ABSTRACT
Cross-sectional study was conducted from December, 2008 to April, 2009 to determine the sero prevalence of foot and mouth disease (FMD) virus in West and South West Shoa zones of Oromia regional state, central Ethiopia. The samples were processed with the 3 ABC ELISA kits that can able to identify natural infected animal from vaccinated animals. From the total sera of 421 tested, the overall sero prevalence of FMD in cattle was 15.0% (63/421). The prevalence rate was higher (22%) in the South west shoa zone than West shoa zone (5.6%). The difference was statistically significant (ρ<0.05). At district level, the highest sero prevalence were recorded at Dawo (26.7%), Alemgana (26.2%), Kokir (24.4%) and Haramaya (21.4%) districts, which were located at south west shoa zone. The difference among districts were statistically significant (ρ<0.05). Statistically difference was also observed between different age groups (ρ<0.05); being higher in adult (19.3%) followed by calves (9.3%). The sero prevalence of male and female were 16.0 and 14.1%, respectively, and which was not statistically significant (ρ>0.05). The sero prevalence of FMD was found higher in mid highland (16.8%) than highland attitude (6.7%) which was statistically significant (ρ<0.05). The result of this study indicated that FMD is highly prevalent in the South West Shoa zone than West Shoa zone due to contact of different origin of animals, and free animals movement in search of feed and water in zone. Age and attitude were also found as an important risk factor for the diseases. Finally, it is recommended that, sero typing of the virus circulating in the study area should be undertaken for effective control of the disease.
Key words: FMD, Sero prevalence, West Shoa, South west shoa, 3 ABC ELSA.
Ethiopia has the largest livestock inventories in Africa, possessing more than 31 million cattle, 48 million small ruminants, 1.5 million camels, 7 million equines and 52 million chickens (Desta,1999). Livestock is an integral part of all farming system in it. They provide milk, meat, hides and skin, and the drought power required for tilling the farm land (Asseged, 2005). However, due to the higher prevalence of different diseases like foot and mouth diseases (FMD) and poor management system, the country is not utilizing this huge potential in livestock resource (Hussen, 2006). FMD, which is also known as aphthous fever (Kahrs, 2001), is a major global animal health problem (Murphy et al., 1999). It is the most contagious transboundary animal disease (TAD) affecting cloven hoofed animals (FAO, 2007) characterized by the formation of vesicles in the mouth, at coronary band and skin of inter digital cleft (Radostits et al., 2000).
The disease is an infection, worldwide in incidence, caused by an entero virus of the genus aphtho virus, family picornaviridae which occurs as seven major A, O, C, SATI, SAT2 , SAT3 and Asia 1 (Radostits et al., 2000). Each serotypes of FMD virus is antigentically district (Kitching, 1987). All the seven (7) serotype produce a disease that is clinically indistinguishable but immunologically district and infection with one serotypes does not confer immunity against the other (Richard, 1998). According to the office of international des epizootics (OIE, 1990), FMD ranks first among the notifiable infection disease of animals (OIE, 2000). The disease is notoriously contagious that it can be spread as much as 50 (fifty) miles downwind from one out break area to another (Sainsbury, 2000). Introduction of the virus types or subtypes to regions where they were previously absent, lead to epidemics of varying magnitude (Gibbs, 1981). When the disease breaks out in susceptible cattle, it spreads very rapidly, and the morbidity rates approximate 100%. The disease is rarely fatal expect in young anima (Kahan and Scottlline, 2005).
The disease has an incubation period of 3 to 14 days, and excretion of the virus from infected animals in all secretion and excretion usually begins before the appearance of the visible clinical signs (kitching and Donaldson, 1987). Initial virus multiplication occurs mainly in the pre-pharyngeal area and the lungs (Burrows et al., 1981). Acutely infected cattle salivate profusely and develop a nasal discharge (mucoid and muco purulent). Following pyrexia (about 40°C) vesicles appears on the dorsum of the tongue, hard palate, dental pad, lips, gums, muzzle, coronary band and inter digital space with consequent lameness. The lesions are susceptible to secondary bacterial infection. At this stage, it also include linking, cannot eat and move. Other signs include licking of the feet or shifting weight from one leg to other, holding one hoof off the ground, lagging behind the herd, lying down and reluctance to rise (Woodbury, 1995). Vesicles may also be seen on the teats of lactating animals.
Morbidity is high and young caves may die before the appearance of clinical signs due to virus infection of the developing heart muscle and the production of a severe myocarditis (Woodbury, 1995). However, most animal recover within 2 weeks. FMD has a great potential for causing severe economic loss in susceptible cloven-hoofed animals (OIE, 2000). Greater losses can result from refusal of FMD free countries to import livestock and livestock products from infected regions (Kahrs, 2001). Adult mortality is not very high but causes heavy economic losses (losses of flesh, diminished milk production, mastitis and calf mortality and infertility etc). This combined with the time and money spent treating animals, and their long convalescence contributes to consider it as the most important animal disease in a world context (Solomon, 1980). The disease occasionally transmitted to humans causing a self limited, febrile illness, characterized by pain in the limps excessive salivation and the appearance of vesicular lesions on the buccal or lingual epithelium and on the skin of hand, feet and other parts of the body (Lennette et al., 1979).
Very few cases have been reported even among people working with infected carcasses and laboratories. However, humans can also be vehicle for transmission of the disease to animals (Radostits et al., 2000). No specific treatment exist for FMD; however, proper animal husbandry practice and treatment of secondary bacterial infection reduce losses (Hirsh and Zee, 1996), treatment with mild disinfectants and protective dressing to inflamed areas to prevent secondary bacterial infection is recommended in endemic countries where slaughter policy is not in force. If, however a disease outbreak occurs in suckling calves, no treatment will be possible (Seifert, 1990). Given the law absolute production of pastoral herds compared with commercial or semi commercial dairy with, some workers assume that FMD is relatively minor disease in pastoral. However, at certain time of years pastoralist rely heavily on milk for food and therefore, they often prioritize FMD due to its impact on milk supply. They also associate FMD with mortality in claves and “Chronic FMD” cases showing heat intolerance, reduced fertility and other signs (Rufael, 2006).
There is no reliable figure for the prevalence of FMD in different countries. The disease generally occurs in the form of an outbreak that rapidly spread from herd to herd before it is controlled (Radostits et al., 2000). The disease is endemic in Ethiopian, the main incidence to the stress of harvesting and trashing (Solomon, 1980). The occurrence of FMD in Ethiopia is increasing, and in 1999 almost 10% of cattle were under risk of infection, and in 2000 and 2001 a total of 27 and 88 disease outbreaks were reported, respectively (Esayas et al., 2005). Four of the seven serotype of FMD of virus were recorded in Ethiopia. The four identified serotype were O, A, C and SAT2. SAT2 was first identified in 1989 from bovine sample collected from Leben Ranch, Borena area, southern Ethiopia. Similarly, serotype C identified in 2005 from virus was identical to a virus identified in 1971.
Serotype SAT1 and 3 have not been identified until 2005 (Esayas et al., 2005). Similarly, from the sample recover in 2005 by the WRL for FMD at institute of animal health, pirbright (UK), 22 types O,9 types A and 4 types C were recovered from Ethiopia. Therefore, FMD is an highly contiguous disease, it is a very serious disease as it spreads rapidly, causes large scale economic losses and halt exports of all animals products as well as agricultural products used as animal feed in the country (Bouxton, 1977). Although, numerous researches were conducted on sero prevalence of FMD in the country, still there is lack of information in the study area. This is due to the controlling of FMD in endemics area that requires a good understanding of the status of the disease within the zones. Hence, this study was conducted to meet the following objectives:
1. To determine the Sero prevalence of foot and mouth disease in selected districts of West and South West Shoa zones.
2. To identify major risk factors of the disease.
Study areas description
The study was conducted in South West and West Shoa zones of Oromia regional state, Ethiopia which located in West of Addis Ababa. The Zones have got a total land area of about 2.17 million hectare of land, and divide into three agro climatic zone. The low land located below 1500 m a.s.l which caver 17% of the total land area, the mid high land attitude from 1500 to 2500 m a.s.l covers 61% of the total land area and the high land cool temperature located above 2500 m a.s.l that covers 22% of the study area . The study area has two rainy seasons, the long rainy season covering most of the place and occurs from June to September and the short rainy season occurs March to April with an average annual rain fall being 2900 mm. It is presumed generally that the climate of the study area is suitable for both agriculture and livestock production.
The livestock population of the study area is estimated at 2.3 million of cattle, 619,000 Sheep, 172,000 Goat, 283,000 Equine and 1.4 million poultry. Agriculture, which is the main economy sector of activities of the zones, provides livelihood for more than 90% of the population. Almost 85% of the total land coverage used for crop production where 15% is used for animal grazing. Animal health problems specially infections disease such as FMD, pasteurellosis, lumpy skin disease are important in the study area due to increase livestock population, transhumance way of movements from high land to low land area during long rainy season for season for grazing and low level of veterinary services. In addition external, internal parasites and tryp.\anomiasis are of considerable economic importance of veterinary concern in the area.
The study animals
The study conducted on cattle that were kept under majority of livestock production system in the Zones. Study animals were selected from West Shoa Zone of cattle population in ten (10) districts of West Shoa Zone namely: Welmera, Dendi, chelia, Ejere, Ambo, Nano, Metarobi, Jaldu Ada’aBarga, and Bake and from South West Shoa Zone cattle in seven (7) districts namely: Kersa Kondaltit, Alemgana, Dawo, Kokir, Wonchi,Weliso and Hamaya from which 180 and 241 animals were selected, respectively. Approximately 15 animals from each peasant associations (PAs) were selected randomly to be included in the study. Accordingly, 28 Pas and 421 animals were included in the study.
Sample size determination
The study was conducted on cattle that were kept under mixed farming system in which the sample size was determined by considering a prevalence of 50% to get the maximum number required to determine the prevalence in simple random sampling because there was no previous work of FMD in the study area. The precision was decided to be 5 to 95% confidence level. The sample size was estimated by the formula described by Thrusfield, (1995).

Even if 384 were the minimum sample size required, 421 cattle from West and south West Shoa zones area were considered in the study prevalence of FMD.
Study Animals and Characterization
A total of 421 samples were randomly collected from animals presented to grazing area. Animals were grouped into three categories based on agro climatic zone, age groups and sex. The animals were classified into three age groups (6 month to 2 years, 2 to 4years and >4years) according to Ken and Tony (1993).
Study design and methods
Study design
A cross sectional study was under taken from December, 2008 to April, 2009. During the laboratory work, a total of 421 sera samples collected from herds of West and South West Shoa zones of cattle, and were examined using 3 ABC ELISA for detection of FMD antibodies.
Sample collection and submission
Cattle blood samples were collected from mixed farming system of herds of cattle in South West and West Shoa zones for analysis of foot and mouth disease antibody. Blood samples were collected form jugular veins of individual animals using plain vacutainer tube of 10 ml capacity, 38 mm length and 11/2 gauge sterile vacutainer needle. The owners handled the animals properly. After taking the sample code was given to test tube which contains the sample. Then blood/sample was allowed to clot by lacing it over night at room temperature. The serums were collected from clotted blood and transported using an icebox to national Animal health diagnostic and investigation center (NAHDIC) Sebeta, then transferred in a single sterile cryo vials and labeled with specific laboratory number. The sera sample was stored at -20°C until laboratory investigation.
Laboratory analysis
During laboratory work, total of 421 sera were examined by the SVANOVIR foot and Mouth disease virus 3 ABC- EILSA kit to detect foot-and-mouth disease virus (FMDV) specific antibodies in bovine serum samples. The kit procedures were based on a solid phases indirect ELISA. In this procedure, samples were exposed to non structural FMDV antigen (NSP 3 ABC) coated wells on micro titer plates. FMDV antibodies (if present in their sample bind to the antigen in the well) horseradish peroxidase (HRP) conjugate added subsequently forms a complex with the FMDV antibodies.
Unbound materials were removed by raising PBS-buffer before the addition of substrate solution, subsequently a blue-green color was developed which is due to the conversion of the substrate by conjugate. The reaction was stopped by addition of stop solution. Within 15 min, the result was read by micro plate photometer, where the optical density (OD) was measured at 405 nm. The diagnostic relevance of the result was obtained by comparing the optical density (OD) which develops in wells containing the samples with the OD from the wells containing the positive control as it was read by the ELISA reader.
Data analysis
The data collected was entered into M-excel and coded for analysis, the laboratory investigation for prevalence result were analyzed using statistical package for the social sciences (SPSS) statistical package. Variation for the prevalence between the two different zones of Shoa (West and South West Shoa), districts, sex, age and altitude were analyzed by using chi-square (χ2) test. In all the analysis, confidence level was at 95% and ρ<0.05 set for significance.
Sero prevalence result of total 421 cattle sera from West and South West Shoa zones of Oromia regional state were assessed for the presence of non structural FMDV protein (antibodies) (Table1). The overall sero prevalence of FMD in cattle was 15.0% (63/421). The prevalence rate was higher in the South West Shoa zone 22.0% (53/241) as compared to West Shoa 5.6% (10/180) was significantly different (ρ<0.05). From seventeen district investigated, 10 were from West Shoa seven, 7 were from South West Shoa (Table 2). The highest sero prevalence were recorded at Dawo (26.7%), Alemgana (26.2%), kokir (24.4%) and Hamaya (21.4%) districst of South West Sho,a and no sero positivity was recorded in Enjere, Dendi, Bake and Ada Berga in West Shoa zone.
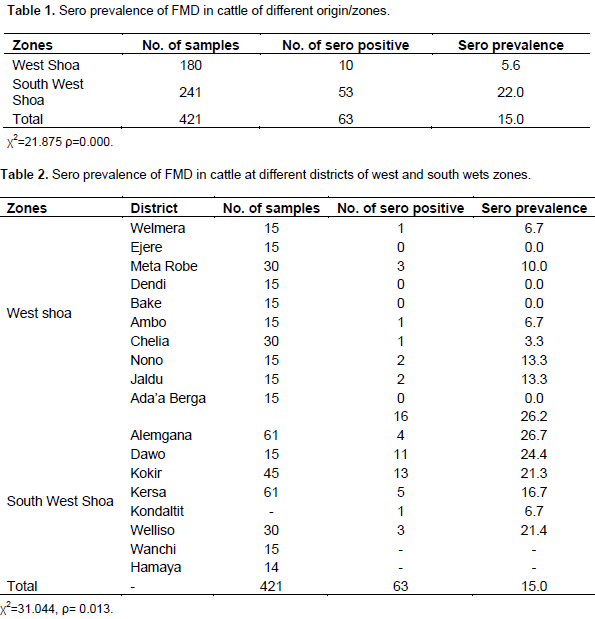
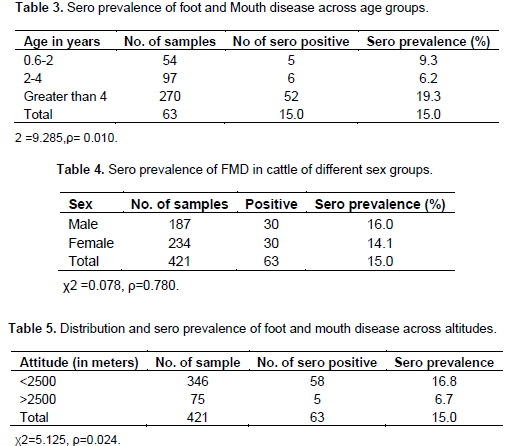
The difference among district is statistically significant (ρ<0.05). The sero prevalence rate of FMD at West and South West Shoa zones are statistically significant difference (ρ<0.05) were recorded between the three age groups. Being highest in >4 years (Table 3). The sero prevalence male and female were 16.0 (30/187) and 14.1% (33/234), respectively. However, statistically significant difference were not observed between the sexes (ρ>0.05) (Table 4). On the other hand, higher disease prevalence 16.8% (58/346) was observed in mid-high highland than cool highland 6.7% (5/75) with statistically significant variation (ρ < 0.05) (Table 5).
According to this study the sera prevalence of FMD in the study area was high, and statistically significance difference was observed between the two zones districts, age and altitude but not in sexes. Although, knowledge on the serotyping of the FMD is very important, this study has limitation in identifying the types FMD Sero types circulating in the study area. Currently Ethiopia exports beef and live animals to Egypt and Middle East from different parts of Ethiopian in which foot and mouth disease is one the most important diseases that cause restriction on trade of animals both locally and internationally thereby threatening the livelihood of mixed farming system pastoralists area and national agricultural economy in general (Rufael et al., 2007). The overall sero prevalence rate of FMD in West and South West Shoa zones were recorded (15%) in individual animals. The study was less prevalence with previous findings from Ethiopia (Sahle et al., 2004) in which seropositivity of 26.5% (Rufael, 2006) reported 21.0% in Borana pastoral system.
The increased in prevalence from the previous study may be associated to pastoral production system that with nature of increased live stock movement result in high rate of contact between animals at common grazing places as well as at watering point (Rufael et al., 2007). The sero prevalence of South West Shoa Zone (22.0%) was higher as compared to west shoa zone (5.6%) (Table1). The different is statistically significant (ρ<0.05). This is probably due to the fact that South West Shoa zone has long border with the Southern Nations Nationalities and Peoples' Regional State (SNNPR) region and western part of Ethiopia which have two broad roads passing through it, and where free cattle movement across the boundaries for grazing and watering, and also by illegal trade thus promoting the concept that FMD peaked in cattle associated with cattle movement (Rufeal et al., 2007).
Among the sampled districtes, the highest sero prevalence was found in those districts of Alemgana, Kersa kondaltit, Dawo and kokir that are found in the south West Shoa Zone. These districts are located on the road of livestock trade root passed through, and found close to Addis Ababa. This is probably due to fact that South West Shoa Zone has long border with the SNNP and western part of Ethiopia. That has two cattle trek roots for trade from SNNP and western part of Ethiopia to Addis Ababa cattle Market. In this zone, it can cause high concept that FMD peaked in cattle associated with cattle movement (Rufael et al., 2007). But this is in agreement with the previous study indicating that all age groups are susceptible to the disease (Oluthemi and Mastiga, 1988).
A significant difference was observed in seroprevalence of FMD among age group of animal in the west Shoa Zones (Table 3). This may be due to the fact that those cattle with age group over four years had practiced more exposures to FMD at grazing, watering point and at market than in age group less than 2 years. Therefore, adult animals might have acquired infection from multiple sero type, and could produce anti bodies against all serotypes of FMD. The low prevalence in young and calves may be indicative of persistence passive immunity and less frequency of exposure of the animal to the disease as the farmers keep their calves around the home areas (Rufael et al., 2007). Although, the variation in sero prevalence was seen in between sexes there was no significant different observed (Table 4), and the observation of statistical insignificant difference on prevalence rate across the sex was supported in the study of Rufael (2006).
This observation could be due to both sex groups are equally exposed to the environment at same time and place in addition, significant difference found between areas of different altitude with the prevalence of 16.8 and 6.6% at mid high land and high lands altitude respectively on (Table 5). This variation may be due to the fact that in low land and mind high lands, cattle have to move long distance in search of good pasture and source of water (Rufael, 2006) tend to be contacted at different origin, which is the predominant factor for the transmission of the disease. Therefore, from this study the level of sero prevalence indicated that FMD is one of the economically important diseases in the study areas which needs further attention to reduce the economic impact of the disease on the country’s economy.
The presence of sero prevalence of 15.0% of FMD in the study area shows that FMD is one of the economically important diseases in the region. The study has proven that FMD is highly contagious, and a serious impediment for cattle production. Moreover, the study shows that age and altitude are major risk factors for the distribution of FMD in the study area. Movements of animal in the border regions and Zones freely due to illegal trade and seasonal movements of animal are also the major contributing factor for FMD virus transmission, and circulating in the Zones. Based on this study high prevalence of FMD in adult indicates that it causes loss of production that has significant economic impact on the country.
1. Further study on FMD virus isolation should be conducted. Recommendation and sero typing should be conducted to fill the gap of informational on the incidence of FMD.
2. Presence of foot and mouth disease in livestock population affects the economy at large by limiting international trade of live animals and animal by products, and consideration of this situation is important in controlling the disease.
3. Policymakers and economy analysts have to be provoked to put their relentless effort in the control of such disease that has serious impact on international trade.
4. Active surveillance should be conducted in all regions of the country so as to control the disease on time before its outbreak.
5. Control measures through vaccination and restriction of animal movements remain the mast important to minimize the risk of FMD in the study area.
The authors are grateful to the management of the National Animal Health Diagnostic and Investigation Center (NAHDIC) for providing an enabling environment for conducting this study. The authors also acknowledge the NAHDIC laboratory personnel for their support at all levels.
The authors have none to declare.
REFERENCES
|
Asseged B (2005). Review of foot mouth disease: An in depth discourse of Global, Sub Saharan and Ethiopian status; Addis Ababa University. FVM. Research and graduate studies Debrezeit, Ethiopia. pp.3-49
|
|
|
|
Burrows R, Mann JM, Greig A, Goodridge D (1981). The pathogenesis of natural and simulated natural foot and mouth disease infection in Cattle. J. Comp. Pathol. 91:599-609.
Crossref
|
|
|
|
|
Bouxton A, Faser G (1977). Animal microbiology. Black well scientific publication Ltd, Ed. In burgh. 2:611-619
|
|
|
|
|
Desta S (1999). Diversification of livestock assets for risk management in the Borana pastoral system of southern Ethiopia, PhD thesis Utah state University, Logan Utah. Pp10-31: Cited in ; Rufael T. (2006). Participatory appraisal and seroprevalence study of foot and mouth disease in borana pastoral system, southern Ethiopia, Msc theis FVM, AAu, debrezeit, Ethiopia pp.1-89
|
|
|
|
|
Esayas G, Berhanu B, Gelagay A (2005). Foot and mouth disease serotypes identified in Ethiopia. Ethiop. Vet. Assoc. J. 9(1):75-77.
|
|
|
|
|
FAO EMPRES and EUFMD commission (2007 ) Foot and mouth disease distribution Worldwide and major epidemiological events in 2005- 2006.Contributors; sumption K,Dinto J., Lubroth J., morzaria S., Murray T., Dela Racquet S., Nijeumi F., No 1:1-9.
|
|
|
|
|
Gibbs PJ (1981). Foot and Mouth Disease in: Viral diseases of food animals. Aworld geography of epidemiology and control. Vol II Academic press Inc.
|
|
|
|
|
Hirsh DC, Zee YC (1996). Foot and Mouth Disease in: Tropical animal health. Kluwer Academic Publisher, Netherlands. pp. 426-431
|
|
|
|
|
Hussen BM (2006). Seroprevalence study of Foot and disease in Export Bulls of Borana and Jimma origin, Ethiopia, DVM Thesis Faculty of Veterinary Medicine. AAU.Pp.1-21
|
|
|
|
|
Kahrs FF (2001).Foot and Mouth Disease in :Viral diseases of cattles. IOWA state Universitypress Pp.271-275
|
|
|
|
|
Kahan CM, Scottline (2005). Foot and Mouth disease in: The merck veterinary manual.9thEdition. Merck and Co. Inc. Whitehouse Stations. NJ USA Pp 509-511.
|
|
|
|
|
Ken, Tony P (1993). Feedlot ting: A guid for beef producer s. National Library of Australia Cataloguing –in- Publication dat. P17.
|
|
|
|
|
Kitching RP, Donaldson AI (1987). Collection and transportation of specimens for vesicular. Virus investigation. Rev. Sci. Tech. Off. Int. Epiz., 6,Pp.263-272.
|
|
|
|
|
Lennette EH, Nathalie MD, Schmidit J (1979). Foot and Mouth Disease in: Diagnostic procedures for viral, Rickettsial and Chlamydial infections. 5th Ediion. Washington Dc. Am. Public Health Assoc. Inc Pp.1013-1016
|
|
|
|
|
Murphy AF, Gibbs JE, Horzinec CM, Studdert JM (1999). Foot and Mouth Disease in: Veterinary virology 3rd Edition California, Academic press USA. pp. 521-537
|
|
|
|
|
OIE (2000). World organization for Animal health. Manual of standards for diagnostic test and vaccines. Office International des Epizooties (OIE), Paris, France, pp. 77-86.
|
|
|
|
|
OIE (1990). Recommended Diagnostic techniques and requirements for Biological products. Office international des Epizooties (OIE), Paris, France. pp. 1-7
|
|
|
|
|
Oluthem AW, Mastiga WN (1988).Viral Disease of Animals in Africa OAU/ST, RC, Scientific publication, Legos, Nigeria. pp. 178-199.
|
|
|
|
|
Radostits OM, Gay CC, Blood , Hinchliff KW (2000). Veterinary Medicine A textbook of the diseases of cattle, sheep, pigs, goats and houses. Foot and Mouth Disease, 9thEdition China Elsevier LTD Pp. 1059-106
|
|
|
|
|
Richard P (1998) Foot and Mouth Disease. The mark veterinary manual 8th Edition mark and Co Inc. White house station NJ, USA, 457-459
|
|
|
|
|
Reid SM, Ferris NP, Hutchings GH, Zhang Z, Belsham GJ, Alexanderson S (2002). Detection of all seven serotype of FMDV by real time fluorogenic reverse transcription polymerase chain reaction assay. J. Virol. Methods. 105:1-80.
Crossref
|
|
|
|
|
Rufael T (2006).Participatory appraisal and seroprevalence study of Foot and Mouth Disease in Borana pastoral system, Southern Ethiopia, MSc thesis FVM AAU, Deberezeit, Ethiopia. pp.1-89.
|
|
|
|
|
Rufael T, Catley A, Bogale A, Sahle M, Shiferaw Y (2007). Foot and Mouth Disease in the borana pastoral system southern Ethiopia and implication for livelihoods and international trade. Trop. Anim. Health Prod. 40:29-38.
Crossref
|
|
|
|
|
Sahle M, Venter EH, Warka RM, Voslo W (2004). Molecular epidemiology of serotype of foot and mouth disease viruses isolated from cattle in Ethiopa between 1979-2001. Onderstepoort. J. Vet. Res. 71(2):129-38.
Crossref
|
|
|
|
|
Sainsbury D (2000). Foot and mouth Disease in: Animal Health. 2ndEdition Blackwell science Pp. 142-160.
|
|
|
|
|
Seifert HSH (1990). Tropical Animals Health. Kluwer Academic Publisher Netherlands. pp. 410-425.
|
|
|
|
|
Solomon H (1980). Animal health review Ethiopia 1979 Ministry of agricultural livestock research development department veterinary service division Pp. 275-276.
|
|
|
|
|
Thrusfield M (1995). Veterinary Epidemiology 2nd ed. Black well science Ltd. pp. 178-198.
|
|
|
|
|
Woodbury EL (1995). A review of the possible mechanisms for the persistence of Foot and Mouth Disease virus. Epidemiol. Infect. 114:1-13.
Crossref
|
|