ABSTRACT
A cross-sectional study was conducted on the major skin diseases of cattle in Hawassa city with the objectives of estimating the prevalence and assessing the effect of different risk factors in the occurrence of these skin diseases in the study area. The study was conducted with thorough physical examination of cattle and laboratory examination of samples collected from all encountered cases. Out of 662 cattle examined for the presence of skin diseases, 146(22.05%) cattle had different skin diseases, namely ring worm (6.2%), lice infestation (6%), tick infestation (5.3%), wart (4.4%), lumpy skin disease (0.6%), mange mites (0.3%) and dermatophilosis (0.2%).There was a significantly higher (p<0.001) prevalence of skin diseases in young (38.5%) than the old (10.3%) animals. However, the other considered risk factors namely; sex, origin and management were not statistically associated with the occurrence of skin diseases. In general, unless feasible control measures are timely implemented the encountered skin diseases can have varied and adverse effect on cattle production, tanning industry and health of the public and concomitantly pose huge economic loss.
Key words: Skin diseases, ring worm, ectoparasites, wart, risk factors, cattle, Hawassa.
The livestock subsector of Ethiopia is the second major sources of foreign currency, through export of live animals and hides and skins (Ayele et al., 2003; Teshome and Derso, 2015). Although the country produces about 2.7 million of hide, 8.1 million of sheepskin and 7.5 million of goatskin per annum for tannery industry, yet as many as one quarter to one –third of all the skins processed at tanneries are unsuitable for export due to various defects (Kassa et al., 1998; Abebayehu et al., 2011). Up to 65% of these defects are believed to occur in the pre-slaughter stage of production while the animal is still alive (Kassa et al., 1998; Wondwossen, 2000). The most common cattle skin diseases reported in Ethiopia are dermatophilosis, lumpy skin disease, dermatophytosis, pediculosis, acariasis, photosensitization and warts (Chalachew, 2001; Teshome and Derso, 2015). Skin diseases are accountable for significant and varied socio-economic impacts. Apart from quality degradation of hides and skin, skin diseases induce associated economic losses due to reduction of wool quality, meat and milk yield, losses due to culling and occasional mortalities related with cost of treatment and prevention of the diseases (Yacob e al., 2008; Salih et al., 2015).
In addition, some skin diseases such as ringworm and sarcoptic mites are potential zoonosis (Quinn et al., 2002; McDaniel et al., 2014). The huge impact of socioeconomic perspective still demands the nationwide detailed investigation of the distribution of important skin diseases and their determinants. Though some research works on bovine skin diseases have been done in some part of the country (Chalachew, 2001; Yacob et al., 2008; Teshome and Derso, 2015), most of them were based on tannery information, some others were focused only on dermatophilosis (Berhanu and Woldemeskel, 1999; Woldemeskel,2000; Woldemeskel and Taye, 2002; Kassaye et al., 2003) and most of them were done in areas other than the current study site and hence there is still information gap in some geographic location and on the remaining skin diseases.Therefore, this study was conducted to assess the prevalence of major skin diseases of cattle in Hawassa city and to assess the effect of different risk factors associated with these skin diseases.
Study area
The study was carried out from October 2014 to May 2015 in Hawassa city, which is the capital of south nation nationalities and people regional state (SNNPRS). Hawassa is located at 38°29’E and 7°05’N, 275 Km south of Addis Ababa. The elevation is about 1790 m above sea level and the respective minimum and maximum average temperature is 12 and 28°C. The mean annual rain fall is 960 mm. The short rainy season/‘belg’ start from February to May and the long rainy season /‘meher’ start from mid-June to October. The total livestock population of the zone was 2,172,015 cattle, 858,206 sheep and goat, 155,356 equines and 2,123,579 poultry (CSA, 2017). The two rural villages, Chefe and Dato, are characterized by water lodged and marshy land and are located north east main part of the city following the water course tributary to Lake Hawassa called ‘Tikur weha’. The area is known for huge indigenous cattle population and cash crops like Catha edulis (Chat) and ‘Enset’.
Study animal and sampling method
The sampling was made first by randomly selecting small-scale rural and urban dairy farms from Hawassa, Chefe and Dato and then the study animals, comprising both sexes, both local and crossbred and different age group were selected using simple random sampling. Accordingly, a total of 662 cattle, composed of 213 male and 449 female, were selected for physical and laboratory based examinations. Majority (528) of the study animals were cross bred and the rest (134) were local (Zebu) breeds. The age of the animals was estimated based on teeth eruption as suggested by Muylle (2016). The average age of the examined cattle was 2.6 years. For simplicity sake, the study animals were categorized into young (less than 2 years) and adult (above 2 years). The management was classified as poor and better based on the availability of additional feed, shelter and medicament when needed.
Study methodology
History taking and physical examination
History regarding, age, presence of itching, course and progression of the disease, measures taken, if any; the number of animal having the same problem, type of lesion and\or clinical signs observed and their chronology were recorded. The entire skin of the animals was thoroughly inspected and palpated for the presence of papules, vesicles, pustules, erythema, scabs, pigmentation abnormalities, alopecia, tumorous growth and ectoparasites. Distribution of the lesion on the body of the animal and symmetry were also considered.
Samples collection and processing
Depending on the case, appropriate samples were collected for microbiological and parasitological examinations. Lumpy skin disease was diagnosed tentatively based on the characteristic clinical findings and previous serological confirmation made in the area.
Skin scraping: Skin scraping was collected from representative sites of cases presented with alopecia, Itching and erythematous lesions. Skin scrapings collected from suspected cases were placed in dry clean sterile test tubes. In addition to superficial skin scraping taken from the periphery of the circular lesion, hair specimens were also collected from lesions suspected of dermatophytosis using a pair of forceps. Similarly, for dermatophilosis suspected cases, exudative crusts were also collected by pairs of forceps along with a deep scraping with clean surgical blade. Moreover, the nodular lesions of demodicosis suspected cases were pressed in between two digits until the pus is expressed, the pus was then placed on a slide and crushed in between two glass slides or after placing cover slip (Greiner, 2012; Abu-Samra et al., 2014). The collected samples were properly labeled, packed and transported to the Veterinary Parasitology and Pathology Laboratory, Hawassa University for examination.
Tick and lice collection and examination: Ticks and lice were manually collected by searching on different regions of the animals’ body. Various predilections/feeding sites namely; base of the tail, ear, perianal area, sternum, scrotal area and the belly were the targeted sites. The collected ticks and lice were placed in universal bottles, preserved in 70% ethyl alcohol, labeled with the necessary information and transported to the laboratory for further identification.
Laboratory investigation: The squash smears prepared from demodicosis suspected cases were examined for the presence of demodectic mites at 10x magnification of light microscope (Greiner, 2012). Each scraping was transferred to clean and dry petri-dish and examined directly under stereomicroscope for the presence of moving sarcoptic mites. Positive sample was then treated with few drops of 10% KOH and examined based on the standard procedures, mites were picked with dissecting needle along with some content, placed on clean slide, covered with cover slip and examined under 10x magnification to characterize and identify the mites according to their morphology (Urquhart et al., 1996; Greiner, 2012).
Exudative crusts and/or deep skin scrapings taken from dermatophilosis suspected cases were moistened with warm saline solution on clean slide, smeared, dried with heat and subjected to Giemsa staining for demonstration of Dermatophilus congolensis (Quinn et al., 2011).The collected ticks and lice were examined under stereomicroscope and identified following the procedures given by Hoogstral (1956) and Walker et al. (2003). Scrapings and hairs collected from ringworm suspected cases were mounted for direct examination of dermatophytes in 25% KOH or NaOH mixed with 5% glycerol, heated for 1 h at 51 to 54°C to emulsify lipids, and examined under 40X magnification for fungal structures (Weitzman and Summerbell, 1995).
Data analysis
Data collected from the field and laboratory examination were recorded, entered in Microsoft Excel spread sheet and analyzed using STATA version 11 (STATA corp., College Station, TX). In the analyses the confidence level was held at 95% and p<0.05 was set for establishing significance. Association with the considered risk factors (age, sex, management system, and origin) was assessed with chi square analysis.
Out of 662 cattle examined for the presence of skin diseases, 146 (22.05%) cattle were positive for different skin problems, namely ring worm (6.2%), lice infestation (6%), tick infestation (5.3%), wart (4.4%), lumpy skin disease (0.6%), mange mites (0.3%) and dermatophilosis (0.2%) (Table 1, Plates 1-3). The common lice species/genera identified were Bovicola bovis, Linognatus and Haematopinus spps. Moreover, Rhipicephalus (Boophilus) decolaratus, Rhipicephalus evertsi-evertsi, Amblyomma variegatum, A. coherence and A. lepidium were the identified tick species. The variation in the prevalence of skin diseases were statistically significant (p<0.001) between age categories, young cattle being more frequently affected (38.5%) than their adult counterparts (10.3%). Hence, young cattle were 5.44 times more likely to have skin diseases than adults (Odds Ratio (OR) =5.44, 95% CI=3.62, 8.18). However, the other considered risk factors namely; sex, origin and management were not showing statistically significant effect in the occurrence of the problem (Table 2). Age was having statistically significant (p<0.001) effect on the occurrence of ringworm, lice infestation and wart. Moreover, the occurrences of wart and lice infestation were significantly influenced by both management and origin of the examined cattle.
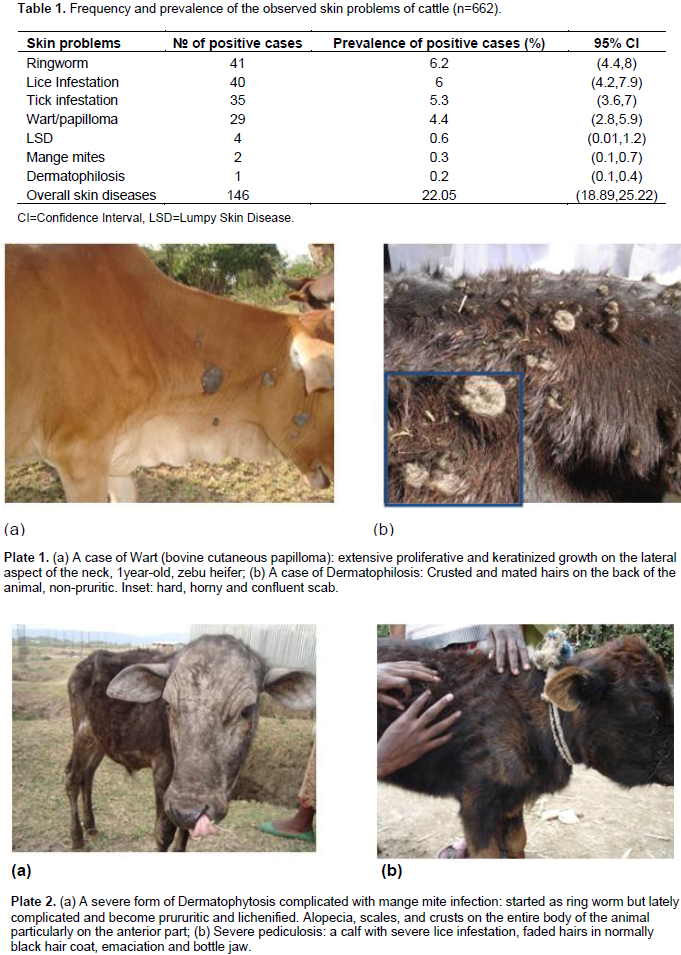
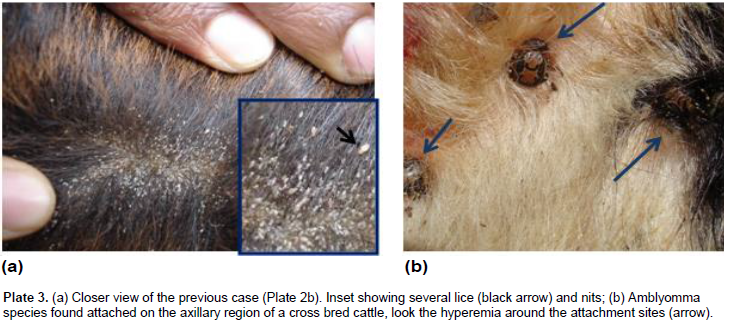
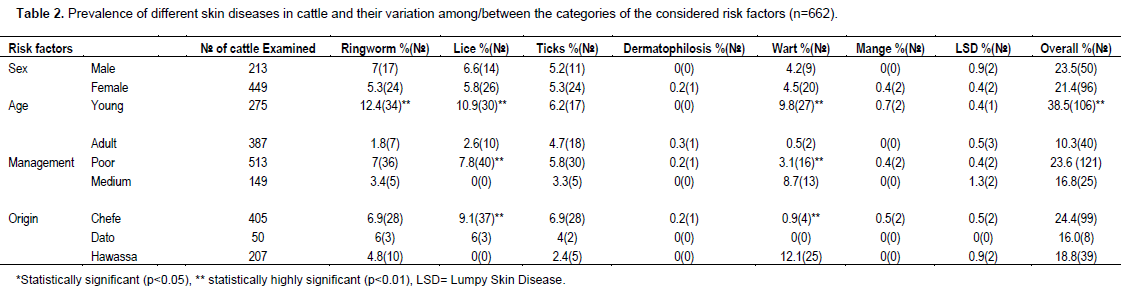
This study revealed that, skin diseases caused by parasites, bacteria, viruses and fungus were common in Hawassa city in cattle with an overall prevalence of 22.05%. This finding is relatively higher than the report made by Yakob et al. (2008) and lower from that of Teshome and Derso (2015), who reported 15.41 and 27.68% from veterinary clinics of Adama and University of Gondar, respectively. The observed difference among the studies could be partly explained by the difference in the geographic location (that is, ecology and climate), cattle management practice and the study sites preferred for sampling. Although sampling at the veterinary clinics potentially incurs bias, the prevalence current reported and the previous work are still high because of several reasons. The prevailing poor veterinary services, poor animal husbandry, lack of awareness on the problem, the use of communal watering and grazing sites, improper application of acaricides by non-professionals are the major reasons to induce and augment this endemic situation.
Among the different skin diseases, ringworm (aka Dermatophytosis) ranked to be the first with a prevalence of 6.2%. Dermatophytosis in domestic animals is an infection of keratinized tissues by one of the two genera fungi, Microsporum and Trichophyton (Quinn et al., 2002; Kahn et al., 2005). Although information is lacking in Ethiopia, it is believed to pose the greatest economic and human health consequence in most developed countries (Bradford, 1996). In the present study, the prevalence of the disease was significantly higher (p<0.01) in animals less than two years of age (12.4%, OR=7.66, 95% CI=3.34, 17.55) than the older age group (1.8%). This variation in the prevalence can be partly explained by the fact that, dermatophytosis in adult and healthy animals is self-limiting but in young and debilitated animals the infection is wide spread and persistent. Moreover, animal susceptibility is determined largely by immunological status, and hence young animals are most susceptible (Radostits et al., 2000; Quinn et al., 2002).
The overall prevalence of cattle skin parasites (11.6%) was markedly lower than the prevalence reported from southern rangelands (73.3%) by Abebayehu et al. (2011). This could be attributed to the differences in the management practice, level of owners’ awareness about the problem, ecology and climate of sampling sites. Ticks require moisture and warm environment for survival and the activity of most ticks commences during spring (Mekonnen et al., 2001). It is admitted that tick infestation, perhaps lice and mange mite infestation too, potentially predispose to other skin diseases (Scott, 2007) and hence even low prevalence could not be underestimated. The prevalence of pediculosis observed in the present study (6%) was in agreement with previous reports made by Yacob et al. (2008) and Regasa and Abebe (2008), who reported 3.94 and 5.13%, respectively. As previously reported (Regasa and Abebe, 2008; Yacob et al., 2008), no significant sexual predisposition was noted (p>0.05) in the prevalence of lice infestation.
Usually mild cases are not considered as, being having any pathogenic effect, but heavy infestations are associated with extensive hide damage and blood loss (Urqhart et al., 1996). All age groups of cattle can be infested with lice, but the heaviest infestations are usually seen on calves, yearlings or in older unthrifty animal. In line with these, young animals were frequently affected (OR=4.77, 95% CI=2.27,10.04) than older animals, this is perhaps because they possess a higher ratio of accessible surface to body volume, inefficient grooming behavior and other defense capabilities (Urqhart et al., 1996; Zewdu et al., 2015). Besides, 2 cases of mange (Demodectic and Sarcoptic, one case each) were also observed in this study. The prevalence of mange (0.3%) obtained in this study was in agreement with previous report made by Regasa and Abebe (2008). Among all skin diseases encountered in this study, wart was the fourth main skin disease with prevalence of 4.4%.
In the present study, the prevalence of the disease was significantly higher (p<0.01, OR=24.72, 95% CI=5.69, 107.34) in animals less than two years of age (9.8%) than the older age group (0.5%). This is because, most virus-induced papilomas are self-limiting and are usually found in young animals (Goldschmidt and Hendrick, 2002), which develop good immunity afterwards. The prevalence of lumpy skin disease in the current study (0.6%, 4 cases) was lower than the report of Ayelet et al. (2014) and Gari et al. (2010), who reported a prevalence of 13.61% in central Ethiopia (Mojo, Adama, Welenchiti and Wenji) and 8.1% in different agro-climatic zones of Ethiopia, respectively. This difference could be associated with the variation in agro-climatic condition, season and vector population during the study periods. A 6 years retrospective data collected and analyzed by Ayelet et al. (2014) suggest that, outbreaks of lumpy skin disease frequently occur between September and December than in November to June, the period at which the current study was conducted.
In the current study, the prevalence of dermatophilosis was 0.2%. Comparable prevalence ranging from 0.0 to 4.8%, were reported in zebu cattle in different part of the country (Berhanu and Woldemeskel, 1999; Woldemeskel, 2000; Yacob et al., 2008; Meseret and Sefinew, 2011; Teshome and Derso, 2015). This low prevalence could be due to the fact that, the pathogen may not yet established its spore in the premises or the animals were less exposed to the possible predisposing factors such as ticks, insects, thorny bushes, ox-pecker birds, rain, etc. Owing to the difficulty of control and eradication of established dermatophilosis, the current prevalence should not be underestimated. Because of budget and time limitations, this study were conducted mainly during the dry period, hence we couldn’t see the effect of season on the prevalence of the different skin diseases including dermatophilosis.
In the study area, different skin diseases were prevalent and among the major diseases affecting cattle production. Compared to the clinical physical examination, assessment at wet blue stage in the tanneries would have indicated more problem/defects that can’t be detected while the animal is still alive. The higher prevalence of dermatological problems in young animals warrant special attentions in the implementation of feasible control and prevention measures at young age categories even before animals reach the age of slaughter. In general, unless feasible control measures are timely implemented the encountered skin diseases can have varied and adverse effect on the cattle production, tanning industry and health of the public and concomitantly pose huge economic loss. To design appropriate control strategy, further studies should be carried out on the seasonal occurrence and socio-economic impacts of these diseases, on large sample size and wider study sites. Moreover, regular application of acaricides and timely treatment of clinical infectious cases were suggested to reduce further impact.
The authors have not declared any conflict of interests.
REFERENCES
|
Abebayehu T, Endris F, Berhanu M, Endrias, Z (2011). Study on the prevalence of ectoparasite infestation of ruminants in and around Kombolcha and damage to fresh goat pelts and wet blue (pickled) skin at Kombolch Tannery, Northeastern Ethiopia. Ethiop. Vet. J. 15(2):87-101.
|
|
|
|
Abu-Samra MT, Shuaib YA (2014). A study on the nature of association between demodex mites and bacteria involved in skin and meibomian gland lesions of demodectic mange in cattle. Vet. Med. Int. 2014:413719.
Crossref
|
|
|
|
|
Ayele S, Assegid W, Jabbar MA, Ahmed MM, Belachew H (2003). Livestock marketing in Ethiopia: A review of structure, performance and development initiatives. Socio-economic and Policy Research Working Paper 52, ILRI (International Livestock Research Institute), Nairobi, Kenya. P 35.
|
|
|
|
|
Ayelet G, Haftu R, Jemberie S, Belay A, Gelaye E, Sibhat B, Skjerve E, Asmare K (2014). Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of the virus. Rev. Sci. Tech. Off. Int. Epiz. 33(3):877-887.
Crossref
|
|
|
|
|
Berhanu D, Woldemeskel M (1999). Bovine dermatophilosis and its influencing factors in central Ethiopia. Transbound. Emerg. Dis. 46(10):593-597.
Crossref
|
|
|
|
|
Bradford P (1996). Large animal internal Medicine, 2nd edition, St. Louis, Mosby year book Inc, USA.
|
|
|
|
|
Chalachew N (2001). Study on skin diseases in cattle, sheep and goat in and around Wolayita Soddo, Southern Ethiopia, DVM thesis, AAU-FVM, Debre Zeit, Ethiopia.
|
|
|
|
|
Central Statistical Agency (CSA) (2017). Agricultural Sample Survey 2016/17. Federal Democratic Republic of Ethiopia Central Statistical Agency. Volume II, Report on Livestock and Livestock Characteristics, Statistical bulletin 585, Addis Ababa, Ethiopia.
|
|
|
|
|
Gari G, Waret-Szkuta A, Grosbois V, Jacquie P, Roger F (2010). Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol. Infect. 138:1657-1666.
Crossref
|
|
|
|
|
Goldschmidt MH, Hendrick MJ (2002). Tumors of the skin and soft tissues. In: Meuten DJ (ed) Tumors in Domestic Animals, 4th edition, Iowa State Press, A Blackwell Publishing Company. pp. 45-70.
Crossref
|
|
|
|
|
Greiner E (2012). Diagnosis of arthropod parasites. In: Zajac AM, Conboy GA (Eds) Veterinary clinical parasitology, 8th edition, John Wiley & Sons, Inc. pp. 217-219.
|
|
|
|
|
Hoogstral H (1956). Africa Ixoid ticks of Sudan with special reference to equatorial province and preliminary reviews of the genera Boophilus, Margaropus and Hyalomma species, Washington D.C. Department of Navy, Bureau of medicine and surgery. pp. 263-266.
|
|
|
|
|
Kahn CM, Line S, Aiello SE (2005). Merck Veterinary Manual. National Publishing Inc., Philadelphia, pp. 1233-1235.
|
|
|
|
|
Kassa B, Bisrat M, Asegedech S, Africa J (1998). Control of "Ekeke" skin defects in sheep by insecticides and shearing, EVA proceeding 12th annual conference, Addis Ababa, Ethiopia. pp.104-109.
|
|
|
|
|
Kassaye E, Moser I, Woldemeskel M (2003). Epidemiological study on clinical bovine dermatophilosis in northern Ethiopia. DTW. Deutsche tierarztliche Wochenschrift 110(10):422-425.
|
|
|
|
|
McDaniel CJ, Cardwell DM, Moeller RBJr, Gray GC (2014). Humans and Cattle: A Review of Bovine Zoonoses. Vector-Borne Zoonotic Dis. 14(1):1-19.
Crossref
|
|
|
|
|
Mekonnen S, Hussein I, Bedane B (2001). The distribution of Ixodid ticks (Acari: Iodidae) in central Ethiopia. Odnderstepoort J. Vet. Res. 68:243- 51.
|
|
|
|
|
Meseret A, Sefinew A (2011). Study on clinical bovine dermatophilosis and its potential risk factors in North Western Ethiopia. Int. J. Anim. Vet. Adv. 3(1):33-36.
|
|
|
|
|
Muylle S (2016). Estimation of age by examination of the teeth. In: Allen DG, Constable PD, Dart A, Davies PR, Quesenberry KE, Reeves PT, Sharma JM (Eds). The Merck Veterinary Manual, 11th edition, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Available at:
View
|
|
|
|
|
Quinn PJ, Markey BK, Carter ME, Donnelly WJC, Leonard FC (2002). Veterinary Microbiology and Bacterial Disease. Black Well Science, London. P 381.
|
|
|
|
|
Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, Fitzpatrick ES (2011). Veterinary Microbiology and Microbial Disease, 2nd edition,Wiley-Blackwell, UK.
|
|
|
|
|
Radostits OM, Gay CC, Blood DC, Hinchcliff KW (2000). Veterinary Medicine. A textbook of diseases of cattle, sheep, pigs, goats and horses, 9th edition, Harcourt publishers limited, London Philadelphia. pp. 701-967, 1476-1478.
|
|
|
|
|
Regasa C, Abebe W (2008). Major skin diseases of cattle coming to Nekemte Veterinary Clinic, Western Ethiopia. DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia, 2003.
|
|
|
|
|
Salih DA, El Hussein AM, Singla LD (2015) Diagnostic approaches for tick-borne haemoparasitic diseases in livestock. J. Vet. Med. Anim.Health 7(2):45-56.
Crossref
|
|
|
|
|
Scott DW (2007). Color atlas of farm animal dermatology, 1st edition, Blackwell Publishing Professional, 2121 State Avenue, Ames, Iowa 50014, USA.
|
|
|
|
|
Teshome D, Derso S (2015). Prevalence of major skin diseases in ruminants and its associated risk factors at university of Gondar Veterinary Clinic, North West Ethiopia. J.Vet. Sci. Technol. S:S13-002.
|
|
|
|
|
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996). Veterinary parasitology, 2nd edition, Blackwell publisher, UK.
|
|
|
|
|
Walker AR, Bouattour A, Camics JL, Estradopena A, Horak IG, Latif AA, Pegram RG, Preston PN (2003). Ticks of Domestics Animals in Africa; a Guide to Identification of species, Bioscience reports, Scotland, U.K. pp.1-27.
|
|
|
|
|
Weitzman I, Summerbell RC (1995). The dermatophytes. Clin. Microbiol. Rev. 8(2):240-259.
|
|
|
|
|
Woldemeskel M (2000). Dermatophilosis: A threat to livestock production in Ethiopia. DTW. Deutsche tierarztliche Wochenschrift 107(4):144-146.
|
|
|
|
|
Woldemeskel M, Taye G (2002). Prevalence of bovine dermatophilosis in a tropical highland region of Ethiopia. Trop. Anim. Health Prod. 34:189-194.
Crossref
|
|
|
|
|
Wondwossen A (2000). Sheep and goat skin diseases control initiatives in Amhara region. Ethiopian veterinary epidemiology newsletter. pp. 1-9.
|
|
|
|
|
Yacob HT, Nesanet B, Dinka A (2008). Prevalence of major skin diseases in cattle, sheep and goats at Adama Veterinary Clinic, Oromia regional state, Ethiopia. Revue Méd. Vét.159(8-9):455-461.
|
|
|
|
|
Zewdu S, Tsegaye T, Agerie A (2015). Ectoparasites prevalence in small ruminants in and around Sekela, Amhara Regional State, Northwest Ethiopia. Hindawi Publishing Corporation. J. Vet. Med. 2015:216085.
|
|