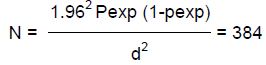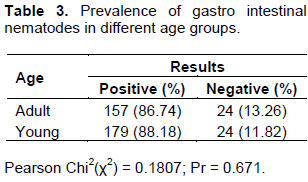ABSTRACT
A study by qualitative fecal examination of 384 fecal samples (201 sheep and 183 goats) was conducted from November 2011 to April 2012 with the objective to determine the major gastrointestinal (GIT) nematodes of small ruminants and their prevalence in sheep and goats in Guto Gida District. The study showed that 186 (92.5%) sheep and 150 (81.97%) goats were found to harbor eggs of GIT nematodes. Both sheep and goats were infected with identical parasites species, but with different level of infection. The six genera of nematodes were identified with prevalence of 21.87, 14.87, 12.5, 10.67, 11.19 and 7.29% for Haemonchus, Trichostrongylus, Trichuris, Oesophagostomum, Bunostumum and Strongloides, respectively. There was a significant difference (p<0.05) in the prevalence of GIT nematodes between sex and species of animals but not for different age group. The study showed that GIT nematodes are major problems of small ruminants in the study area. Therefore, comprehensive study on GIT nematodes, cost effective control strategy and awareness creation to the farmers should be instituted in the area.
Key words: Sheep, goats gastrointestinal nematodes, Guto Gida District, Nekemte.
In Ethiopia, helminth infections in ruminants are characteristically chronic and insidious in nature. The parasites attract very little attention, including research funds, when compared with viral, bacterial and some protozoan diseases. This is despite of the fact that they undoubtedly exert a heavy toll on the health and productivity of a vitally important livestock resource with obvious implications for the rural and national economy of the country. Gastro intestinal parasites are a worldwide problem for small and large scale farmers, and their impact is major for sub-Saharan Africa in general and Ethiopia in particular. This is due to the range of agro-ecological factors suitable for diversified host and parasite species (Regassa et al., 2006). Endoparasites are responsible for the deaths of one third of calves, lambs and goat kids, and considerable production losses due to parts of carcasses, being condemned during meat inspection. It is well recognized that in a resource poor country, helminth infections of sheep and goats are factor responsible for economic losses through reductions in productivity (Abunna et al., 2009, Abouzeid et al., 2010). Although helminth parasites of small ruminants are ubiquitous in the climatic zones of Ethiopia where prevailing weather provides favorable conditions for their survival and development, their presence does not mean that they necessarily cause overt disease (Abunna et al., 2009).
Among the diseases that constrain the survival and productivity of sheep and goats, gastrointestinal nematode infection rank highest on the global scales, with Haemonchus contortus recognized as a major parasite for both small and large scale small ruminant production (Abunna et al., 2009). These disease have major impact on morbidity and mortality rates with annual losses as high as 30 to 50% of the total value of livestock production of Ethiopia (Abunna et al., 2009). With little inputs, sheep and goats play an important role in rural economy through the provision of meat, milk, blood and cash, accumulating capital, fulfilling cultural obligations, manure and contribution to the national economy through export of live animals meat and skins (Abunna et al., 2009).
The prevalence of gastro intestinal parasite, the genera of helminthes parasites involved species and the severity of infection also vary considerably depending on local environmental and managements practices (Singla 1995; Taylor et al., 2007). Therefore, the distribution and prevalence of the disease should be represented by geographical areas that could roughly correspond to climatic conditions (Regassa et al., 2006). These, information are important in the formulation of parasites control strategies since the degree of infection varies according to the parasites involved and other factors (Urquhart et al., 1996; Wadhawa et al., 2011). Thus, the study was designed to determine the prevalence the major GIT nematodes of sheep and goat in Guto Gida Woreda to recommend the most effective control strategies.
The study was conducted from November 2011 to April 2012 in Guto Gida District, East Wollega zone, Oromia regional state. It is located in western part of Oromia at latitude of 09° 04׳; 957×´N, longitude of 36°, 32׳, 928×´ E and altitude of 2124 m above sea level. It is located at 331 km far from our capital city Addis Ababa. It is annual rainfall range from 1800 to 2200 mm. The maximum temperature is 25°C and the minimum temperature of the area is about 20°C. The area receives long rainy season from June to September and short rainy season from March to May. The area is rich in natural vegetation that comprised of tropical rain forest trees, all grasses and brushes (data obtained from agricultural office).
Study animals and design
A cross-sectional study was conducted on small ruminants of all age and sex category to determine the prevalence of nematodes parasites by collecting fecal samples from individual grazing animals.
Sample size determination
Simple random sampling strategy was followed to collect feces from individual animals. The sample size was decided based on the formula described by Thrusfield (2005) with 95% confidence interval at 5% desired absolute precision and by assuming the expected prevalence of 50%. The estimated sample size was calculated by the formula:
Where N = required sample size; pexp = expected prevalence; d = desired absolute precision.
Parasitological examination
Laboratory diagnosis using flotation, fecal culture and Baermann technique conducted for the identification of the parasite.
Collection of eggs
Faecal pellets collected from the rectum of sheep and goats were placed in small vial. Warm water was slowly added to the faeces and the pellets stirred until a relatively uniform homogenate was obtained and liquid suspension was obtained. The suspension was filtered through sieve with 3 mm aperture. The resulting suspension was again made to pass through a sieve of 150 μm pore size. The suspension was then poured into 15 ml test tubes and centrifuged for 2 min at 377 g and the supernatant was decanted. The tube was agitated by vortex mixer to loosen the sediment. Saturated sodium chloride was then added to the test tube until the meniscus forms above the test tube on which the cover slip was placed. After 3 to 5 min, the cover slip was carefully taken off the tube and put into microscope slide for observation (Bowman, 1999).
Fecal culturing
The method of incubating fecal samples at room temperature to hatch egg to larvae and development to L3 was followed, so that the larvae hatched can be indentified according to the criteria listed as follows: Taking certain amount of feces in a tray; incubating it under suitable moisture content for 14 to 21 days with continuous moistening at an interval of 3 days. The recovery larvae (L3) were studied and identified and the criteria used for identification were based on shape of larvae, head, number and shapes of gut cells, presence or absence of retractile bodies, larvae sheath coverage and length of sheath tail. Then L3 were harvested using Baerman apparatus after 14 days of incubation and were identified (Annon, 2005).
Data management and analysis
The collected sample was entered into Microsoft excel and analyzed using statistical software packages for social science (SPSS). Descriptive statistics like percentage can be used to determine prevalence of GIT nematode and Chi-square (χ2) used to check the association between prevalence of GIT nematodes and risk factors. In the analysis, confidence level was held at 95% and p<0.05 was set for significance.
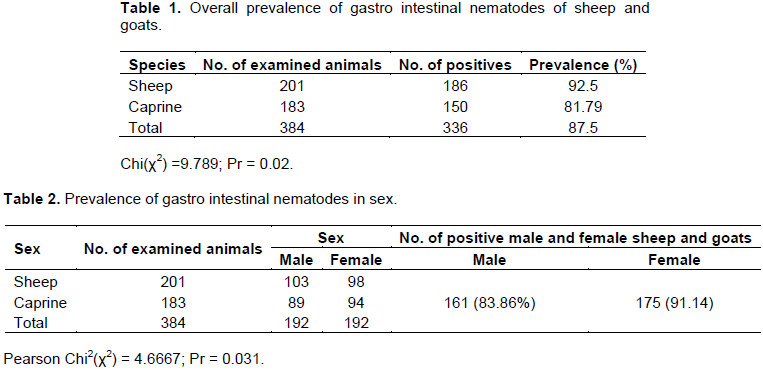
The overall prevalence of gastro intestinal nematodes infection was 87.5% and the species wise is 92.5 and 81.97% in sheep and goats, respectively, which was a significant difference in infection rate between the two animal species (Table 1). Prevalence of gastro intestinal nematodes of infection of sheep and goats on the basis of sex is shown in Table 2. There is statistical significant difference (P<0.05) in prevalence of gastro intestinal nematodes which was 175 (91.15%) and 161 (83.85%) in female and male, respectively. In this study species, sex and age were considered as a risk factors and revealed significant difference (p<0.05) with variation in species, and sex of animals while in the case of age, there is no significant difference (p>0.05) in sheep and goats but there is higher number of young sheep and goats infected than old age group.In this study, 6 types of gastro intestinal nematodes were identified during the study period based on their morphology described (Urquart et al., 1996). During the study period, fecal samples were cultured to determine genera prevalent as shown in Table 4. Haemonchus (21.87%) were the most frequently identified helminthes followed by Trichostrongylus, Trichuris with Strongloides been the least (7.29%) identified in the study.
In the present study, the prevalence of gastro intestinal nematodes was 87.5% (Table 1) in sheep and goats. This result coincides with the result of Gebreyesus (1986) (96.38%) at Ogaden range lands; Esayas (1988) (90.41 and 82.13%) in sheep and goats in and around Wolayita soddo; Tesfalem (1989) (88.1 and 84.32%) in sheep and goats in and around Mekelle; Melkamu (1991) (91.435%) in sheep in and around kombolcha; Bayou (1992) (90.94 and 94.855%) in sheep and goats of Gonder; Yoseph (1993) (92.23 and 94.1%) in sheep and goats in Mendayo district of Bale; Genene (1994) (93.22 and 92.24%) in sheep and goats of four Awarajas of Eastern shoa; Getachew (1998) (90.23 and 88.3%) in sheep and goats of Buno province of Iluababor; Tefera et al. (2009) (91.32 and 93.295%) in sheep and goats in and around Bedelle, respectively.
Statistically significant difference (p<0.05) (Table 1) was recorded between sheep and goats with relative lower number of goats infected than sheep which may be due to different habits of grazing by these two species of animals. This study also indicates significant different was observed between sex of animals and females are more infected than male (Table 2). Thus, pregnant or lactating ewes/does become the major source of infection for the new born. In some manner, other studies in Africa have shown that the age and immune status of the host animals have significant influences on the GIT eggs pus (Magona and Musis, 2002). On the contrary, there was no statistical significant difference (p>0.05) recorded in this study between young and adult but relatively higher number of young animals are infected than older once (Table 3). This could be due to equal exposure of both age groups of animals and they are from similar agro-ecological area.
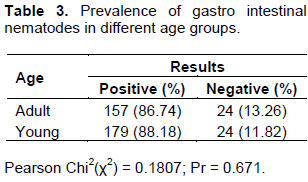
The prevalence of Haemonchus species was 21. 87% (Table 4) in sheep and goats in the study area which disagreed with the work of different scholars: Ahmed (1988) (88.23% prevalence in East Wollega zone); Kumsa and Wossene (2006) (95.1% in ogaden region); Naod et al. (2006) (81.18% in Awassa in Hawassa in sheep and goats). The prevalence of Trichostrongylus species was 14.8% in sheep and goats. This result is disagreed with Abunna et al. (2009) who reported prevalence of 90.45% in sheep and goats; Tefera et al. (2009) (43.5 and 55% in sheep and goats in and around Bedelle). But, this study is consistent with the finding reported by Sissay et al. (2007), where Haemonchus contrortus was the most prevalent parasites of GIN; this could be associated partly with breed susceptibility, biological plasticity and high environmental adaptability.
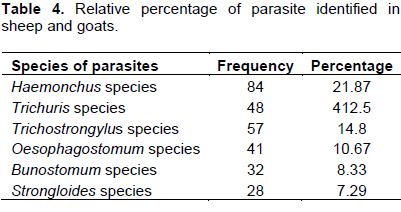
The prevalence of Strongloides species was detected to be 7.49% in sheep and goats in the study area and this is in line with the work of Tefera et al. (2009) (13.04%) in sheep and 20% in goats in Bedelle and the prevalence of Bunostomum species was detected to be 8.33% in sheep and goats in the study area and this was in disagreement Tefera et al. (2009) (26.1 and 35% in sheep and goats in and around Bedelle). This difference in the prevalence of parasite in different area might be attributed to the difference in breed of sheep and goats and different agro-ecological zones where the animals are kept.
CONCLUSION AND RECOMMENDATION
The result of present study showed the high prevalence rate of gastro intestinal nematodes infection in small ruminants in Guto Gida District, East Wolloega. There was highly significant difference in infection level on the basis of sex and species. This shows that the general scientific facts on those bases can be recognized in the study area and the traditional husbandry and animal management have also valuable contribution in the detection of gastro intestinal nematodes. The existence of these parasites has an impact on the productivity and can hamper the sustainability of the revenue generated from small ruminant production by small holder society of East Wollega zone. Therefore, based on the result obtained the following recommendation are forwarded:
1. To get clear epidemiological picture of GIN parasites, comprehensive study should be launched in the area.
2. Effective utilization of available of feed resource such as agricultural by products, natural pasture and appropriate nutritional supplements program for young and lactating animals.
3. Detail study should be conducted to identify the parasite using more sensitive and specific methods such as postmortem examination.
4. Strategic nematode control practice should be implemented.
5. Field veterinarian should be aware of the importance and burden of GIN in sheep and goats.
The authors declare that they have no conflict of interest.
REFERENCES
|
Abouzied N, Saalium AM, Heady KEL (2010). The prevalence of gastro intestinal parasite infection sheep zoo garden and sinai district and study the efficacy of ant helminthic drugs in treatment of these parasites. Faculty of veterinary medicine zagazing university, Egypt.
|
|
|
|
Abunna F, Tsedeke E, Kumsa B, Megersa B, Regessa A, Debala E (2009). Abomsal nematode: prevalence in small ruminants slaughtered at Bishoftu town, Ethiopia. Int. J. Vet. Med. 7:1.
|
|
|
|
|
Ahmed N (1988). Prevalence of GIT helminthes and the comparative efficiency test of nematocidal drugs in goats of Wollega administrative region at mechara settlement area. DVM. Thesis Addis Ababa University Faculty of veterinary medicine Ethiopia.
|
|
|
|
|
Annon (2005). Small ruminants research strategy. Ethiopian agricultural research organization (EARO), animal science research directorate, Addis Ababa, Ethiopia.
|
|
|
|
|
Bayou A (1992). Prevalence of gastro intestinal helminthes of small ruminants in Buno provine llubabor administrative region. DVM Thesis Addis Ababa University Faculty of veterinary medicine Ethiopia.
|
|
|
|
|
Bowman DD (1999). Georgies parasitology for veterinarians, 7th ed. W.B. Sconders, Philadelphia.
|
|
|
|
|
Esayas T (1988). Study on prevalence of GIT helminthes in Ogaden goats. DVM thesis, Faculty of veterinary medicine, Addis Ababa University, Ethiopia.
|
|
|
|
|
Gebreyesus M (1986). Prevalence of gastro intestinal helminthes of small ruminants (sheep and goats) in Gonder administrative region. DVM. Thesis, Faculty of veterinary medicine, Addis Ababa, Ethiopia.
|
|
|
|
|
Genene R (1994). A study on prevalence of ovine GIT helminthes in and around kombolcha. DVM. Thesis, Faculty of veterinary medicine, Addis Ababa University Ethiopia.
|
|
|
|
|
Getachew G (1998). Prevalence of ovine and caprine GIT helminthes in mekelle and its surrounding. DVM Thesis, Faculty of veterinary medicine, Addis Ababa University, Ethiopia.
|
|
|
|
|
Kumsa B, Wossene A (2006). Abomsal nematodes of small ruminants of ogaden region, Eastern Ethiopia, prevalence, worm burden and species composition. Revue Med.Vet. 157:12:27-32.
|
|
|
|
|
Magona JW, Musis G (2002). Influence of age, grazing system, season and agro climatic zones on the prevalence and intensity of gastro intestinal strongloides in Uganda goats. Small Ruminates Res. 44:285-290
Crossref
|
|
|
|
|
Melkamu T (1991). Prevalence of gastro intestinal helminthes of small ruiminants in four awrajas of Eastern shoa administrative regions, DVM, thesis, AAU, Debrezeit, Ethiopia.
|
|
|
|
|
Naod T, Teshale S, Bersisa K (2006). Prevalence of abomsal nematodes and dicytocaulus filarial of sheep and goats slaughtered in Awassa restaurant. AAU, FVM, Ethiopia.
|
|
|
|
|
Regassa F, Sori T, Dhuguma R, Kassa Y (2006). Epidemiology of gastro intestinal parasite of Ruminants in Western Oromia, Ethiopia. Int. J. Appl. Res. Vet. Med. 4(1):51.
|
|
|
|
|
Singla LD (1995). A note on sub-clinical gastro-intestinal parasitism in sheep and goats in Ludhiana and Faridkot districts of Punjab. Indian. Vet. Med. J. 19:61-62.
|
|
|
|
|
Sissay MM, Uggla A, Waller PJ (2007). Epidemiology and seasonal dynamics of gastrointestinal nematode infections of sheep in a semi-arid region of eastern Ethiopia. J. Vet. Parasitol. 143:311-321
Crossref
|
|
|
|
|
Taylor MA, Coop RL, Wall RL (2007). Veterinary Parasitology 3rd ed UK; Black well science.
|
|
|
|
|
Tefera M, Batu G, Bitew M (2009). Prevalence of gastro intestinal parasite of sheep and goat in and around Bedele, south Western Ethiopia. Internet J. Vet. Med. 8:2.
|
|
|
|
|
Tesfalem T (1989). Prevalence of GIT helminthes of small ruminants in mendayo province Bale administrative region, Ethiopia, DVM thesis AAU, Ethiopia.
|
|
|
|
|
Thrusfield M (2005). Veterinary Epidemiology 3rd edition, UK, Black well science.
|
|
|
|
|
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jenning FW (1996). Veterinary Parasitology, 2nd, Black well. Science, UK. P 307.
|
|
|
|
|
Wadhawa A, Tanwar RK, Singla LD, Eda S, Kumar N, Kumar Y (2011) Prevalence of gastrointestinal helminths in cattle and buffaloes in Bikaner, Rajasthan, Indian. Vet. World 4(9):417-419.
Crossref
|
|
|
|
|
Yoseph S (1993). Prevalence of ovine GI helminthes in and around Asella, Ethiopia. DVM thesis, AAU, Ethiopia.
|
|