, testis, caput, corpus epididymis. The relationship between cauda epididymal sperm motility, mass activity and protein concentrations in sperm cells and fluids in post pubertal WAD and SR bucks account for the fact that sperm cells undergo ripening to aid buffering process (Egbunike et al., 1985; Butswat and Zaharadeen, 1998).
Even though the age of the animals used in this study is just after puberty, the relationship between activity in the testis and the sperm store house were normal. Therefore, data generated from this study has highlighted some attributes in relationship between in situ sperm production and sperm reserves in the testes. It is concluded from these results, that even though bucks of these breeds may be relatively low in sperm storage capacity at the post pubertal phase, they could be used cautiously as sires and genetic resources for conservation and improvement.
The authors have not declared any conflict of interests.
REFERENCES
|
Amann RP (1970). Sperm production rates in the testes, vol, pp 443-482 .Eds A.D Johnson, W.R Gomez & N.L Van Demard Academic press. New York.
|
|
|
|
Amann RP, Almquist JO (1962). Reproductive capacity of dairy bulls IV. Effect of unilateral vasectomy and ejaculation frequency on sperm reserves, aspects of epididymal physiology. J. Reprod. Fertil. 3:206-268.
Crossref
|
|
|
|
|
Bitto II, Egbunike GN, Akusu MO (2008). Seasonal Variations in the Histometric Characteristics of the Reproductive Organs of Pubertal West African Dwarf Bucks in their Native Tropical Environment. Int. J. Morphol. 26(2):397-401.
Crossref
|
|
|
|
|
Bitto II, Osayande UD (2013). Gonadal and extragonadal sperm reserves of Post Pubertal West African Dwarf and Sokoto red bucks in their native tropical environment. Proceedings of 4th International Conference on Sustainable Animal Agriculture for Developing Countries (SAAD), Lanzhou, China. pp. 87-88. Available at: https://www.cabdirect.org/?target=%2fabstracts%2f2013340%2fcabdirect%2fabstract%2f20133405753%2fcabdirect%2fabstract%2f20133405753
|
|
|
|
|
Butswat IS, Zaharadeen DT (1998). Comparism of some reproductive parameters in Red Sokoto and Kano brown breeds of bucks. Niger. J. Anim. Prod. 25(1):1-5.
|
|
|
|
|
Carew BA, Egbunike GN (1980). Sperm production rates in Maradi goats extensively managed in a tropical environment. Proc. 9th Congress on Animal Reproduction and A.I., Madrid. pp. 608-611.
|
|
|
|
|
Corteel JM (1973). Artificial insemination of goats: Physiological basis of present state and future prospects. Word Rev. Anim. Prod. 9:73-99.
|
|
|
|
|
Dot HM, Skinner JD (1967). A reassessment of extra gonadal sperm reserves in Suffolk rams. J. Agric. Sci. 69(02):293-295.
Crossref
|
|
|
|
|
Egbunike GN (1980). Sperm storage capacity of the indigenous West African boar. Int. J. Androl. 3:210-216.
Crossref
|
|
|
|
|
Egbunike GN, Togun VA, Agiang EA (1985). Sperm production in ruminants in humid climates. World. Rev. Anim. Prod. 21(3):11-17.
|
|
|
|
|
Ewuola EO, Jimoh OA, Bello AD, Bolarinwa AO (2014). Testicular Biochemical, sperm reserve and daily sperm production of West African Dwarf bucks fed varied dietary Aflatoxin. Anim. Reprod. Sci. 148:182-187.
Crossref
|
|
|
|
|
Gall CH, Steir CH, Younan M (1992). Small ruminants in developing countries. Target for biotechnology symposium of potentials and limitations of biotech for technology for livestock breeding and production in developing countries. Merlense Germany. pp. 99-130.
|
|
|
|
|
Gallaha RN, Pitman ND (2001). Concentration of forage in the tropics and subtropics. In: A Sotomayor-Rios and W.D Pitman (Editors).Tropical forage plants: Development and use. CRC press LLC, Boca Rason. pp. 233-250.
|
|
|
|
|
Inegedu EO, Ezekwe AG, Igboeli G (2005). Gonadal and Extragonadal sperm reserves of unilateral cryptorchid and normal West Africa bucks (Capra hircus) reared in Nsukka. Proc. Of the 30th ann. Conf. of the Nig. Soc. For Anim. Prod. 20th to 24th March, 2005.Vol 30. pp. 27-29.
|
|
|
|
|
Jindal SK, Panda JN (1980). Epididymal sperm reserves of the goat. (Capra hircus). J. Reprod. Fert. 59:469-471.
Crossref
|
|
|
|
|
Ngere LO (1983). The small ruminant of West Africa. A review, FAO Animal genetic resources in Africa, OAU/STRC/IBAK Publ:112.
|
|
|
|
|
Oyeyemi MO, Ubiogoro O (2005). Spermiogram and morphological characteristics in testicular and epididymal spermatozoa of large boar in Nigeria. Int. J.Morphol. 23(3):235-239.
Crossref
|
|
|
|
|
Sharma AR, Gupta RC (1978). Epididymal sperm reserves of buffalo bulls (Bulbalis bulbalis). Andrologia 10:479-483.
Crossref
|
|
|
|
|
SAS (Statistical Analysis System Institute) (2013). SAS User's Guide. SAS Institute Cary, NC, USA. Available at: https://support.sas.com/documentation/onlinedoc/stat/131/glimmix.pdf
|
|
|
|
|
Ugwuegbu SO, Oke BO, Akusu MO, Aire TA (1985). Gonadal and extragonadal sperm reserves of the Maradi (Red Sokoto) Goat. Bull. Anim. Health Prod. Afr. 5(33):139-141.
|
|
|
|
|
Weichselbaum JE (1946). An accurate and rapid method for the determination of proteins in small amounts of blood or plasma. Am. J. Clin. Pathol. 16: 40.
|
|
|
|
|
Weisgold AD, Almquist JD (1979). Reproductive capacity of beef bulls. VI. Daily spermatozoa production, spermatozoa reserves, dimensions and weight of reproductive organs. J. Anim. Sci. 48:351-358.
Crossref
|
|
|
|
|
White IG (1973). Biochemical aspects of Spermatozoa and their environment in the male reproductive tract. J. reprod. Fert. 4:471-474
|
|
|
|
|
Wildeus S, Enwistle KW (1982). Post pubertal changes in gonadal and extragonadal sperm reserves in Bos indicus stain. Theriogenology 17:655-667.
Crossref
|
|