ABSTRACT
Very little information is available in Algeria on Q fever and chlamydial abortion sheep, two zoonosis caused by Coxiella burnetii and Chlamydophila abortus and their main reservoirs are domestic ruminants. This study aimed at investigating the seroprevalence of these two diseases in sheep flocks from six Daïra (Telagh, Tanira, Moulay Slissen, Marhoum, Ras Elma and Merine). A serological survey was conducted in 39 flocks with a history of abortions, which were classified by size. A total 180 sera were collected from the aborted ewes. Q fever indirect ELISA kit and C. abortus indirect ELISA kit (ID Screen®) kits were used to know the percent prevalence in sheep. The results showed that 28% (N = 50/180) of sheep were seropositive for Q fever and 31% (N = 55/180) of sheep were seropositive for chlamydial abortion. Twenty eight herds (72%) showed at least one seropositive animal for Q fever and 29 herds (74%) showed at least one seropositive animal for chlamydial abortion. Larger herds led to more infected herds of small and medium for Q fever. These results showed that infection with Q fever and chlamydial abortion were common in the study area, therefore encouraging efforts are needed to propose measures to reduce the spread and zoonotic risk.
Key words: Q fever, chlamydial abortion, seroprevalence, enzyme-linked immunosorbent assay (ELISA), zoonosis.
Chlamydial abortion and Q fever are two zoonoses caused by two small obligate intracellular Gram-negative bacteria, Chlamydophila abortus and Coxiella burnetii (Maurin and Raoult, 1999; Rodolakis, 2006: Aitken and Longbottom, 2007) which grow in the cytoplasm of eukaryotic cells. They are the most important causes of reproductive failure in sheep and goats (Berri et al., 2001, 2005; Arricau-Bouvery and Rodolakis, 2005). These infections cause abortion, stillbirth, delivery of weak offspring and infertility in the small ruminant (Rodolakis et al., 2004; Agerholm, 2013). The losses caused by these two agents evaluated on several levels. In economic terms, the non-sale of the product (lamb or goat), the non-renewal of young breeding (antenaise or goat) and decreased milk production (dairy farming) are the most negative impact on the scale of livestock. In health terms, the main concerns are the risk of contamination of several lots on livestock, as well as professional zoonotic transmission. Both agents were the subject of increasing research from the years 2002 in small ruminants, due to their proven implication in human focus (Wallenstein et al., 2010).
The present study attempted to investigate the prevalence of chlamydial abortion and Q fever at the level of district Sidi Belabbes through sero-prevalence studies in flocks that have experienced abortions and correlate its possible association with managerial (flock size, region, type of farming, and contact with other flocks) risk factors. In conjunction, the study try to clarify the interpretation of complementary investigations required by veterinary practitioners by confirming diagnostic tools available for both agents searched.
Animals and blood sampling
This study was carried out from September to December 2013 (season of lambing); this study was based on the declaration of 1, 2 or 5 abortions on a period less than or equal to 4 weeks as equivalent to an abortion episode. While those with two reported abortions at 5 weeks of interval were eliminated from this study.
From 39 sheep flocks, a total of 180 blood samples were collected by jugular venous puncture in 4 ml sterile vacutainer tubes using Tubes BD Vacutainer® secs ”BD, France” from aborted ewes aged between 1 and 6 years. After storage at room temperature for 1 h, blood samples were centrifuged at 3000 rpm for 5 min at room temperature. Sera were carefully harvested and stored at -20°C until assayed. Selected flocks ranged in size from 42 to 450 sheep. Flocks sizes were <100, 100-200 and >200 sheep for 6, 22 and 11 flocks, respectively, in order to establish the sero-prevalence of chlamydial abortion and Q fever via an indirect diagnosis. All flocks visited only once, no vaccine against chlamydial abortion or Q fever used.
Serological techniques
For the detection of antibodies against C. burnetii and C. abortus, two different ELISAs were used. For Q fever, indirect ELISA kit and C. abortus indirect ELISA kit (ID Screen® France) were used. Positive and negative control provided by the manufacturer and an internal positive laboratory reference was included in each test. Results were expressed as a percentage of the optical density reading of the test sample (% OD) calculated as % = 100 × OD sample / OD positive control. Recommended readings OD%<40 as negative, OD%>40, OD%<50 as doubtful, OD%>50<80% as positive and OD>80 highly positive for C. burnetii, and OD%<50 as negative, OD%>50<60 as doubtful, and OD%>60 as positive for C. abortus.
Statistical analysis
All data were entered and validated using a Microsoft Excel package. To bring out the association between a supposed risk factor and the disease, the odds ratio (OR) and relative risk (RR) were calculated.
The odds ratio is the probability of having the disease according to the presence or absence of risk factors and allows for addition to the degree of significance of the association, the direction and strength of the association.
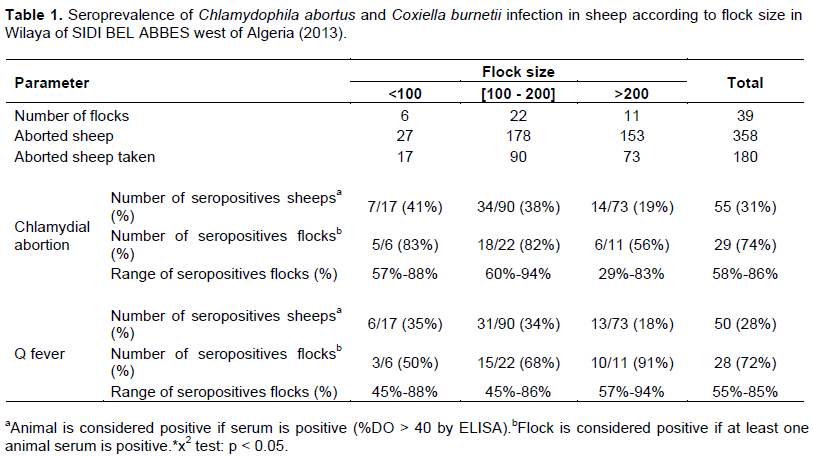
According to the experimental design, all 39 flocks studied had a history of abortions and stillbirths, 180 aborted ewes belonging to these 39 flocks were examined for antibodies against C. abortus and C. Burnetii. The seropositivity results towards for these two bacteria obtained in aborted sheep at individual and flock levels were summarized in the Table 1.
At the farm level, 74% (29/39, 95% CI: 58 to 86) of farms had at least one seropositive sheep to C. abortus and 72% (28/39, 95% CI: 55-84) of farms had at least one seropositive sheep to C. burnetii. The seroprevalence of C. abortus infection in ewes is not associated (P > 0.05) with the three flocks size groups, it was the same for C burnetii. The seroprevalence rate ranged from 29 to 88% and from 45 to 95% for C. abortus and C burnetii, respectively.
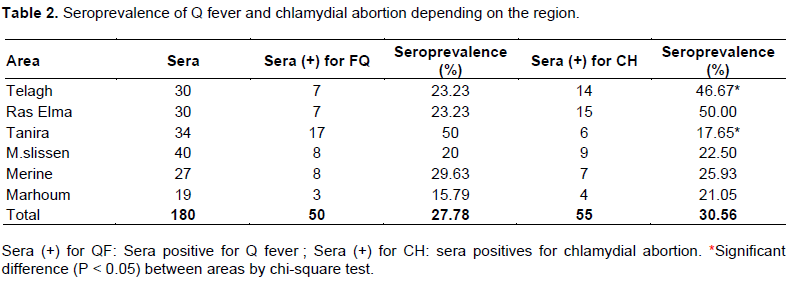
Overall, the sheep level seroprevalence was 31% (55/180, 95% CI: 23, 92 -37, 84) for C. abortus and was 28% (50/180, 95% CI: 21, 37 -34, 93) for C. burnetii. In sheep level, there was signiï¬cant difference (P < 0.05) between the seroprevalence of chlamydial infection and the location (Table 2). The highest prevalence rate (46.67%) of chlamydial infection was observed in Telagh area, while the lowest rate (17, 65%) was observed in Tanira area.
Exposure of sheep to C. abortus and C. burnetii was evaluated by testing for the presence of antibodies with an indirect ELISA test. The detected antibodies in this study imply a natural response to exposure to the microorganisms because there is no vaccination program against ruminant chlamydiosis or Q fever in Algeria before. The survey design provided data on seroprevalence at the flock and the animal level in this area. Results of the present study revealed an animal-level seroprevalence to C. abortus of 31% and to C. burnetii of 28% in Sidi Bel Abbes region, Algeria. This figure is higher than that reported from other Algerians regions (Khaled et al., 2016; Hireche et al., 2014; Merdja et al., 2014). Also, several authors had previously reported high prevalence in Maghreb countries such as Marocco (El Jai et al., 2003), Tunisia (Russo et al., 2005), Egypt (Abdel-Moein and Hamza, 2017) and in world countries such as Turkey (Kennerman et al., 2010), Italy (Francesca et al., 2016), Slovakia (Trävnicek et al., 2001), Spain (Mainar-Jaime et al., 1998) and Jordan (Al-Qudah et al., 2004). The overall seroprevalence rate at the flock level in our survey was 74% to chlamydial abortion and 72% to Q fever. These rates are higher than those reported by Francesca Rizzo et al. (2016) in flocks 38% in Italy and Angela et al. (2012) with 28% in Germany. However, due to numerous parameters such as differences in study design and inclusion criteria (e.g. high abortion rates), flock size and management, prevalence of other abortifacient agents (e.g. Brucellae, Salmonellae, Toxoplasma, Chlamydia, Campylobacter) it is virtually impossible to compare the present study’s prevalence findings with the aforementioned studies (Masala et al., 2004).
A higher prevalence rate was revealed for flocks with more than 200 animals compared with that of small flocks (91 and 50%, respectively) for Q fever, while, no significant correlation was revealed between flock size and the rate of seroprevalence for chlamydial abortion. The difference rate of seroprevalence revealed for the flock high size for Q fever might be that due to animal overcrowding in livestock buildings. Also may be related to the high number of lambing at lambing season, which increases the total population at risk and, subsequently, the risk of pathogen introduction and transmission, where high density may influence animal welfare and the occurrence of infectious diseases. The study showed that there is a signiï¬cant difference (P < 0.05) between chlamydial infection in sheep and areas of northwest of Algeria (Table 2). Tanira and Ras Elma areas having the highest rate of chlamydial infection in sheep may be explained by the behavior of these species breeding in these sites (frequency of herding group belonging to several farmers in the same village). These factors favor the rapid spread of infection.
The geographic distribution of C. burnetii and C. abortus indicate that both pathogens are present throughout the district of Sidi Bel Abbes. The highest percentage of positive samples was found for chlamydial abortion in Ras Elma (50%), and for Q fever in Tanira (50%). It seems that abortions in sheep following infection with C. burnetii and C. abortus have a higher frequency, even in young animals. Q fever and chlamydial abortion are a public health problem in Algeria. To for better control both in animals and humans, veterinary and public health sector should strengthen their collaboration for the establishment of a national program to fight against the major zoonoses in general and against Q fever and chlamydial abortion in particular.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Moein KA, Hamza DA (2017). The burden of Coxiella burnetii among aborted dairy animals in Egypt and its public health. acta tropica.166:92-95.
|
|
|
|
Agerholm JS (2013). Coxiella burnetii associated reproductive disorders in domestic animals- a critical review. Acta Vet. Scand. 55(1):13.
Crossref
|
|
|
|
|
Aitken ID, Longbottom D (2007). Chlamydial abortion. In: Aitken, I. (Ed.), Diseases of Sheep. Blackwell Publishing Ltd., Oxford. pp. 105-112.
Crossref
|
|
|
|
|
Al-Qudah KM, Sharif LA, Raouf RY, Hailat NQ, Al-Domy FM (2004). Seroprevalence of antibodies to Chlamydophila abortus shown in Awassi sheep and local goats in Jordan. Vet. Med.-Czech 12:460-466.
|
|
|
|
|
Angela H, Gernot S, Hannah L, Udo M, Roland D, Andreas F, Lothar H, Steffen H, Michael E, Herbert T, Klaus H, Heinrich N, Lisa DS (2012). Prevalence of Coxiella burnetii in clinically healthy German sheep flocks. BMC Research Notes. 5(1):152.
Crossref
|
|
|
|
|
Arricau-Bouvery N, Rodolakis A (2005). Is Q fever an emerging or reemerging zoonosis? Vet. Res. 36:327-349.
Crossref
|
|
|
|
|
Berri M, Crochet D, Santiago S, Rodolakis A (2005). Spread of Coxiella burnetii infection in a flock of sheep after an episode of Q fever. Vet. Rec. 157(23):737-740.
Crossref
|
|
|
|
|
Berri M, Souriau A, Crosby M, Crochet D, Lechopier P, Rodolakis A (2001). Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet. Rec. 148 :502-505.
Crossref
|
|
|
|
|
El Jai S, Bouslikhane M, El Idrissi AH (2003). Suivi épidémiologique des avortements de petits ruminants dans les zones pastorales du Maroc. Revue Marocaine des Sciences Agronomiques et Vétérinaires 23(2):95-100.
|
|
|
|
|
Francesca R, Nicoletta V, Marco B, Vitaliano B, Camilla L, Laura C, Maria LM (2016). Q fever seroprevalence and risk factors in sheep and goats in northwest Italy. Prev. Vet. Med. 130:10-17.
Crossref
|
|
|
|
|
Hireche S, Bouaziz O, Djennad D, Boussen S, Imgur R, Kabouia R, Bererhi E (2014). Seroprevalence and risk factors associated with Chlamydophila spp. infection in ewes in the northeast of Algeria. Trop. Anim. Health Prod. 46:476-473.
Crossref
|
|
|
|
|
Kennerman E, Rousset E, Gölcü E, Dufour P (2010). Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comp. Immunol. Microbiol. Infect. Dis. 33(1):37-45.
Crossref
|
|
|
|
|
Khaled K, Sidi-Boumedine H, Merdja S, Dufour P, Dahmani A, Thiéry R Rousset E, Bouyoucef A (2016). Serological and molecular evidence of Q fever among small ruminant flocks in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 47:19-25.
Crossref
|
|
|
|
|
Mainar-Jaime RC, De La Cruz C, Vázquez-Boland JA (1998). Epidemiologic study of chlamydial infection in sheep farms in Madrid, Spain. Small Rumin. Res. 28:131-138.
Crossref
|
|
|
|
|
Masala G, Porcu R, Sanna G, Chessa G, Cillara G, Chisu V, Tola S (2004). Occurrence, distribution, and role in abortion of Coxiella burnetii in sheep and goats in Sardinia, Italy Vet. Microbiol. 99:301-305.
Crossref
|
|
|
|
|
Maurin M, Raoult D (1999). Q fever. Clin. Microbiol. Rev. 12:518-553.
|
|
|
|
|
Merdja S-E, Khalid H, Dahmani A, Bouyoucef A (2015). Chlamydial abortion in algerian small ruminants. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Vet. Med. 72(1):23-26.
|
|
|
|
|
Rodolakis A (2006). Chlamydiosis and Q fever, similarity and difference between these two zoonoses. Renc. Rech. Ruminants, 13:365-401. Available at :
View
|
|
|
|
|
Rodolakis A, Berri M, Rekiki A (2004). Le point sur le diagnostic et la prevention de la chlamydiose et la fièvre Q Journée nationales GTV, Tours, 751-754.
|
|
|
|
|
Russo PS, Pepin M, Rodolakis A, Hammami S (2005). Enquête sérologique sur les principales causes d'avortements infectieux chez les petits ruminants en Tunisie. Revue Méd. Vét. 156(7):395-401.
|
|
|
|
|
Trävnicek M, Kovacova D, Zlbricky P, Cislakova L (2001). Serosurvey of sheep and goats to Chlamydia psittaci in Slovakia during the years 1996-2000. Vet. Med. -Czech 46:281-285.
|
|
|
|
|
Wallenstein A, Moore P, Webster H, Johnson C, Van derburgt G, Pritchard G, Ellis J, Oliver I (2010). Q fever outbreak in Cheltenham, United Kingdom, in 2007 and the use of dispersion modelling to investigate the possibility of airborne spread. Euro. Surveill. 15(12):19521.
|
|