ABSTRACT
An investigation was done to observe follicle development and ovulation by ultrasound in a synchronization protocol in Bunaji cows using ovatide. Cows (n=16), aged 4 to 6 years with average body condition scores of 2.5 to 3.5 and weighing between 250 and 350 kg were used. They were managed according to the routine management practice of the Diary Research Programme NAPRI. Only cycling cows at 75 days post-partum with palpable CL were included in the study. Cows were randomly assigned to 1 or 2 treatment groups for synchronization of ovulation. Treatment group 1 comprising Bunaji (n=8) received 50 µg of GnRH and 25 mg of PGF2α. While, treatment group 2 comprising Bunaji (n=8) received 50 µg of ovatide and 25 mg of PGF2α. The treatment was as follows: Group 1: (Day 0, 50 µg GnRH; Day 7, 25 mg PGF2α and day 9, 50 µg GnRH), group 2: (Day 0, 50 µg ovatide, Day 7, 25 mg PGF2α and Day 9, 50 µg ovatide). Ultrasound examinations were conducted. Examinations were conducted at the time of second gonadotropin injections, to determine presence of one or more antral follicles > 10 mm in diameter and at 48h after second gonadotropin injections, to determine absence of 1 (single – ovulation) or 2 (double – ovulation) of those earlier antral follicles. Results showed synchronization rate for ovatide was 75%, while that of GnRH (Cystorellin) was 62.5% (p>0.05). Double ovulation rate for both groups was 0%. It was concluded that 50 µg Ovatide in Ovsynh protocol has synchronization potentials in Bunaji cows. Further studies on gonadotropins of fish origin are recommended.
Key words: Ovatide, follicle, sonographic, synchronization, Bunaji, cows.
Ovatide is an indigenous, cost-effective and new hormonal formulation for induced breeding of fishes. It is also effective in breeding major carps. The dosages for females are 0.20 to 0.40 ml/kg for rohu and mrigal, 0.40 to 0.50 ml/kg for catla, silver carp and grass carp, the dosages for males are 0.10 to 0.20 ml/kg for rohu, mrigal, 0.20 to 0.30 ml/ kg for catla and 0.20 to 0.25 ml/kg for silver carp and grass carp (Naipagropediaraichur, 2012). It is a new, highly potent and ready to use injectable formulation containing a synthetic peptide analogous to the naturally occurring hormone, salmon GnRH. The formulation also contains a dopamine antagonist, whereas the GnRH analogue stimulates the pituitary to release gonadotropins and trigger the process of reproduction, the dopamine antagonist inhibits the release of dopamine and makes sure that the secretion of gonadotropins is not inhibited. The use of Ovatide, thus constitutes the latest and the most advanced technology employed for induced breeding of fishes and production of high quality fish seed. It is composed of Gonadorelin A (GnRH A) 20 mcg, Domperidone BP 10 mg and benzyl alcohol IP 1.5% v/v (HemoPharma, 2014).
Gonadotropic releasing hormone (GnRH) is labeled for treating follicular cysts in cows at a dosage of 100 µg (Merial Animal Health, Duluth, GA) in the United States. This is why the Ovsynch protocol utilizes a 100 µg dose. Administration of 100 µg GnRH after PGF2α injection increases the rate of synchronized ovulation in bovines (Pursley et al., 1995). A study by Navanukraw et al., (2002) reported a 37.5% pregnancy rate at 42 post AI using Ovsynch with two half dose of GnRH. Also, Fricke et al., (2003) found similar pregnancy rates using half dose Ovysynch on second service animals.
Another study conducted using Holstein Friesian cows in Wisconsin compared 100 µg dose of GnRH to lower 50 µg dose and reported no statistical difference between treatment groups (Fricke et al., 1998). Thus, 50 µg dose of GnRH decrease total hormone cost from $16. 10 to $9.70 and the total cost per pregnancy was reduced from $47.88 to $27.61, making it comparable to the cost of PGF2α only program (Fricke et al., 1998). Because of the increasing demand for the application of AI in Nigeria’s indigenous cattle breeds and the need for fixed timed AI, the cost of using conventional hormones plays a negative role in disseminating the technology and making it available to poor farmers. The application of ovatide, which is a synthetic analogue of GnRH of fish (Salmon) origin in a synchronization protocol in bovines, may be a potential relief from the exorbitant cost of imported hormones. If cows respond to ovatide well in a synchronization protocol, there will be hope that the hormone extracted from live catfish can replace the expensive analogues. The pituitary of live catfish can be harvested from fish processing plants, currently the bye products of such processing plants are being wasted in our society. This work is designed to investigate follicle development and ovulation by ultrasound in a synchronization protocol in Bunaji cows using ovatide.
Study location
This study was carried out at the cattle farm of Diary Research Programme (DRP) of the National Animal Production Research Institute (NAPRI), Shika, Ahmadu Bello University, Zaria. Shika is situated in the Nothern Guinea Savannah between Latitudes 11° and 12° and Longtitudes 7° and 8° E at elevation of 659 m above sea level with an average annual maximum and minimum temperature of 31.0± 3.2 and 18.0±3.7°C, respectively. It has two distinct seasons: dry season (November to April) with mean daily temperature ranging from 15 – 36°C and rainy season (May to October) with average annual rainfall of 1100 mm and mean relative humidity of 72% (Rekwot et al., 1998).
Research animals and management
The animal experiments followed the principles of the laboratory animal care (CACC, 1993). Bunaji cows (n=16) aged 4 to 6 years and weighing between 250 to 350 kg were used. Selected cows with average body condition scores (BCS) of 2.5 to 3.5 using 0 to 5 scale from the most emaciated to the fattest (Pullman, 1978). Cows were identified by means of plastic ear tags. They were managed according to the routine management practice of the Diary Research Programme. Two transrectal examinations a month apart were carried out to ensure cyclicity of the cows before commencement of the study. Only cycling cows at 75 days post-partum with palpable CL were included in the study.
Experimental design
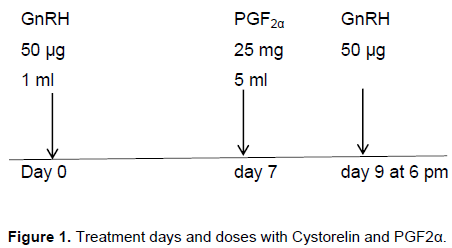
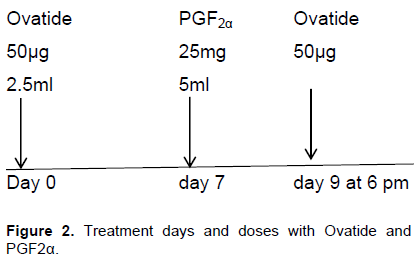
Cows were randomly assigned to 1 or 2 treatment groups for synchronization of ovulation. Treatment group 1 comprising Bunaji (n=8) received 50 µg of GnRH (Cystorellin; Nerial, Ltd., Iselin, NJ) and 25 mg of PGF2α (Lutalysethe Pharmacia – Upjohn Co., Kalamazoo, MI). While, treatment group 2 comprising Bunaji (n=8) received 50 µg of ovatide (Hemo Pharm. PVT Ltd., Mahalaxmi, Mumbai) and 25 mg of PGF2α. The treatment is as illustrated below.
Treatment Group 1
Day 0, 50 µg GnRH; Day 7, 25 mg PGF2α and day 9, 50 µg GnRH. Bunaji cows administered first injection of 50 µg of GnRH on the day 0, followed by 25 mg of PGF2α 7 days later and a second injection of 50 µg GnRH 48 h after PGF2α administration (Figure 1).
Treatment group 2
Day 0, 50 µg Ovatide; Day 7, 25 mg PGF2α and Day 9, 50 µg ovatide. These cows were administered first injection of 50 µg ovatide on day 0, followed by 25 mg of PGF2α 7 days later and a second injection of 50 µg ovatide 48 h after PGF2α administration (Figure 2)
Ultrasonography and rectal palpation
Ultrasound examinations were conducted using ultrasound machine equipped with a transrectal 7.5 MHz linear – array transducer (Aloka 500v; Corometrics Medical Systems, Inc., Willing Ford, (CT). Examinations were conducted at the time of second GnRH injection, to determine presence of one or more antral follicles > 10 mm, in diameter and at 48 h after second GnRH injection, to determine absence of 1, (single – ovulation) or 2 (double –
ovulation) of those earlier antral follicles.
Transrectal palpation was conducted twice at a month interval to select cycling cows within 5 to 12 days of the estrous cycle before initiating Ovsynch protocol (Voh, 1997).
Fertility rates
Synchronization rate
Synchronization rate was calculated as the number of cows that ovulated at least 1 follicle within 48 h of the second GnRH injection, expressed as a percentage of the total number of cows that received the Ovsynch protocol.
Double ovulation rate
Double- ovulation rate was calculated as the number of cows that ovulated 2 follicles within 48 h of the second GnRH injection, expressed as a percentage of synchronized cows.
Statistical analysis
Data obtained on follicle development, synchronization rate, double ovulation rate were expressed in percentages and represented in charts. Differences in the parameters between treatment groups were analyzed using Chi-squares test. Values of P <0.05 were considered significant. A data analysis was carried out using Statistical Package for Social Sciences (SPSS) Version 17.0.0 (SPSS Inc. Chicago IL, USA).
Synchronization rate (SR)
SR = No. of ovulations 48 h after 2ndGnRH/total no. of animals that received the treatment.
Ovatide = 6/8 x100 = 75%; GnRH = 5/8 X100 = 62.5%; P = 1.00, O.R = 1.80.
Double ovulation rate (DR)
DR = No. of cows that ovulated 2 follicles within 48 h of the 2ndGnRH injection divided by the number of synchronized cows. Ovatide = 0.6 x100 = 0%; GnRH =
0.5 x100 = 0%.
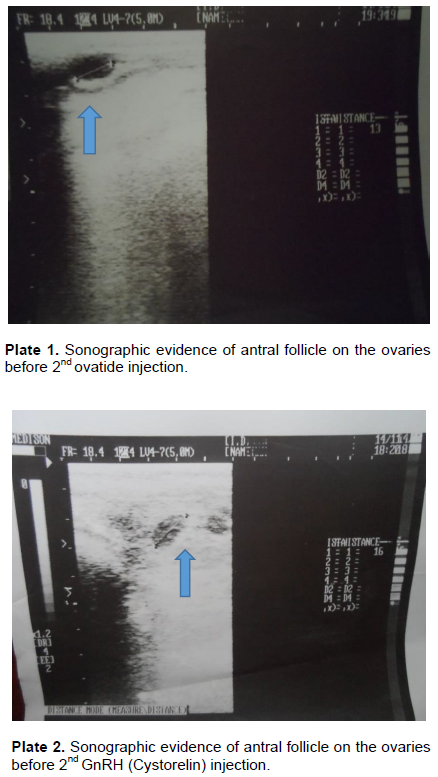
The use of 50 µg of ovatide in the fixed time artificial insemination synchronization protocol showed that there was adequate follicular development (Figure 4 and Plate 1). The GnRH (Cystorelin) at 50 µg also showed adequate follicular development by day nine just before the 2nd gonadotropin injections (Figure 4 and Plate 2). This means that the bunaji cows responded equally to both treatments in respect of follicle development. This response may be complementary to prostaglandin injections on day seven. Subsequently, the number of cows that ovulated 48 h following 2nd gonadotropin injections did not differ significantly between the two groups (P>0.05), this means that both treatments were capable of luteinizing the developed follicles at the treatment doses of 50 µg. A synchronization rate of 75% was recorded for the ovatide while 62.5% was recorded for the GnRH (Cystorelin) (Figure 3). Synchronization rate between the two groups did not differ significantly. The implication of this is that ovatide has synchronization potentials in the Bunaji cows just like Cystorelin.
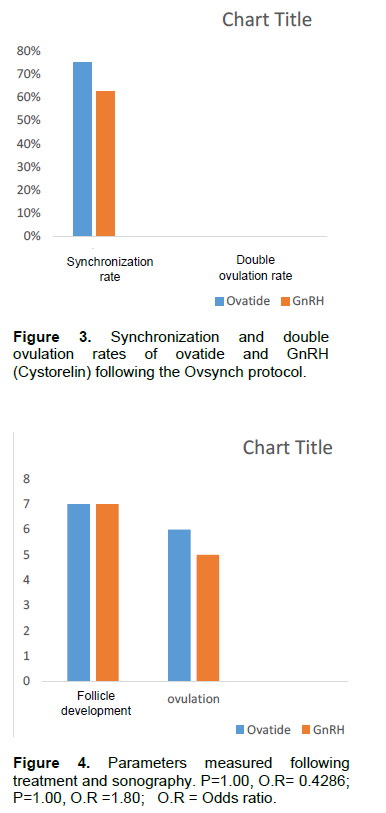
The emergence of a new follicular wave is synchronized only when GnRH treatment causes ovulation (Martinez et al., 1999). If the first GnRH does not synchronize follicular wave emergence, ovulation following the second GnRH may be poorly synchronized (Martínez et al., 2002a), resulting in disappointing pregnancy rates following TAI (Martínez et al., 2002b). The ovatide showed a higher value of synchronization rate (75%) as compared to Cystorelin (62.5%), this may be attributed to factors such as poor compliance and dose.
Anytime you add another cow handling to a program, you are likely increase the probability that not every cow will be treated with the right product at the right time. Keeping things simple and understandable for everyone involved in the breeding program is priority. Knowing the difference between products to be used, using proper syringe and needle sizes (18 g, 1.5 inch), and following instructions are key to a high rate of compliance and good AI conception rates (Jeff, 2016). The recommended dose of Cystorelin for cows is 100 µg. In this study, using 50 µg may have contributed to the lower synchronization rate of 62.5% as compared to 75% of ovatide. It has been reported that the dose of ovatide used in fish affects fertility parameters in fish. A study was conducted to evaluate ovatide doses (0.6, 0.8 and 1.0 ml/kg body weight of female) on breeding performance of Clarias batrachus in the subtropical region of Hisar. The breeding
performance was judged on the basis of the total weight of stripped eggs, net fecundity, fertilization, hatching and survival. To judge the egg quality, the per cent fertilization, hatching and survival of fry were considered. The results indicated that the total weight of stripped eggs and spawning fecundity were the highest (p < 0.05) when females were injected with 1 ml of ovatide per kg body weight (BW) as compared to those injected with other dose levels. The lowest stripping response was observed with injection of 0.6 ml ovatide per kg BW of female brood fish. At the 1 ml dose, the percentages of total fertilized egg and hatching were 82.33 and 55.35% respectively, which were the highest (p < 0.05) among all treatments. The net survival of fry was found to be 98.52% at 1 ml ovatide per kg BW. Therefore, it has been recommended that 1 ml of ovatide per kg BW of female brood fish was found optimum among the three experimental doses for best breeding performance and egg quality in C. batrachus (Sharm et al., 2010). Similarly, GnRH is recommended for treatment of follicular cysts at 100 µg. This report means that there is an optimum dose of gonadotropin (Ovatide or Cystorelin) that is required for ovulation to occur in a particular species as reflected in the total number of stripped eggs in fish.
Both ovatide and GnRH (Cystorelin) showed sonographic evidence of follicular development on day nine of the treatments, before the 2nd gonadotropin injections. Ovulations occurred in both treatment groups which were not significantly different. A synchronization rate of 75 and 62.5% were recorded for ovatide and GnRH (Cystorelin), respectively. It was concluded that treatment of bunaji cows with 50 µg ovatide in Ovsynh protocol has synchronization potentials. Based on the outcome of this study, it was recommended that further studies be carried out using pituitary extract of C. gariepinus (African catfish) in a fixed time AI synchronization protocol in bunaji cows.
The authors have not declared any conflict of interests.
REFERENCES
|
Canadian Council on Animal Care Guide (CACC) (1993). Second edition.
|
|
|
|
Fricke PM, Caraviello DZ, Weigel KA, Welle ML (2003). Fertility of dairy cows after resynchronization of ovulation at three intervals following first timed insemination. J. dairy sci. 86(12):3941-3950.
Crossref
|
|
|
|
|
Fricke PM, Guesther JN, Wiltbank C (1998). Efficacy of decreasing the dose of GnRH used in a protocol for synchronization of ovulation andtimed AI in lactating dairy cows. Theriogenology 50(8):1275-1284.
Crossref
|
|
|
|
|
HemoPharma (2014). Ovatide: A new highly potent hormonal formulation for induced breeding of fishes at low cost. Hemo pharmaceuticals PVT. Ltd.
|
|
|
|
|
Jeff S (2016). What is the best timed AI program? Reproduction. Hoard's Dairy man.
|
|
|
|
|
Martinez MF, Adams GP, Bergfelt DR, Kastelic JP, Mapletoft RJ (1999). Effect of LH or GnRH on the dominant follicle of the first follicular wave in beef heifers. Anim. Reprod. Sci. 57(1):23-33.
Crossref
|
|
|
|
|
Martinez MF, Kastelic JP, Adams GP, Mapletoft RJ (2002b). The use of a progesterone-releasing device (CIDR-B) or melengestrol acetate with GnRH, LH, or estradiol benzoate for fixed-time AI in beef heifers. J. Anim. Sci. 80(7):1746-1751.
Crossref
|
|
|
|
|
Naipagropediaraichur (2012). Induced breeding of Fishes with Ovatide. Available at: Agropedia.iitk.ac.in/content/induced-breeding-fishes-ovatide.
|
|
|
|
|
Navanukraw C, Reynolds LP, Grazul-Bilska AT, Redmer DA, Fricke PM. (2002). Effect of presynchronization on pregnancy rate to a timed artificial insemination protocol in lactating dairy cows. J. Dairy Sci. 85(Suppl 1):263.
|
|
|
|
|
Pullman NB (1978). Condition scoring of White Fulani cattle. Trop. Anim.Health Prod. 10:118-120.
Crossref
|
|
|
|
|
Pursley JR, Mee MO, Wilkbank MC (1995). Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology 44(7):915-923.
Crossref
|
|
|
|
|
Rekwot PI, Oyedipe EO, Barje PP, Rwuaan JS (1998). Factors affecting the reproductive performance of cattle in Nigeria. Niger. Vet. J. 19:66- 77. Sharma K, Yadava NK, Jindal M (2010). Effect of different doses of ovatide on the breeding performance of Clarias batrachus (Linn.). Livest. Res. rural Dev. 22(4).
|
|
|
|
|
RJ (2002a). The use of progestins in regimens for fixed-time artificial insemination in beef cattle. Theriogenology 57(3):1049-1059.
|
|
|
|
|
Voh AA Jr (1997). Fertility and embryonic mortality rates of Zebu cows following oestrus synchronization and artificial insemination. Ph.D. Thesis, Ahmadu Bello University, Zaria.
|
|