ABSTRACT
A preliminary survey was conducted to determine the prevalence of some tick-borne haemoparasitic diseases (TBHDs) and their effects on the haematological parameters of sheep and goats in Maiduguri abattoir. A total of 200 blood samples were collected from sheep (n = 100) and goats (n = 100) from November 2015 to May 2016. Giemsa stained blood smears were prepared and examined under light microscope, to screen for haemoparasites. Packed cell volume (PCV) was determined by microhaematocrit centrifugation technique while haemoglobin (Hb) concentration was determined by Sahli’s method. The total white blood cell (WBC) and red blood cell (RBC) counts were estimated with Neubauer hemocytometer while erythrocyte indices were calculated. The results showed 13.5% overall prevalence of tick-borne haemoparasitic diseases in sheep 13 (6.5%) and goats 14 (7.0%). There was no significant (p>0.05) differences in prevalence of haemoparasites between sexes and age groups of sheep and goats. Anaplasma ovis and Babesia ovis were identified in the study of which A. ovis [23 (11.5%)] was higher (p<0.05) than B. ovis [2 (1.0%)]. A single co-infection of A. ovis and B. ovis was encountered in sheep. The mean values of PCV, Hb and RBC counts of infected sheep were lower (p<0.05) than the uninfected sheep. Similarly, the mean values of Hb and WBC of were significantly (p<0.05) lower in infected goats. This stud has reports important tick-borne haemoparasitic diseases in sheep and goats. We recommend tick control using suitable acaricides, periodic screening and treatment of small ruminants in Maiduguri.
Key words: Anaemia, Anaplasma ovis, Babesia ovis, haemoglobin, packed cell volume, white blood cell count.
Sheep and goats are common household livestock in Nigeria. They are particularly important in the northern region, where a greater proportion can be found (Blench, 1999). Generally, three breeds of goats (Sahel, Sokoto red and West African dwarf) and four breeds of sheep (Balami, Ouda, Yankasa and West African dwarf) are recognized in Nigeria (Blench, 1999).The socio-economic importance of sheep and goats varies in different parts of the country, but they generally have agricultural, cultural and economic values (Lawal-Adebowale, 2012; Adamu and Balarabe, 2012). Most commonly, their flesh is recognized as sources of protein for human consumption, and their hides and skin also generates revenue (Lawal-Adebowale, 2012).
The productivity of sheep and goats in the Sahel zone of Nigeria is threatened by diseases and inclement weather conditions. Among parasitic diseases, sub-clinical gastrointestinal parasitism is responsible for great economic losses (Singla, 1995; Singh et al., 2017a, b). The incidence of parasitic infections with special reference to tick-borne intracellular haemoparasites of the genus Anaplasma, Babesia and Theileria has been linked with significant losses in productivity of small ruminants in the tropics and subtropical areas of the world (Soulsby, 1982; Jatau et al., 2011; Adamu and Balarabe, 2012; Demessie and Derso, 2015; Salih et al., 2015; Sumbria and Singla, 2017). Anaplasma species are mainly transmitted by various species of the genus Amblyomma, Dermacentor, Ixodes and Rhipicephalus (Soulsby, 1982) and occasionally by biting flies of the genus Tabanus (Radostits et al., 2007).
The disease is caused by Anaplasma ovis in small ruminants and is characterized by anaemia, high fever, weight loss, breathlesness, incoordination, abortion and death (Khan, 2005). A. ovis has a worldwide distribution and is responsible for huge losses in sheep and goats stock, with considerable impact on the economy of developing countries in tropics and subtropics, which rely heavily on small ruminant production (Rymaszewska and Grenda, 2008). Babesiosis in sheep and goats is caused by Babesia motasi, Babesia foliata, Babesia taylori and Babesia ovis (Soulsby, 1982). In sheep and goats, the disease is characterized by fever, anaemia, icterus, haemoglobinuria, anorexia, and death (Demessie and Derso, 2015).
Other species of haemoparsites such as Theileria hirci (Metenawy, 1999), Theileria ovis (Okaiyeto et al., 2008), Trypanosoma vivax, T. congolense and T. brucei (Samdi et al., 2008) have also been reported in small ruminants in Nigeria. Sheep and goats contribute significantly to food security and value chain of the Nigerian economy (Lawal-Adebowale, 2011), but their productivity is threatened by ticks and associated haemoparasitic diseases, especially Babesiosis and Anaplasmosis (Okaiyeto et al., 2008; Jatau et al., 2011). This study was therefore conducted to investigate the prevalence of tick-borne haemoparasites and the associated changes in haematological parameters of slaughtered sheep and goats in Maiduguri abattoir.
Study area
This study was conducted in Maiduguri, the capital city of Borno state, located in the Sahel savannah zone of North-eastern Nigeria, between latitude 11°50′48″N and longitude 13°09′25″E of the equator. The climate of Maiduguri is characterized by a short period of rainfall from June to October, followed by a long period of dry season for the rest of the year (Hess et al., 1995). Sheep and goats are among the important household livestock in Maiduguri and its environs. They are mainly raised under traditional semi-intensive or free-range management systems in low income communities (Figure 1).
Sample collection
A total of 200 blood samples was randomly collected from sheep (n=100) and goats (n=100), consisting of 50 males and females. 5 mls of blood was collected immediately after slaughter from the severed jugular vein into vacutainer tubes, containing 1 mg of ethylene diaminetetra-acetic acid (EDTA). The age, sex and specie of each animal were also identified based on morphometric characteristics and recorded in a case book. The samples were transported on icepacks at 4°C to the veterinary parasitology and clinical pathology laboratories, University of Maiduguri for parasitological and haematological examinations.
Laboratory examination
In the laboratory, thin and thick blood smears were prepared on clean glass slides and stained with Giemsa according to standard protocol described by Soulsby (1982), to screen for the presence of haemoparasites. The stained blood films were examined with oil immersion objective (×100) of a compound microscope (Gupta and Singla, 2012). Identification of haemoparasites was performed using morphologic characteristics (Soulsby, 1982). The packed cell volume (PCV) was determined by microhaematocrit method; haemoglobin (Hb) by Sahli’s method; the total white blood cell (WBC) and red blood cell (RBC) counts by Neubauer hemocytometer while erythrocyte indices, mean corpuscular volume (MCV), mean corpuscular haemoglobin(MCH) and mean corpuscular haemoglobin concentration (MCHC) were calculated using standard formula (Brar et al., 2000).
Statistical analysis
Chi Square test was computed with statistical package for social sciences (SPSS) version 22, to determine the prevalence of haemoparasites and its associations with age and sex of sheep and goats. The student’s t-test was performed to determine the difference between mean haematological parameters of infected and uninfected sheep and goats. Significant differences were declared at P<0.05.
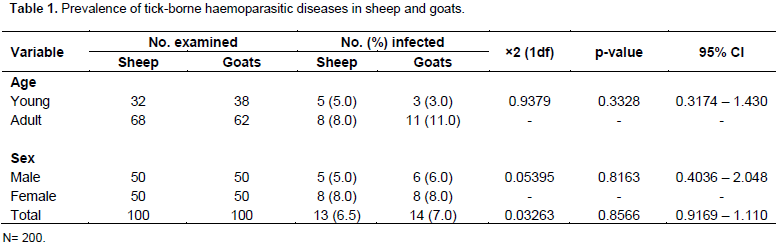
The results obtained from this study has shown that out of 200 blood samples of sheep and goats examined, 27(13.5%) were positive for various tick-borne haemoparasites. The results further revealed 13(6.5%) and 14(7.0%) of infected animals were sheep and goats, respectively. There was no significant difference (p>0.05) in prevalence of tick-borne haemoparasites among the different sexes and age groups of sheep and goats(Table 1). The two species of haemoparasites identified in this study were tick-borne A. ovis and B. ovis. There was no significant difference (p>0.05) in prevalence of infection with haemoparasites in sheep and goats. However, the prevalence of A. ovis (11.5%) in both sheep and goats was significantly (p<0.05) higher than B. ovis (1.0%).
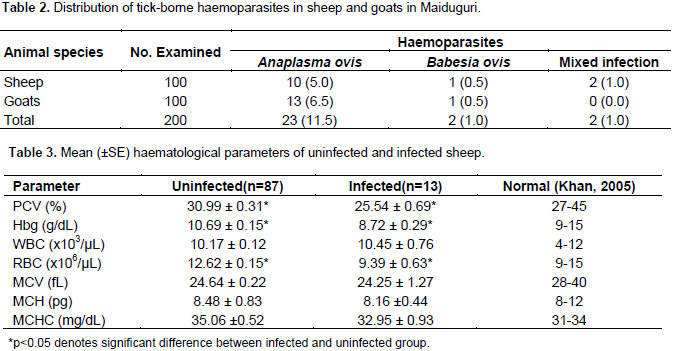
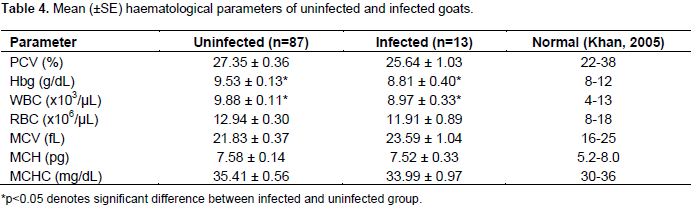
Furthermore, 2(1.0%) sheep had co-infection with A. ovis and B. ovis (Table 2).The mean values of PCV, Hb and RBC counts of infected and uninfected sheep were significantly (p<0.05) different. Furthermore, the mean PCV (25.54 ± 0.69) was below normal range (27 to 45) of values for sheep (Table 3). Similarly, the mean Hb concentration and WBC counts of infected and uninfected goats were significantly (p<0.05) different but fell within normal range of values for goats (Table 4). Other haematological parameters of infected and uninfected sheep and goats were comparable (p>0.05).
The results obtained from our present study revealed that tick-borne haemoparasites are prevalent in both sheep and goats examined at slaughter in Maiduguri. The study further revealed a numerically higher prevalence in female adult sheep and goats than their counterparts. The occurrence of haemoparasites in both sheep and goats in this study may be associated with previous reports on high prevalence of ixodid ticks in livestock in Maiduguri and environs (James-Rugu and Jidayi, 2004; Oparah and Ezeh, 2011; Musa et al., 2014; Paul et al., 2017). Ticks of the genus Amblyomma, Rhipicephalus including subgenus Boophilus, Hyalomma and Dermacentor, which are potential vectors of Anaplasma and Babesia species in sheep and goats were previously reported in Borno state (James-Rugu and Jidayi, 2004; Oparah and Ezeh, 2011; Musa et al., 2014; Paul et al., 2017). Furthermore, the sheep and goats slaughtered in Maiduguri are raised under extensive and semi-intensive management systems in outdoor environments graze alongside with cattle. These increase their exposure to the arthropod vectors.
Both A. ovis and B. ovis were identified in this study. A. ovis was the most prevalent species in both sheep and goats. This finding agrees with previous reports (Okaiyeto et al., 2008; Jatau et al., 2011; Adamu and Balarabe, 2012). The prevalence of these parasites elsewhere in Nigeria was linked with suitable microclimate favouring the propagation of their arthropod vectors (Jatau et al., 2011). Similarly, the prevalence of haemoparasites in slaughtered cattle in Maiduguri was linked with conditions favouring the bionomics of ixodid ticks (Paul et al., 2016). Moreover, A. ovis is a ubiquitous organism that has been reported in all the six continents (Rymaszewska and Grenda, 2008) and especially in the tropics and subtropics, due to the abundance of its tick vectors (Jongejan and Uilenberg, 2004). The low prevalence of B. ovis recorded in both sheep and goats in this study agreed with previous reports (Bell-Sakyi et al., 2004; Jatau et al., 2011).
This finding could be attributed to the enzootic occurrence of babesiosis in indigenous animals in Nigeria. Sheep and goats usually develop strong immunity in early life and resist subsequent challenges favourably by preventing establishment of the parasite (Soulsby, 1982). This study revealed that, infection with haemoparasites in sheep caused a significant (p<0.05) reduction in PCV, Hb and total RBC counts. Furthermore, our results show a significant (p<0.05) reduction in the Hb concentration and total WBC count of goats. The anaemia observed in this study characterized by a reduction in PCV and Hb concentration of infected sheep and goats, is consistent with previous reports. Anosa (1988) reported that, anaemia is a predominant feature that often serves as a reliable indicator for severity of haemoparasitic infections. Rymaszewska and Grenda (2008) observed that progressive anaemia usually develops during anaplasmosis and babesiosis.
Furthermore, Anumol et al. (2011) reported that haemoparasites are responsible for most cases of anaemia in goats. The pathogenesis of anemia in haemoparasitic infections is multifactorial in nature; emergence of parasites from RBC, mechanical rupture of RBC, spontaneous lysis of RBC due to increased osmotic fragility, direct removal of non-infected erythrocytes by phagocytosis and adsorption of circulating antigen-antibody complexes to the surface of RBC, leading to their removal by phagocytosis as described by Soulsby (1982). The observed reduction in WBC counts of infected goats in this study has been previously reported. A significant reduction in total WBC counts of dromedary camels infected with babesiosis in Saudi Arabia was described by Swelum et al. (2014). This finding could be linked with concurrent infections and stress which may lead to immune suppression. Helminthosis and bacterial infections are usually encountered concurrently with haemoparasitic infections under field conditions in Nigeria (Okaiyeto et al., 2008; Jatau et al., 2011), which may complicate the clinical course of haemoparasitic infections.
This study has revealed the presence of important tick-borne haemoprotozoan parasites in slaughtered sheep and goat in Maiduguri. The prevalence of A. ovis and B. ovis was accompanied by anaemia in sheep and goats. This finding suggests that A. ovis and B. ovis are common causes of anaemia leading to decreased productivity of sheep and goats in Maiduguri and environs.
Molecular studies used to characterize Anaplasma and Babesia genotypes and specific tick vectors responsible for the transmission of these parasites in Maiduguri, in other to aid proper planning of effective control measures is recommended. Meanwhile, vector control with effective acaricides and the periodic screening and treatment of sheep and goats in Maiduguri with suitable antiprotozoal drugs will reduce the impact of tick-borne haemoparasitic diseases which enhance their productivity.
The authors have not declared any conflict of interests.
This work was made possible with the full support received from the Veterinary officer in charge of Maiduguri central abattoir, to which we are very grateful. We are especially grateful to Mallam Ismaila Gadaka in the Veterinary Clinical Pathology Laboratory and Mallam Ya’uba Mohammed in the Parasitology and Entomology Laboratory, both in the Faculty of Veterinary Medicine, for their technical support in the laboratory analysis of the samples.
REFERENCES
|
Adamu BS, Balarabe LM (2012). Prevalence of haemoparasites of sheep and goats slaughtered in Bauchi Abattoir. Int. J. Appl. Biol. Res. 4(1&2):128-133.
|
|
|
|
Anosa VO (1988). Haematological and biochemical changes in human and animal Trypanosomosis. Part 1 Revue d'Elevage Medicine Veterinarie des pays Tropicaux 41:65-78.
|
|
|
|
|
Anumol J, Tresamol PV, Saranya MG, Vijayakumar K, Saseendranath MR (2011). A study on aetiology of anemia in goats. J. Vet. Anim. Sci. 42:61-63.
|
|
|
|
|
Bell-Sakyi L, Koney EBM, Dogbey O, Walker AR (2004). Ehrlichia ruminantum seroprevalence in domestic ruminants in Ghana. I. Longitudinal survey in the greater Accra region. Vet. Microbiol. 100:308-313.
Crossref
|
|
|
|
|
Blench R (1999). Traditional Livestock Breeds: Geographical distribution and dynamics in relation to the ecology of West Africa: Overseas Development Institute Portland House Stag Place London. 122:7-21.
|
|
|
|
|
Brar RS, Sandhu HS, Singh A (2000). Veterinary Clinical Diagnosis by Laboratory Methods (1st Ed) India: Kaylani Publishers. pp. 29-150.
|
|
|
|
|
Demessie Y, Derso S (2015). Tick Born Diseases of Ruminants: A review. Adv. Biol. Res. 9(4):210-224.
|
|
|
|
|
Gupta SK, Singla LD (2012). Diagnostic trends in parasitic diseases of animals. In: Veterinary Diagnostics: Current Trends. Gupta RP, Garg SR, Nehra V and Lather D (Eds), Satish Serial Publishing House, Delhi. pp. 81-112.
|
|
|
|
|
Hess TM, Stephens W, Maryah UM (1995) Rainfall trends in the North East Arid Zone of Nigeria 1961-1990. Agric. For. Meteorol. 74:87-97.
Crossref
|
|
|
|
|
James-Rugu NN, Jidayi S (2004). A survey on the ectoparasites of some livestock from some areas of Borno and Yobe States. Niger. Vet. J. 25 (2):48-55.
|
|
|
|
|
Jatau ID, Abdulganiyu A, Lawal AI, Okubanjo OO, Yusuf HK (2011). Gastrointestinal and haemoparasites of sheep and goats at slaughter in Kano, Northern Nigeria. Sokoto J. Vet. Sci. 9(1):7-11.
|
|
|
|
|
Jongejan F, Uilenberg G (2004). The global importance of ticks. Parasitology 129: 129(S1):S3-S14.
|
|
|
|
|
Khan CM (2005). The Merck veterinary Manual. 9th ed. USA: Merck and Company Incorporated: pp.18-32.
|
|
|
|
|
Lawal-Adebowale AO (2012). Dynamics of ruminant livestock management in the context of Nigerian Agricultural System. InTech. pp. 61-80.
Crossref
|
|
|
|
|
Metenawy TM (1999). Blood parasites of sheep and goats. Parkistan Vet. J. 19(1):43-45.
|
|
|
|
|
Musa HI, Jajere SM, Adamu NB, Atsanda NN, Lawal JR, Adamu SG, Lawal EK (2014). Prevalence of tick infestation in different breeds of cattle in Maiduguri, North-eastern Nigeria. Bangladesh J. Vet. Med. 12(2):161-166.
Crossref
|
|
|
|
|
Okaiyeto SO, Tekdek LB, Sackey AKB, Ajanusi, OJ (2008). Prevalence of haemo and Gastrointestinal parasites in sheep kept by the Nomadic Fulanis in some Northern states of Nigeria. Res. J. Anim. Sci. 2(2):31-35.
|
|
|
|
|
Opara MN, Ezeh NO (2011). Ixodid ticks of cattle in Borno and Yobe States of Northeastern Nigeria: breed and coat colour preference. Anim. Res. Int. 8(1):1359-1365.
|
|
|
|
|
Paul BT, Bello AM, Haruna NM, Dauda J, Ojo TD, Gadzama MA (2017). Infestation of Zebu cattle (Bos indicus Linnaeus) by hard ticks (Acari: Ixodidae) in Maiduguri, North eastern Nigeria. Persian J. Acarol. 6(3):213-224.
|
|
|
|
|
Paul BT, Bello AM, Ngari O, Mana HP, Gadzama MA, Abba A, Malgwi KD, Balami SY, Dauda J, Abdullahi AM (2016). Risk factors of haemoparasites and some haematological parameters of slaughtered trade cattle in Maiduguri, Nigeria. J. Vet. Med. Anim. Health 8(8):83-88.
Crossref
|
|
|
|
|
Radostits OM, Gay GC, Hinchiff KW, Constable PO (2007). Veterinary Medicine: A text book of the disease of cattle, sheep, goat, pigs and horses. 10th ed. Saunders Elsevier London, New York pp. 2047-1533.
|
|
|
|
|
Rymaszewska A, Grenda S (2008). Bacteria of the genus Anaplasma- characteristics of Anaplasma and their vectors: A Review. Vet. Med. 53(11):573-584.
|
|
|
|
|
Salih DA, Hussein AM El, Singla LD (2015) Diagnostic approaches for tick-borne haemoparasitic diseases in livestock. J. Vet. Med. Anim. Health 7(2):45-56.
Crossref
|
|
|
|
|
Samdi S, Abenga JN, Fajinmi A, Kalgo A, Idowu T, Lawani F (2008). Seasonal variation in Trypanosomosis Rates in small Ruminants at the Kaduna Abattoir, Nigeria. Afr. J. Biomed. Res. 11:229-232.
|
|
|
|
|
Singh E, Kaur P, Singla LD, Bal MS (2017a). Prevalence of gastrointestinal parasitism in small ruminants in western zone of Punjab, India. Vet. World 10(1):61-66.
Crossref
|
|
|
|
|
Singh R, Bal MS, Singla LD, Kaur P (2017b). Detection of anthelmintic resistance in sheep and goat against fenbendazole by faecal egg count reduction test. J. Parasitic Dis. 41(2):463-466.
Crossref
|
|
|
|
|
Singla LD (1995). A note on sub-clinical gastro-intestinal parasitism in sheep and goats in Ludhiana and Faridkot districts of Punjab. Indian Vet. Med. J.19:61-62.
|
|
|
|
|
Sumbria D, Singla LD (2017). Thwack of Worldwide Weather Transformation on Vector and Vector-Borne Parasitic Infections. ARC J. Anim. Vet. Sci. 3(2):1-10.
|
|
|
|
|
Soulsby EJL (1982). Helminths Arthropods and Protozoa of Domesticated Animals 7th ed. London, U.K: Bailliere Tindall. P 809.
|
|
|
|
|
Swelum AA, Ismael AB, Khalaf AF, Abouheif MA (2014. Clinical and laboratory findings associated with naturally occurring babesiosis in dromedary camels. Bull. Vet. Institute Pulawy. 58(2):229-233.
Crossref
|
|