ABSTRACT
This study was conducted to determine the prevalence of haemoparasites and some haematological parameters of slaughtered trade cattle in Maiduguri abattoir. A total of 120 blood samples were randomly collected from cattle between January and July, 2014. The samples were screened for haemoparasites by examining Giemsa stained thin blood films. Microhaematocrit centrifugation technique was used for determination of packed cell volume (PCV) while total red blood cell (RBC) counts and total white blood cell (WBC) counts were determined by cyanmethaemoglobin and hemocytometer methods. An overall prevalence of 10.8% (CI=0.064, 0.017) was recorded for Anaplasma (5.8%), Babesia (4.2%) and Trypanosoma species (0.8%). Young cattle had significantly (p<0.05) higher prevalence of 9.2% (CI= 0.052, 0.157) compared to adults with 1.7% (CI= 0.005, 0.059). Among different sexes, females had a significantly (p<0.05) higher prevalence of 7.5% (CI: 0.040-0.136) than males with 3.3% (CI= 0.130, 0.083). Rahaji breed had a significantly higher (p<0.05) prevalence of 7.5% (CI= 0.040, 0.136) compared to Ambala with 1.7% (CI= 0.005, 0.059), Kuri and Adamawa gudali each with a prevalence of 0.8% (CI= 0.002, 0.046). Cattle with moderate body condition scores had significantly (p<0.05) higher prevalence of 6.7% (CI= 0.034, 0.126) compared with those that have good body condition scores with 4.2% (CI= 0.018, 0.094) while thin and fat cattle were not infected with haemoparasites. Even though all the haematological parameters were within range of normal values, there was a significant difference (p<0.05) in mean packed cell volume (PCV) and total white blood cell (WBC) counts between infected and un-infected slaughtered cattle. It was concluded from this study that haemoparasites are endemic in cattle populations in Maiduguri and the prevalence of haemoparasites may be associated with changes in PCV and WBC count.
Key words: Haematological parameters, haemoparasites, Maiduguri, Prevalence, risk factors, trade cattle.
The Nigerian livestock resources was conservatively estimated to the tone of USD 6 billion (Anon, 2006; Akande et al., 2010) and contributes significantly to the Agricultural component of the Gross Domestic Product (GDP) of which cattle production contributes up to 40% (McIntyre et al., 1992). Cattle production provides essential source of protein through meat and milk, generates employment, income, farm power and organic manure for arable Agriculture in the Sudano-Sahelian ecological zones of the country (Ikhatua and Asaka, 2000). Among nomadic Fulani and Shuwa-Arab pastoralists, the ownership of cattle also serve as an index of social prestige (Lamorde, 1998). Furthermore, their products such as hide and skins, bones and blood serve as raw materials for industries (Ikhatua and Asaka, 2000).
Among parasitic diseases of cattle, haemoparasitism constitute a disease entity of great economic importance (Jongejan and Uilenberg, 2004; Salih et al., 2015) and has been recognised as a serious threat to food security of Nigeria. The impact of haemoparasites on cattle productivity is also difficult to quantify (Singla et al., 2007; Samdi et al., 2010) but losses in traction power, milk and meat production and costs of control programs have been ascribed to haemoparasites (ILIR, 1997). Haemoparasites and their vectors have a global distribution, and are especially important in Sub-Saharan Africa (Okorafor and Nzeako, 2014). The prevalence of haemoparasites of cattle in Nigeria is generally considered to be very high due to the preponderance of their arthropod vectors (Biu and Kabono, 2005; Kamani et al., 2010; Okorafor and Nzeakor, 2010; Musa et al., 2014). Moreover, 90% of the cattle population in Nigeria are raised under the pastoral husbandry system of Fulani herders (Lorusso et al., 2013). Under this system cattle are extensively grazed on pastures and forests and may be exposed to various arthropod vectors of haemoparasites (Obadiah and Shekaro, 2012). The prevalence of various genera of haemoparasites of cattle (Trypanosomes, Babesia, Anaplasma, and Theileria) was previously reported in different parts of the country (Akande et al., 2010; Kamani et al., 2010; Samdi et al., 2010; Enwezor et al., 2012; Ademola and Onyiche, 2013; Okorafor and Nzeako, 2014; Qadeer et al., 2015) and elsewhere in the world (Alim et al., 2012; Velusamy et al., 2014). Trypanosoma, Babesia and Anaplasma species are listed among the most economically important genera of haemoparasites in Nigeria (Biu and Kabono 2005; Akande et al., 2010; Kamani et al., 2010), and their impact on cattle production and productivity accounts for heavy economic losses to livestock producers in the tropics and subtropics (Soulsby, 1982; FAO, 1984). They are responsible for destruction of erythrocytes leading to anaemia, jaundice, anorexia, weight loss and infertility in livestock (Akande et al., 2010).
There is paucity of information on the prevalence and importance of haemoparasites in trade cattle in Maiduguri. Therefore, this study was conducted to determine the prevalence of haemoparasites of cattle and their associated haematological changes.
Study area
This study was conducted in Maiduguri which is the capital and largest city of Borno State, and has an estimated total population of 521,492 (NPC, 2006). Maiduguri has one of the largest cattle markets in north-eastern Nigeria with a high volume of trade in livestock and livestock products through neighbouring Chad, Cameroun and Niger republics. Maiduguri lies between Latitude 11°N and Longitude 13°E and it is characterized by a long period of dry season which lasts between October and May, with a short period of rainfall between June and September (LCRI, 2007).
Study population and sampling method
A total of 120 trade cattle presented for slaughter at the Maiduguri central abattoir were randomly selected for this study which lasted between January and July, 2014. Characteristics of population such as age, sex, breed and body condition scores were observed and recorded for each sample throughout the study. Sex differentiation was based on the appearance of external genitals while breed identification was based on morphology as described by Yunusa et al. (2013). Ageing was based on rostral dentition as described by Lasisi et al. (2002). Cattle aged less than 3 years old were categorised as young while older ones were considered as adults. The body condition score (BCS) of cattle was evaluated on a 5 point scale based on modification of method described by (DEFRA, 2001).
Collection and preservation of blood samples
5 ml of blood was collected from the jugular vein at the point of slaughter into EDTA containing bottles which were labelled appropriately and placed on ice packs for onward delivery to the side laboratory, Veterinary Teaching Hospital, University of Maiduguri where they were further processed.
Determination of haematological parameters
Packed cell volume (PCV) was determined using microhaematocrit centrifugation technique (MHCT) as described by Brar et al. (2011). Blood was introduced into microhaematocrit tubes by capillary action and one end of each capillary tube was sealed with plasticin. The tubes were spun in a microhaematocrit centrifuge (Hawksley, England) at 13000 g for 5 min. PCV was measured with a hematocrit reader (Hawksley, England), and recorded appropriately (Kamani et al., 2010). Total red blood cell counts (RBC) and total white blood cell counts (WBC) were determined by cyanmethaemoglobin and hemocytometer methods described by Coles (1974).
Preparation of blood film for identification of haemoparasites
A thin blood smear was prepared on a standard microscope glass slide (75 mm by 25 mm), air dried, fixed in methyl alcohol for 3-5 min, stained in 5% Giemsa stain for 30-45 min in a staining jar and rinsed in buffered distilled water (Kamani et al., 2010). Haemoparasites were identified by direct microscopic examination based on morphologic keys as described by Soulsby (1982), using a compound microscope (Olympus, USA).
Data analysis
Data generated were summarized and presented in tables using descriptive statistics. Prevalence of haemoparasites was estimated as p =d/n (%). Where p= prevalence, d = number of individuals having disease at a particular point in time and n = number of individuals in the population at risk at that point in time (Thrusfield, 2005). The two sided 95% confidence interval (Newcombe, 1998) for various sexes, age groups, breeds and body condition scores of slaughtered cattle were computed on VassarStats.® (Website for statistical computation), and p<0.05 was considered significant.
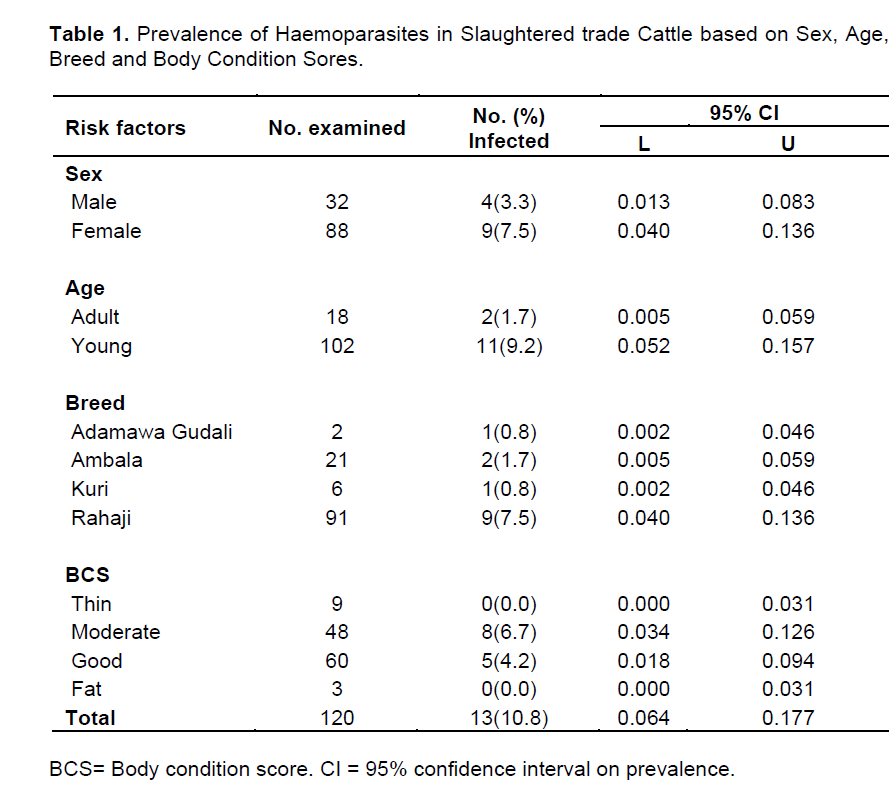
The distribution of haemoparasites in slaughtered trade cattle by sex, age, breed and body condition scores is presented in Table 1. Out of 120 blood smears examined, 13 representing 10.8% (95% CI= 0.064, 0.177) were positive for various genera of haemoparasites. Sex-wise, the prevalence of haemoparasites was significantly higher (p<0.05) in females (7.5%: 95% CI= 0.04, 0.136) than males (3.33%: 95% CI= 0.013, 0.083). Age-wise, the prevalence of haemoparasites was significantly higher (p<0.05) in young representing 9.2% (95% CI= 0.052, 0.157) compared with older cattle which represent 1.7% (95% CI: 0.005-0.059). Among the different breed of cattle examined in this study, the prevalence of haemoparasites was significantly higher (p<0.05) in Rahaji which represents 7.5% (95% CI= 0.040-0.136) while the lowest prevalence was recorded in Adamawa gudali and Kuri breeds which both had a prevalence of 0.83% (95% CI= 0.002, 0.046). Similarly, a significantly higher (p<0.05) prevalence was recoded in cattle with moderate and good body condition scores which represent 6.7% (95% CI= 0.034, 0.126) and 4.2% (95% CI= 0.018, 0.094), respectively. Both thin and fat cattle examined in this study were not infected with haemoparasites.
Some haematological parameters of infected and uninfected slaughtered trade cattle is shown in Table 2. Mean values of packed cell volume (PCV), total white blood cell counts (WBC) and red blood cell count (RBC) of infected and uninfected cattle examined in this study were within normal range. However, there was a significant difference (p<0.05) in mean packed cell volume (PCV) and total white blood cell counts (WBC) between infected and uninfected slaughtered trade cattle. On the other hand, no significant difference (p>0.05) was observed in mean RBC between infected and uninfected cattle.
The prevalence of various genera of haemoparasites in slaughtered trade cattle in Maiduguri is presented in Table 3. The three genera identified in this study were Anaplasma (5.8%), Babesia (4.2%) and Trypanosoma (0.8%).
The results obtained in this study indicates that haemoparasites are endemic in cattle populations within Maiduguri and its environs, even though the overall prevalence is not comparable with previous reports. The preponderance of infection with haemoparasites may be attributed to a high prevalence of cattle ticks in Maiduguri (Musa et al., 2014). Biu and Kabono (2005) reported a higher prevalence of haemoparasites in cattle from the same study area. This difference could be attributed to changes in farm management practices, including more aggressive measures of vector control in the last decade. Our result is also at variance with Okorafor and Nzeako (2014) who reported a prevalence of 6.7% for various species of haemoparasites of cattle and Ademola and Onyiche (2013) who reported a prevalence of 5% in Oyo state, Nigeria. Also, our overall prevalence does not agree with Kamani et al. (2010) who reported a higher prevalence of 25.7% for haemoparasites in North-central, Nigeria. These discrepancies could be attributed to local differences in prevalence of haemoparasites due to variations in geographical location (Velusamy et al., 2014) which determines the distribution of the arthropod vectors of the parasites (Shah-Fischer and Say, 1989; Agbede, 2013). However, our result is comparable to Onoja et al. (2013) who reported an overall prevalence of 9.5% for bovine Babesiosis in slaughtered cattle from Zaria, Nigeria and Kumar et al. (2015) who reported an overall prevalence of 9.3% from cattle in Punjab, India.
The effects of risk factors such as age, sex and breed on prevalence of haemoparasites has been previously reported (Kamani et al., 2010; Alim et al., 2011; Ademola and Onyiche, 2013; Okorafor and Nzeako, 2014). The higher prevalence in females (7.5%) than males (3.3%) agrees with Kamani et al. (2010) who observed a similar trend and attributed their finding to the fact that female animals were generally herded much longer for the purpose of breeding and milk production, thereby prolonging their exposure to challenges of disease. Also, younger cattle less than 3 years old had a higher prevalence compared to their adult counterparts. This finding agrees with Ademola and Onyiche (2013) and could be due to development of immunity in adult cattle with previous infection. The higher prevalence of haemoparasites recorded in Rahaji breed could be attributed to the fact that it is the most numerous breed in Borno State because of their adaptation to arid and semi-arid conditions (Blench, 1999). Moreover, they are usually herded by pastoralists under transhumant conditions which exposes them to the vectors of haemoparasites thereby increasing the risk of infection
.
This study has also revealed that haemoparasites were prevalent only in cattle with moderate and good body condition scores but thin and fat cattle were not affected. This finding may be due to the fact that fewer numbers of thin and fat cattle were encountered in the study. Additionally, fat animals usually come from feed lots where they are probably treated against various parasites and other diseases, thereby minimizing the chances of detecting haemoparasites among them. By contrast, thin cattle may originate from extensively managed herds with poor nutritional background, which increases risk of haemoparasites among them. Moreover, poor nutrition and management are significant risk factors for acquiring other debilitating diseases by extensively managed cattle under transhumant conditions, and could be responsible for loss of body condition.
The mean PCV and total white blood cell counts of infected and uninfected slaughtered trade cattle examined in this study were within normal range (Merck Manual, 2012). However, there was a significant difference (p<0.05) in PCV between infected and uninfected cattle, and this could be attributed to the effects of haemoparasites on blood cells. This finding agrees with Kamani et al. (2010) who reported that infection with Babesia, Anaplasma, Theileria and Trypanosoma species, either singly or in combination caused a significant reduction in mean PCV of cattle. It also is known that infection with most haemoparasites leads to destruction of erythrocytes and anemia (Soulsby, 1982; Ademola and Onyiche, 2013). The higher mean total white blood cell counts recorded in infected cattle could be explained on the basis of immune response to presence of haemoparasites. Eosinophilia was previously reported in haemoparasitic infections of ruminants (Ademola and Onyiche, 2013) which may contribute to the observed differences in WBC count between infected and uninfected cattle in the present study.
The three genera of haemoparasites identified in this study (Anaplasma, Babesia and Trypanosoma) were previously reported in domestic animals in Nigeria (Abenga et al., 2004; Biu and Kabono 2005; Kamani et al., 2010; Ademola and Onyiche, 2013; Okorafor and Nzeako, 2014; Qadeer et al., 2015) and elsewhere in the world (Soulsby, 1982; Alonso et al., 1992; Bock et al., 2004; Alim et al., 2011; Sitotaw et al., 2014). The higher prevalence of Anaplasma (5.8%) and Babesia (4.2%) in this study could be attributed to the availability of suitable environmental conditions which favour multiplication and survival of their tick vectors (Soulsby, 1982; Shah-Fischer and Say, 1989). However, the low prevalence of Trypanosomes in the present study is not unusual because Maiduguri is located in the Northern limit of tsetse distribution (Abenga et al., 2004). Previously, cases of trypanosomosis have been reported in the Sahel around Maiduguri (Maxie et al., 1979). These unusual occurrences have been linked to movement of cattle from tsetse infested to tsetse free zones (Anene et al., 1991). Moreover, mechanical vectors such as biting flies have been incriminated in transmission of trypanosomosis in tsetse free zones (Soulsby, 1982).
A number of antiparasitic drugs have been used for the treatment and control of Bovine haematozoa (Soulsby, 1982) but only diminazene aceturate and imidocarb dipropionate are still in common use (Merck, 2013). These drugs have shown up to 100% efficacy against Babesia, Anaplasma and Trypanosoma species and could be used to effectively reduce the prevalence of these haemoprotozoa in cattle populations within Maiduguri and environs.
Even though we recorded a lower prevalence of haemoparasites in this study compared to the previous report in the last decade, it may be concluded that haemoparasites are endemic in cattle populations within Maiduguri, and their occurrence may be associated with changes in some haematological parameters.
It is therefore recommended that a stringent measure of controlling haemoparasites in food animals should be instituted in Maiduguri and its environs where these animals are sourced. Such measures should include more aggressive chemotherapy, chemoprophylaxis and control of arthropod vectors through the use of effective insecticides, acaricides and environmental management.
The authors have not declared any conflict of interests.
The authors are very grateful to Yusuf Jairus of Department of Veterinary Teaching Hospital, University of Maiduguri for his support in the technical aspect of this work.
REFERENCES
|
Abenga JN, Enwezor FNC, Lawani FAG, Osue HO, Ikemereh ECD (2004). Trypanosome Prevalence in Cattle in Lere Area in Kaduna State, North Central Nigeria. Rev. Elev. Med. Vet. Pays Trop. 57(1-2):45-48.
|
|
|
|
Ademola IO, Onyiche TE (2013). Haemoparasites and Haematological Parameters of Slaughtered Ruminants and Pigs at Bodija Abattoir, Ibadan, Nigeria. Afr. J. Biomed. Resourc. 16:101-105.
|
|
|
|
|
Agbede RIS (2013). A Guide to Tropical Veterinary Entomology. Mac Chin Multimedia Designers, Zaria, Nigeria, l08 p.
|
|
|
|
|
Akande FA, Takeet MI, Makanju OA (2010). Haemoparasites of Cattle in Abeokuta, South West Nigeria. Science World J. 5 (4):19-21.
|
|
|
|
|
Alim AM, Roy SDK, Sikder MMS, Hassan MM, Siddiki AZ, Hossain MA (2012). Prevalence of Hemoprotozoan Diseases in Cattle Population of Chittagong Division, Bangladesh. Pakistan Vet. J. 32(2):221-224.
|
|
|
|
|
Alonso M, Arellano-Sota C, Cereser VH, Cordoves CO, Guglielmone AA, Kessler R, Mangold AJ, Nari A, Patarroyo JH, Solari MA, Vega CA, Vizcaino O, Camus E (1992). Epidemiology of bovine anaplasmosis and babesiosis in Latin America and the Caribbean. Rev. Sci. Tech. off. Int. Epiz. 11(3):713-733.
|
|
|
|
|
Anene BM, Chime AB, Jibike GI, Anika SN (1991). Prevalence of Trypanosomosis in Zebu cattle at Obudu ranch, a tsetse free zone in Nigeria. Prevent. Vet. Med. 10:257-260.
Crossref
|
|
|
|
|
Anon JE (2006). Opening address by Mallam Adamu Bello at the 5th Nigerian economic Summit Group (NESG), Abuja. Nigerian Federal Ministry of agriculture (1999-2007).
|
|
|
|
|
Biu AA, Kabono A (2005). Prevalence of Bovine Haemoparasites in Maiduguri, Nigeria. J. Experimental Appl. Biol. 6:27-31.
|
|
|
|
|
Blench R (1999). Traditional Livestock Breeds: Geographical distribution and dynamics in relation to the ecology of West Africa: Overseas Development Institute Portland House Stag Place London 122:7-21.
|
|
|
|
|
Bock R, Jackson L, de Vos A, Jorgensen W (2004). Babesiosis of cattle. Parasitology, 2 Ed. UK: Cambridge University Press pp. 247-270.
Crossref
|
|
|
|
|
Brar RS, Sandhu HS, Singh A (2011). Veterinary Clinical Diagnosis by Laboratory Methods,1 Ed. India: Kaylani Publishers, pp. 29-150.
|
|
|
|
|
Coles EH (1974). Veterinary clinical pathology, 1 Ed. U.S: WB Saunders Company, pp. 67-92.
|
|
|
|
|
Department for Environment, Food and Rural Affairs (DEFRA) (2001). Condition Scoring of Beef Suckler Cows and Heifers,
View.retrived 09-03-2016.
|
|
|
|
|
Enwezor FNC, Samdi SM, Ijabor O, Abenga JN (2012). The Prevalence of Bovine Trypanosomosis in Parts of Benue State, North-Central Nigeria. J. Vector Borne Dis. 39:188-190.
|
|
|
|
|
FAO (1984). Food and Agricultural Organization, Prevention of loss from tick-borne diseases and ticks in cattle imported by developing countries. In: Ticks and Tick-borne Disease Control. A practical field Manual Vol.11, Food and Agricultural Organization, Rome, Italy. pp. 597-621.
|
|
|
|
|
Ikhatua UJ, Asaka ME (2000). Effects of feeding grass forage supplement with rice bran based concentrate diets on the performance and blood characteristic of West African dwarf goats. Unpublished data, cited with permission.
|
|
|
|
|
International Livestock Research Institute (ILIR) (1997). Livestock, People and the Environment. ILIR, Nairobi Kenya.
|
|
|
|
|
Jongejan F, Uilenberg G (2004). The global importance of ticks. Parasitology 129:S3-S14.
Crossref
|
|
|
|
|
Kamani J, Sannus E, Egulu K, Dogo I, Tanko J, Kenza J, Tafariki E, Ghise S (2010). Prevalence and Significance of Haemoparasitic infections of Cattle in North-Central, Nigeria. Vet. World 3(10):445-448
Crossref
|
|
|
|
|
Kumar V, Kaur P, Wadhawan VM, Pal H, Sharma H, Kumar P (2015). Theileriosis in cattle: Prevalence and seasonal incidence in Jalandhar District of Punjab (India). Int. J. Recent Scientific Res. 6(1):2998-2999.
|
|
|
|
|
Lake Chad Research Institute (LCRI) (2007) Annual Weather Report, IFAD-TAG, No 718. 28 p.
|
|
|
|
|
Lamorde AG (1998). Scenario building for the Nigerian Livestock Industry in the 21st Century. A paper presented at the Silver Anniversary Conference of the Nigerian Society for Animal Production-Gateway Hotel, Abeokuta, Nigeria March 21-26, 1998.
|
|
|
|
|
Lasisi OT, Ojo NA, Otesile EB (2002). Estimation of age of cattle in Nigeria Using rostral dentition; Short communication. Tropical Vet. 20(4):204-208.
Crossref
|
|
|
|
|
Maxie MG, Losos GJ, Tabel H (1979). Experimental Bovine Trypanosomosis (T. Vivax and T. Congolense) 1. Symptomatology and Clinical Pathology. Tropical Medical Parasitology 30:275-282.
|
|
|
|
|
McIntyre JD, Bourzat D, Pingel P (1992). Crop-Livestock interaction in sub-Saharan Africa. Regional World Bank, Washington D.C.
|
|
|
|
|
Merck Manual (2012). Haematological reference ranges. Merck Veterinary Manual. http://www.merckmanuals.com, retrieved 01/03/2016.
|
|
|
|
|
Musa HI, Jajere SM, Adamu NB, Atsanda NN, Lawal JR, Adamu SG, Lawal EK (2014). Prevalence of Tick Infestation in Different Breeds of Cattle in Maiduguri, Northeastern Nigeria. Bangladesh J. Vet. Med. 12(2):161-166.
Crossref
|
|
|
|
|
NPC (2006). Nigerian National Population Census Report. National Population Commission, 109 p.
|
|
|
|
|
Newcombe RG (1998). Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods, Statistics in Med. 17:857-872.
Crossref
|
|
|
|
|
Okorafor UP, Nzeako SO (2014). Prevalence of Haemoparasites of Cattle from Three Abattoirs in Ibadan Metropolis, Oyo State, Nigeria. Int. J. Scient. Res. Environ. Sci. 2(7):244-249.
Crossref
|
|
|
|
|
Onoja II, Malachy P, Mshelia WP, Okaiyeto SO, Danbirni S, Kwanashie G (2013). Prevalence of Babesiosis in Cattle and Goats at Zaria Abattoir, Nigeria. J. Vet. Adv. 3(7):211-214.
|
|
|
|
|
Qadeer MA, Gumel MA, Chessed G, Nganjiwa JI, Bernard K, Vandi P, Hakim D, Fadimatu U (2015). A Cross Sectional Study on the Gastrointestinal and Haemoparasites of Trade cattle in Girei and Yola North Local Government Areas of Adamawa State, Nigeria. IOSR J. Agric. Vet. Sci. 8(4):3-5.
|
|
|
|
|
Salih DA, Hussein AM El, Singla LD (2015) Diagnostic approaches for tick-borne haemoparasitic diseases in livestock. J. Vet. Med. Animal Health 7(2):45-56.
Crossref
|
|
|
|
|
Samdi SM, Abenga JN, Attahir A, Haruna MK, Wayo BM, Fajinmi AO, Sumayin HM, Usman AO, Hussaina JZ, Muhammad H, Yarnap JE, Ovbagbedia RP, Abdullahi RA (2010). Impact of Trypanosomosis on Food Security in Nigeria: A Review: Int. J. Animal Vet. Adv. 2(2):47-50.
|
|
|
|
|
Shah-Fischer M, Say R (1989). Manual of Tropical Veterinary Parasitology.CAB International: The Technical Center for Agricultural and Rural Cooperation (CTA), Nairobi, Kenya. Pp. 351-363.
|
|
|
|
|
Singla LD, Aulakh GS and Juyal PD (2007). Haemato-biochemical and clinico-pathological observations on haemoprotists in cattle and buffaloes. Proceedings of National Seminar on Recent Diagnostic Trends and Control Strategies for Haemoprotozoan Infections in Livestock held on February 09-11 at Sardarkrushinagar, Gujarat, pp. 107-110.
|
|
|
|
|
Sitotaw T, Fikru R, Fikre, Abraha GK (2014). Epidemiology significance of major haemoparasites of ruminants in and around Debre-Zeit, Central Ethiopia. Acad. J. 6(2):16-22.
|
|
|
|
|
Soulsby EJL (1982). Helminths Arthropods and Protozoa of Domesticated Animals, 7th Ed. U.K: Bailiere Tindall, London, 809 pp.
|
|
|
|
|
Thrusfield MV (2005). Veterinary Epidemiology. 3rd Ed. UK: Blackwell Science Oxford, London, P 483.
|
|
|
|
|
Velusamy N, Rani GI, Ponnudurai TJ, Harikrishnan T, Anna K, Arunachalam KS, Anbarasi P (2014). Influence of Season, age and breed on prevalence of haemoprotozoan diseases in cattle of Tamil Nadu, India. Vet. World 7:574-577.
Crossref
|
|
|
|
|
Yunusa AJ, Salako AE, Oladejo OA (2013). Morphometric characterization of Nigerian indigenous sheep using multifactorial discriminant analysis. Int. J. Biodiversity Conservation 5(10):661-665.
|
|