ABSTRACT
The present study was an endeavor to isolate and identify the various bacteria localizing pneumonic lungs and the associated tracheas of sheep slaughtered at Addis Ababa Abattoirs Enterprise, Central Ethiopia, in both aerobic and anaerobic conditions. A total of 60 pneumonic lungs and 60 tracheal swabs were examined bacteriologically. From all the samples collected, a total of 440 bacterial isolates (239 from the aerobic culture and 201 from the anaerobic culture) were obtained. The result of aerobic isolates include: Staphylococcus species (31.38%), Pasteurella hemolytica (29.71%), Bacillus species (10.04%), Bibersteinia trehalosi (6.69%), Micrococcus (3.77%), Escherichia coli (3.35%), Streptococcus species (2.51%), Rhodococcus equi (2.93%), Pseudomonas species (2.09%), Klebsiella pnuemonea (0.84%), Actinobacillus species and Bordetella species (1, 29%); whereas Staphylococcus species (26.87%), P. hemolytica (37.81%), Bacillus species (3.98%), B. trehalosi (10.45%), Micrococcus (3.48), E. coli (6.97%), Streptococcus species (0.5%), Rhodococcus equi (0.5%), Klebsiella pneumoniae (2.99%) and Actinobacillus species (1.49%) were among anaerobic isolates. Thus, isolation of multiple bacterial species from the respiratory tracts of pneumonic sheep in this study signifies their possible role in the involvement of respiratory diseases. Appropriate prevention and control methods should be established along with identification of the most pathogenic species by future studies.
Key words: Bacteria, lung, pneumonia, sheep, trachea.
Ethiopia is home to various indigenous sheep breeds. From the total livestock population of the country, sheep owns about 46%. Despite this huge resource, Ethiopian sheep productivity remains far lower than expected. The major biological constraints contributing to low productivity include bacterial and parasitic infections (Kaur et al., 2009; Leta and Meles, 2014; CSA, 2014-15; Fikru and Gebeyehu, 2015; Gebremeskel et al., 2017; Pawar et al., 2017; Singh et al., 2017).
The lungs are continuously exposed to air that contains dust, bacteria, fungi, viruses and various noxious agents and defense against these potentially harmful materials is controlled by a complex of protective mechanisms (Mohan et al., 2013). Stress factors such as inclement of weather, cold and stress of weaning, transportation, poorly ventilated housing and nutritional deficiencies have predisposing roles. In addition, concurrent infections with some viruses, bacteria or parasitic infestation degrade the potential of the host to combat infections (Gebremeskel et al., 2017; Gupta et al., 2009; Radostits et al., 2000).
The impact of respiratory disease is extensive and can be measured as the sum of the direct economic losses occurring due to mortality, morbidity, treatment and prevention costs. Loss of production (reduced animal performance and carcass quality) and the indirect costs such as labor, infrastructures and intangibles (Jim, 2009; Monot et al., 2015).
A number of bacterial, viral and parasitic agents participate in sheep respiratory diseases, however most important include: mycoplasma species such as Mycoplasma ovipneumoniae, Mycoplasma.arginini, Mycoplasma agalactiae (Lin et al., 2008), lung worms (particularly (Dictyocaulus filaria) (Borji et al., 2012) and bacteria like Pasteurella multocida, Manhemia haemolytica, Chlamydia psittaci, Histophilus somni, which can suppress the animal’s immune system, allowing opportunistic microorganisms to colonize the lung and cause the disease (Angen et al.,1998; Radostits et al., 2000; Tesfaye et al., 2013; Fulton, 2009; Alemneh and Tewodros, 2016).
Therefore, objectives this study identifying most is important to identify most bacterial pathogens involved in pneumonic lung of sheep as well to and compare ing and contrasting the types of isolated bacterial species in different sites of the respiratory system both in aerobic and anaerobic environments. objective
Study area
The study was conducted from October 2010 to April 2011 at Addis Ababa abattoir enterprises, central Ethiopia. Geographically Addis Ababa is located 9°2’ N and 38° 42’E having elevation of 2400 above sea level (a.s.l) and mean annual rainfall of 1800 mm. The city has average minimum and maximum annual temperature of 10.7 and 23.6°C, respectively (NMSA, 2005; Jury and Chris, 2013).
Study animals and sampling strategy
The study was conducted on 60 randomly selected sheep lungs with pneumonic lesions and tracheal swabs slaughtered at Addis Ababa Abattoirs Enterprises. Samples were kept separately and transported to the School of Veterinary Medicine, Microbiology Laboratory of Addis Ababa University in a cool box containing ice pack at 4°C.
Sample collection
Tracheal swabs
Samples were taken with sterile cotton swabs moistened with tryptose soya broth from the trachea of sheep. Two swabs were introduced directly into the trachea of slaughtered sheep and rubbed smoothly against the mucosa in a circular motion. The swabs were allowed to remain in contact with the secretions for up to 1 min, and the two swabs collected from each sheep were kept in a tryptose soya broth transport medium and transported to the laboratory (Lees et al., 1990; OIE, 2008).
Pneumonic lung tissue
Samples of pneumonic lung tissue were collected at post mortem for microbial culture. Each piece of tissue was placed in a fully labeled separate sterile screw capped universal bottle. Containers were fully labeled with the date, tissue and sterile instruments (knife, scalple, forceps and scissors) were used for collecting specimens for microbiological cultures. After collection and transportation to the laboratory, the samples were processed immediately (Gebremeskel et al., 2017).
Bacteriological sample processing
Culturing the tracheal swabs
The broth culture samples were incubated overnight under aerobic and anaerobic conditions, respectively. After 24 h of incubation the samples were thoroughly agitated, mixed and a loop of broth cultures was taken and streaked over labeled Petri plates containing blood agar base supplemented with 7% sheep blood as described by Quinn et al. (1994).
Culturing the lung tissues
The outer surfaces of the lungs were first seared with a heated spatula, followed by cutting and mincing of the inner surface of the lungs using sterile scissors and forceps, and then transferred to sterile Petri dish. The minced interior part of the lungs were further incised with sterile scalpel blade, then printed on the blood agar and streaked with wire loop. All bacteriological procedures were conducted in a level two biological safety cabinet.
Cultural characterization and bacteriological examination
The growths of typical colonies on blood agar were characterized based on the presence or absence of hemolysis, the type of hemolysis and general appearance of the colonies (color, shape, size, consistency etc.). On MacConkey agar, the colonies were examined for the presence or absence of growth, general appearance and ability to ferment lactose (Sharma and Adlakha, 1996; Alemneh and Tewodros, 2016). All cultures were incubated under aerobic and anaerobic conditions at 37°C for 24 to 48 h.
Isolation and Identification
Single colony type from pure cultures on blood agar was transferred to nutrient agar for a series of primary and secondary biochemical tests. Primary tests such as Grams staining, motility, catalase, oxidase, and oxidative-fermentative (O-F) tests were conducted. In addition, secondary biochemical tests including indole, methyl red, and citrate utilization tests were performed for further confirmation of the isolates. General procedures for isolation and identification of Gram positive and Gram negative bacteria were as described by Carter (1984) and Quinn et al. (1994).
Data analysis
Descriptive statistics was performed to analyze the data obtained from the study. The number of each species/genera was expressed as a percentage in comparison to the total number of isolates.
Ethical approval
The study considered direct observation of slaughter animals in the abattoirs and took appropriate samples for further microbiological examination. As a result of this study, no animal was subjected to suffer. Nevertheless, ethical approval was conducted by Research Ethical Approval Committee of Addis Ababa University, School of Veterinary Medicine, Ethiopia.
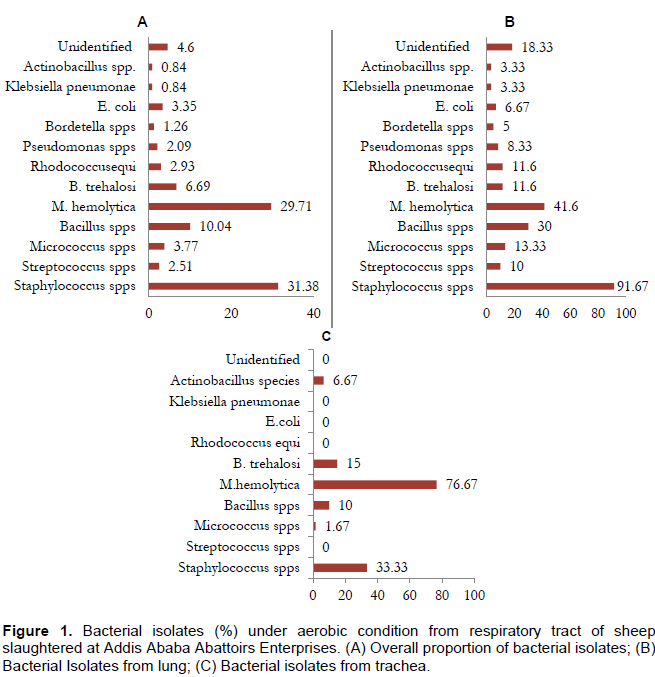
The predominant species among the aerobic isolates were Staphylococcus species (31.38%), followed by M. haemolytica (29.71%), Bacillus species (10.04%), B. trehalosi (6.69%), Micrococcus species (3.77%), E. coli (3.35%), Rhodococcus equi (2.93%), and Pseudomonas species (2.09%). On the other hand Streptococcus, Bordetella, Klebsiella and Actinobacillus were among the least encountered bacterial genera as indicated by Figure 1.
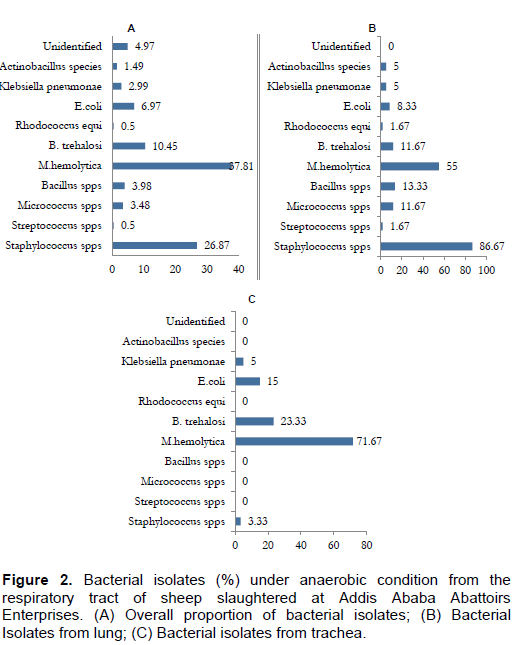
The majority of the isolates (aerobic and anaerobic) colonize the two anatomical sites investigated with Streptococcus, Rhodococcu equi, Pseudomonas, and Actinobacillus as exceptions which were not seen in trachea. However, a general increase in the isolation rate was observed as one that goes down the respiratory tract. In a nutshell, Gram positive bacteria were the predominant species inhabiting the respiratory tract in aerobic condition, whereas Gram negative bacteria predominate in anaerobic conditions.
A total of 191 facultative bacteria were isolated in anaerobic condition with Gram negative bacteria as the dominant isolates. The isolated bacteria with their isolation rate include M. haemolytica (37.81%), Staphylococcus species (26.87%), B. trehalosi (10.45%), E. coli (6.97%), Bacillus species (3.98%), Micrococcus species (3.48%), K. pneumonae (2.99%), Actinobacillus species (1.49%), Streptococcus species (0.5%) and Rhodococcus equi (0.5%) as denoted by Figure 2.
The present study assessed the frequency and type of aerobic and facultative anaerobic bacteria isolated from ovine pneumonic lungs and their associated trachea at Addis Ababa Abattoir Enterprises, Central Ethiopia among which several of them were isolated. A number of workers such as Gebremeskel et al. (2017), Akloul and Mohammed (2016), Sarker et al. (2016), Porter et al. (1994), Al Sultan (1995), Barbour et al. (1997), Almeida et al. (1986), Okolo (1985), Esra et al. (2009) and Richard et al. (1986) have isolated similar bacteria from pneumonic lungs of domestic animals. The invariable isolation of those organisms from pneumonic lungs of various animal species may indicate their significance in various respiratory syndromes in different animal species (Megra et al., 2006).
Among 239 isolates, Staphylococcus species were the dominant bacteria having high proportion accounts (23.25 and 35.95%) of the trachea and lung isolates, respectively. In agreement to this study Staphylococcus species were isolated and reported in high proportion by few studies such as Alley (1975) and Queen et al. (1994). Other investigations includes that by Esra et al. (2009) who isolated Staphylococcus spp. from unhealthy Holstein cattle nasal cavities with high frequency and percentage, and Asaduzzaman et al. (2013) who reported on Staphylococcus species from Black Bengal goat in Bangladesh. These indicate that there is a probability of association between these bacteria and pneumonic syndrome of lung. The bacteria are commensally living in the mucous membrane of the upper respiratory tract of animals and are opportunistic pathogens (Rashid et al., 2014; Quinn et al., 1994).
Bacillus species has been described as ubiquitous microbes found in nature as normal micro-flora. Thus, their role in the pathogenesis of respiratory infections is thought to be insignificant (Carter et al., 1995; Garedew et al., 2010). A high proportion of Bacillus species was obtained mainly from the lungs in this study. Other studies reported similar data such as Asaduzzaman et al. (2013), Garedew et al. (2010), Rajiv et al. (2000) and Shigidi (1973) from various domestic animals.
Micrococcus species is known primarily to be a normal flora of the respiratory tract. However, in most of the infections along with other pathogens, it may flare up and act as a secondary invader (Akloul and Mohammed, 2016; Carter, 1984). Our finding indicated that only 3.77% of Micrococcus species were isolated from ovine pneumonic lungs, with similar studies found to have agreed with present results such as Marru et al. (2013) and Esra et al. (2009).
Streptococcal are resident flora of the upper respiratory tract mucous membrane and commonly associated with suppuration and abscess formation (Quinn et al., 1994; Obasi et al., 2001). Although streptococci have not been associated with respiratory problems in ruminants, they are well known pathogens in humans, equine and camels. In this study, isolation of these bacteria might be an indication of its involvements as opportunistic pathogens in sheep pneumonia. M. haemolytica, is a normal flora of the upper respiratory tract and may play a secondary role after the primary initiating agent suppressed the host’s defense mechanisms, and favors the multiplication of Pasteurella species leading to bronchopneumonia (Aiello and May, 1998; Buxton and Frazer, 1977). Stress factors with or without viral infections interact to suppress the host defense mechanisms which allow the proliferation of commensal bacteria in the respiratory tract of animals (Baker, 1998).
This study revealed that the isolation of multiple bacterial species from the respiratory tracts of pneumonic sheep signifies their possible role in the involvement of respiratory disease complex. However, viruses and mycoplasma species are expected to reside in the respiratory tract. Therefore, the extent of the impact of respiratory diseases on sheep production and a complete understanding of the respiratory microbial flora, both culturable and unculturable condition give future research warranty.
The authors have not declared any conflict of interests.
REFERENCES
|
Aiello E, May A (1998). The Merck Veterinary Manual. 8th ed. Merck and Co., Inc.,NewJersey. USA, pp. 1053-1057.
|
|
|
|
Alley MR (1975). The bacterial flora of the respiratory tract of normal and pneumonic sheep. New Zealand Vet. J. 23(6):113-118.
Crossref
|
|
|
|
Akloul K, Mohammed NM (2016). Pneumonia in Algerian OuledDjellal sheep: Bacteriological study and macroscopic aspect of lung lesions. Afr. J. Microbiol. Res. 10.40:1685-1693.
Crossref
|
|
|
|
Alemneh T, Tewodros A (2016). Sheep and goats pasteurellosis: Isolation, identification, biochemical characterization and prevalence determination in FogeraWoreda, Ethiopia. J. Cell Anim. Biol. 10(4):22-29.
Crossref
|
|
|
|
Al Sultan II (1995). Bacterial isolation from pneumonic lungs in sheep. Iraq J. Vet. Sci. 8(2):213-215.
|
|
|
|
Almeida PFJ, AlvesFS J, Santos LF, Posa JS, DE-Almeida PF (1986). Revista de Micro Biologica, Brazil 17(3):213-215.
|
|
|
|
Angen O, Aherns P, Teptimeicer C (1998). Development of a PCR test for identification of Haemophilus somnus in pure and mixed cultures. Vet. Microbiol. 63:39-48.
Crossref
|
|
|
|
Asaduzzaman KHM, Nazmul HN, Mostafizur R, Khondokar SA (2013). Isolation and identification of bacteria from upper respiratory tract of Black Bengal goat in Bangladesh and investigation of some epidemiological parameters related to pneumonia. Sci. J. Microbiol. 2(11):207-213.
|
|
|
|
Baker JC (1998). Respiratory disease of cattle. In: Merck Veterinary Mannual. 8ed. Merck and Rhone-Poulence Company, pp. 1068-1069.
|
|
|
|
Barbour EKJ, Nabbut NH, Hamadeh SK, Al Nakhli HM (1997). Bacterial identity and characterstics in healthy and diseased respiratory tract of sheep and calves. Am. Vet. Res. Commun. 21(6):401-430.
|
|
|
|
Borji H, Azizzadeh M, Ebrahimi M, Asadpour M (2012). Study on small ruminant lungworms and associated risk factors in northeastern Iran. Asian Pacific J. Trop. Med. 5(11):853-856.
Crossref
|
|
|
|
Buxton, Fraser G (1977). Animal Microbiology Volume 1. Black well Scientific Publications Edinburgh, London, pp. 151-163.
|
|
|
|
Carter GR (1984). Diagnostic procedures in veterinary bacteriology and mycology, 4th ed., pp.12-47.
|
|
|
|
CSA (Central Statistical Authority) (2014-15). Ethiopian agricultural sample survey. Vol. II Report on livestock and livestock characteristics. Statistical Bulletin 388. CSA, Addis Ababa, Ethiopia.
|
|
|
|
Esra S, Yhaya K, Selattin K (2009).Bacterial examination in the nasal cavity of apparently healthy and unhealthy Holstein cattle. J. Anim. Vet. Advan. 8:2355-2359
|
|
|
|
Fikru S, Gebeyew K (2015). Sheep and goat production systems in Degehabur Zone, Eastern Ethiopia: challenge and opportunities. J. Advan. Dairy Res. 3:134.
|
|
|
|
Fulton RW (2009). Bovine Respiratory disease research (1983-2009). Anim. Health Res. Rev. 10(2):131-139.
Crossref
|
|
|
|
Garedew L, Gelagay A, Roman Y, Aschalew Z, Esayas G (2010). Isolation of diverse bacterial species associated with maedi-visnainfection of sheep in Ethiopia. Afr. J. Microbiol. Res 4(1):14-21.
|
|
|
|
Gupta MP, Kumar H, Singla LD (2009). Trypanosomosis concurrent to tuberculosis in black bucks. Ind. Vet. J. 86:727-728.
|
|
|
|
Jim K (2009). Impact of Bovine Respiratory Disease (BRD) from the perspective of the Canadian beef producer. Anim. Health Res. Rev. 10(2):109 -110.
Crossref
|
|
|
|
Jury MR, Funk C (2013).Climatic trends over Ethiopia: regional signals and drivers. Int. J. Climatol. 33(8):1924-1935.
Crossref
|
|
|
|
Gebremeskel AK, Tesema TS, Yegoraw AA, Birhanu BT, Mekuria SA (2017). Isolation and Characterization of Bacterial Species from Respiratory Tracts of Cattle Slaughtered in Addis Ababa City, Central Ethiopia. World Vet. J. 7(1):14-20.
Crossref
|
|
|
|
Kaur S, Singla LD, Hassan SS, Juyal PD (2009). Standardization and application of indirect plate ELISA for immunodiagnosis of paramphistomosis in ruminants. J. Parasitic Dis. 33(1-2):70-76.
Crossref
|
|
|
|
Lees VW, Meek AH, Rosendal S (1990). Epidemiology of Haemophilus somnus in young rams. Can. J. Vet. Res. 54(3):331.
|
|
|
|
Leta S, Mesele F (2014). Spatial analysis of cattle and shoat population in Ethiopia: growth trend, distribution and market access. SpringerPlus 3(1):310.
Crossref
|
|
|
|
Lin YC, Miles RJ, Nicholas RA, Kelly DP, Wood AP (2008). Isolation and immunological detection of Mycoplasma ovipneumoniae in sheep with atypical pneumonia, and lack of a role for Mycoplasma arginini. Res. Vet. Sci. 84(3):367-373.
Crossref
|
|
|
|
Marru HD, Anijajo TT, Hassen AA (2013). Study on Ovine pneumonic pasteurellosis: Isolation and Identification of Pasteurellae and their antibiogram susceptibility pattern in Haramaya District, Eastern Hararghe, Ethiopia." BMC Vet. Res. 9(1):239.
Crossref
|
|
|
|
Megra T, Sisay T, Asseged B (2006).The aerobic bacterial flora of the respiratory passageways of healthy goats in Dire Dawa Abattoir, Eastern Ethiopia. Revue de médecinevétérinaire 157:84-87.
|
|
|
|
Mohan M, Aprajita, Panwar NK (2013). Effect of Wood Dust on Respiratory Health Status of Carpenters. J. Clin. Diagn. Res. 7(8):1589-1591.
|
|
|
|
Monot M, Archer F, Gomes M, Mornex JF, Leroux C (2015). Advances in the study of transmissible respiratory tumours in small ruminants. Vet. Microbiol. 181(1-2):170-177.
Crossref
|
|
|
|
NMSA (2005). National metrology service agency, Ethiopia.
|
|
|
|
Obasi OL, Ragi MA, Adogwa T, Natala AJ (2001). The effect of climaticfactors on the occurrence and gross pathological lesion in bacterial pneumonia of ovine and caprine hosts in Zaria, Nigeria. Glob. J. Pure Appl. Sci. 7:57-60.
|
|
|
|
Office International des Epizooties (OIE) (2008). Manual for terstitial animal disease.
|
|
|
|
Okolo MIO (1985). Pathological conditions found in Goat killed at Slaughter houses in Nusuku. Nig. J. Anim. Prod. 12(125):61-65.
|
|
|
|
Pawar PD, Khasnis MW, hong CD, Khole RP, Mhase PP, Singla LD (2017). Coproprevalence and successful management of parasitic infections in sheep in Pune district of Maharastra.. Proceedings of 25th National Congress of Veterinary Parasitology and International Symposium on Current Concepts in Diagnosis & Control of Parasitic Diseases to Combat Climat Change (15-17 February, 2017) at Veterinary College, Shimoga. P 56.
|
|
|
|
Porter JF, Connor K, Donachie W (1994). Isolation and characterization of Bordetellaparapertussis-like bacteria from ovine lungs. Microbiology 140(2):255-261.
Crossref
|
|
|
|
Queen C, Ward AC, Hunter DL (1994). Bacteria isolated from nasal and tonsillar samples of clinically healthy Rocky Mountain bighorn and domestic sheep. J. Wildlife Dis. 30(1):1-7.
Crossref
|
|
|
|
Quinn PJ, Carter ME, Markery B, Carter GR (1994). Clinical Veterinary Microbiology. London: Wolfe Publishing Company, pp. 178-182.
|
|
|
|
Radostits OM, Gar CC, Blood DC, Hinchcliff KW (2000). Veterinary Medicine.A text book of the Disease of Cattle, Sheep, Pigs, Goats and Horses.9th ed., Harcourt Publishers Limited, London Philadelphia, pp. 701-867.
|
|
|
|
Rajiv K, Katoch RC, Prasenjit D (2000). Bacteriological studies on pneumonic Gaddi sheep of Himachal Pradesh. Ind. Vet. J. 77:846-848.
|
|
|
|
Rashid MM, Ferdoush MJ, Dipti M, Roy P, Rahman MM, Hossain MI, Hossain MM (2014). Bacteriological and pathological investigation of goat lungs in Mymensingh and determination of antibiotic sensitivity. Bangladesh J. Vet. Med. 11(2):159-166.
Crossref
|
|
|
|
Richard YJ, Menour NJ, Cuiguen FJ, Avier CJ, Borges E, Fontain, MJ,Audar J, Brunet J, Pailhac C (1986). Bacteriological study on sheep lungs from the abattoir. Reve De Medecine Veterinaire 137(10):671-680.
|
|
|
|
Sarker CK, Hossen A, Yousuf MA, Uddin MA, Akter MS, Rahman M, Rahman MB (2016). Isolation, identification and characterization of bacterial flora from the respiratory tract of apparently healthy sheep. Asian J. Med. Biol. Res. 1(3):677-685.
Crossref
|
|
|
|
Sharma SN, Adlakha SC (1996). Text book of Veterinary Microbiology. New Delhi: Vikas Publishing House, pp. 63-55.
|
|
|
|
Shigidi AM (1973). Aerobic micro flora of respiratory tract of camels. Sudan J. Vet. Sci. Anim. Husband.14 (1):9-14.
|
|
|
|
Singh R, Bal MS, Singla LD, Kaur P (2017). Detection of anthelmintic resistance in sheep and goat against fenbendazole by faecal egg count reduction test. J. Parasitic Dis. 41(2):463-466.
Crossref
|
|
|
|
Tesfaye B, Tessema TS, Tefera G (2013). Diversity of bacterial species in the nasal cavity of sheep in the highlands of Ethiopia and first report of Histophilus somni in the country. Trop. Anim. Health Prod. 45(5):1243-1249.
Crossref
|