ABSTRACT
Thermostable Newcastle disease vaccine virus strain I2 (NDVI2) was investigated for its efficacy as foodborne vaccine using maize, sorghum and their brans as carriers. Immune response to vaccination and resistance to challenge were assessed by haemagglutination inhibition (HI) test. After primary and secondary vaccination at three and six weeks of age, sera and feather pulp samples were analyzed to determine the antibody titre in the different groups. The highest mean antibody titre of 7.39 ± 0.42 log2 was recorded for serum when the vaccine was administered through treated sorghum coated with gum Arabic (TSGG) and 7.28 ± 0.37 log2 for feather pulp in the group given maize bran (MZB) at eight weeks of age. There was no significant difference (p ˃ 0.05) between the HI antibody titre in the feed groups from feather pulp samples at three weeks of age while a significant difference (p < 0.05) in the serum antibody titre was observed between all the feed groups at five weeks of age. There was correlation in antibody titre between serum and feather pulps only at two weeks after second vaccination. The protection rate after challenge in all the groups was low with the highest rate (14%) recorded when the vaccine was administered in treated maize (TMZ) and TSGG. The study concluded that the vaccine could be effective for the protection of village chickens as food-borne vaccine provided the carrier foods are adequately treated to remove antiviral agents. The use of feather samples as suitable alternative to serum for ND serology was discussed.
Key words: Chickens, maize, sorghum, Newcastle disease, thermostable vaccine.
Newcastle disease (ND) is one of the major important viral disease of poultry which had caused huge economic loses to farmers in recent past (Aamir, 2014). Newcastle disease virus (NDV) has a wide range of hosts, as more than 250 bird species have been found to be susceptible by natural or experimental infections, although wild waterfowl and shorebirds are regarded to be the reservoir of the virus in nature (Kaleta and Baldouf, 1988). Among avian species, the poultry flocks are commonly affected with this disease, chickens are most susceptible while ducks and geese are least susceptible to ND (Khan et al., 2000). ND is presently one of the most important endemic disease of poultry in Nigeria, causing high morbidity, mortality, decrease in eggs production and it constitutes a major constraint to the development of rural poultry production (Abdu et al., 1992).
In Nigeria, ND is controlled by vaccination of commercial birds with live thermolabile lentogenic or mesogenic NDV vaccines containing between 100 and 1000 bird dose per vial. The vaccines are administered intramuscularly, intraocularly or orally in water (Abdu et al., 2012). This is impracticable for a village farmer because the method of administration requires the catching and handling of birds and there is no guarantee that local birds will drink vaccine treated water (Abdu et al., 2012). The vaccine dose format is also not meant for village flocks containing between 7 and 29 birds (Otchere, 1990). Thermostable Newcastle disease virus (NDV) vaccines have been used widely to control ND for village poultry flocks, due to their independence of cold chains for delivery and storage (Guoyuan et al., 2015).
The NDV vaccine strain I2 has undergone laboratory test in several countries and has proved to be protective against local virulent strains. In Vietnam, it has been officially recognized as the NDV vaccine for village chicken after extensive laboratory and village trials (Tu et al., 1998). In Tanzania, it has given protection for at least two months after vaccination (Wambura et al., 2000). Field records in Mozambique indicated that NDVI2 vaccine provides approximately 80% protection in the field of an outbreak when given every month via eye drop (Pangani, 1999). The NDVI2 vaccine is being tested in several African countries (Alders and Spradbrow, 2001).
The vaccine has been used successfully in village chickens populations in many countries in Asia and Africa including Nigeria (Jayawardane et al., 1990; Jagne et al., 1991; Ibrahim et al., 1992; Echeonwu et al., 2008a). Besides, the successes recorded by many researchers using the V4 and I2 thermostable ND vaccine as feed based vaccine (Nasser et al., 1998; Wambura et al., 2000), there are some basic problems reported to be associated with feed-based vaccination (Cumming, 1992). Firstly, not all types of feed are suitable for the delivery of NDV vaccine in terms of suitability to the chicken and delivery of the virus for protection. Secondly, the type of food vehicle to be used is determined by the availability of that particular feed in a locality (Philemon et al., 2007).
The use of feather shafts of chickens for the diagnosis of viral infections and for monitoring vaccine viruses has been reported (Davidson, 2009). Other researchers (Dong-Hun et al., 2016) also detected viral antigens in feathers of chickens infected with viscerotropic velogenic NDV suggesting that feathers could act as source of viral transmission. The threat of ND to the poultry industry requires routine seromonitoring of vaccinated chickens to show that they have been adequately immunized against the disease (Ameh et al., 2016). To do this effectively, serum samples need to be collected at regular intervals. However, farmers are generally reluctant to allow for collection of serum samples from their birds after vaccination. This study was therefore conducted to determine the suitability of maize and sorghum and their respective brans as delivery systems for NDVI2 vaccines and also to study the suitability of using feather pulps as an alternative source of sample for seromonitoring of vaccinated chickens against ND in the study area.
Study area
The study was conducted at the Nutrition Laboratory of the Veterinary Medicine Department of Ahmadu Bello University Zaria, Nigeria. Zaria is located in Kaduna State, Nigeria; it is a part of the central high plains of Northern Nigeria and about 670 m above sea level. Zaria is located at latitude 11.11°N and longitude 7.73°E. It has two distinct seasons: the dry or harmattan season (October to March) and wet season (April to September) with a population of 975,153 (Oladipo, 1985).
Experimental birds
Two hundred day-old unvaccinated cockerels were obtained from the Poultry Research Farm of the National Veterinary Research Institute Vom, Nigeria. The chicks were housed in a brooding room that was cleaned, washed, disinfected and fumigated. All chicks were placed under brooders with chicks mash and water provided ad libitum. At three weeks of age, 18 chicks each were randomly selected and placed in cages with wire mashed floors measuring 56.5 × 56.5 cm until the termination of the experiment.
Experimental design
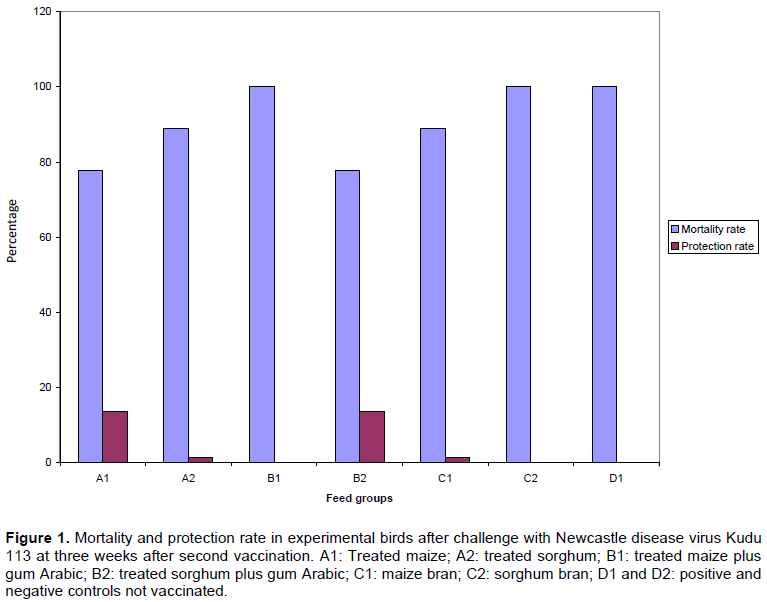
The chicks were divided into 4 groups (A, B, C and D) at three weeks of age. Each group was subdivided into 2 subgroups each consisting of 18 birds. Groups: A1 (treated maize), A2 (treated sorghum), B1 (treated maize plus treated gum Arabic), B2 (treated sorghum plus treated gum Arabic), C1 (maize bran), and C2 (sorghum bran). All birds in subgroups A to C were vaccinated and challenged. Birds in subgroup D1 were not vaccinated but challenged and D2 were unvaccinated and unchallenged and served as positive and negative controls, respectively.
Source of NDVI2 vaccine and challenge virus
The NDVI2 vaccine was obtained from the Viral Research Department, National Veterinary Research Institute (NVRI) Vom, Plateau State, Nigeria. The vials of the vaccines were 50 dose vials meant to be reconstituted in 50 ml of chlorine free water and to be giving orally at 1 ml/bird. The virus strain used for the challenge study was the NDV (Kudu 113 strain) isolated and characterized in a previous study (Echeonwu et al., 1993) with EID50 titre of 107.5. The virus was obtained from the Virology Division of the NVRI, Vom.
Preparation and coating of food carrier with vaccine virus
Five kilograms each of maize, sorghum and their bran and 4 kg of gum Arabic were used. The maize and sorghum were milled once to remove the husk and then crushed into a gritty mash. These were soaked in chlorine free water for 72 h, while changing the water daily. The soaked grains were then washed with clean water, sieved and placed to dry in the sun. They were then weighed and packaged into polythene bags of 1 kg/package and stored at room temperature until used. The maize and sorghum brans were not subjected to any treatment; they were dried, packaged and kept at room temperature until used. About 2 kg of gum Arabic (used as additive) was soaked to dissolve overnight in 1,000 ml of distilled water. The gum Arabic was then boiled for an hour, allowed to cool and then autoclaved at 121°C for 15 min.
The method described by Alders and Spradbrow (2001) was used for coating the feed grain and brans with the vaccine virus. The quantity of grains or brans consumed by 18 birds (10 g per bird) was measured and the time taken to consume the vaccine feed was noted. Three vials of the 50 doses of NDVI2 vaccines were reconstituted in 100 ml of PBS (pH 7.4). Then 50 ml of the treated diluted gum Arabic was thoroughly mixed with the reconstituted vaccine (total 150 ml) and then mixed with the feed in a bowl and then spread on trays and kept at room temperature for 30 min before administrating to the birds.
Vaccinations
First and second dose of NDVI2 coated on the treated grains and bran were given to the birds at 3 and 6 weeks of age, respectively.
Serum samples
About 1 to 2 ml of blood was collected through the wing vein of each bird with a 2 ml syringe and 21 G needles on days 7, 14 and 21 before primary vaccination and at 2 and 3 weeks post vaccination. The blood samples were deposited into sterile test tubes and sera were separated by allowing the blood to clot in the test tubes slanted in racks at room temperature for 1 to 2 h. Sera collected were stored in a freezer at -20°C until tested.
Preparation of feather pulp for serology
The method described by Roy et al. (1998) with slight modification was used for preparing the feather pulp samples. Four down feathers, two from each wing were plucked from each bird, weighed and cut at the base to remove the pulp using a scissor. Laboratory pestle and mortar was used to grind the feather pulp which was then mixed with 2 ml of PBS, centrifuged at 2000 rpm for 5 min and the supernatant tested for NDV HI antibodies.
Haemagglutination (HA) and haemagglutination inhibition (HI) tests
Five millilitres of chicken blood was collected from newly hatched commercial chicks and transferred into 10 ml of Alsever’s solution and gently mixed. The red blood cells (RBCs) were washed three times with PBS pH 7.2 by centrifugation at 2000 rpm for 5 min each. The concentration of the RBCs used was 1% in 99 ml of PBS. The titre of a live La Sota NDV strain antigen obtained from NVRI was determined by the HA test. Four HA units were used in the HI test. All sera collected were tested for NDV specific antibody by the haemagglutination inhibition (HI) test using methods described by OIE (2004). The antibody level for each serum and feather pulp sample was expressed as a log to the base two and recorded. The geometric mean titers (GM) were calculated. In this study, the published cut off value was used for the protective HI antibody titer (HI titer≥log2 3, that is, GM≥3) for ND vaccination in chickens (Alexander et al., 2004; OIE, 2004).
Challenge studies
At nine weeks of age, three weeks after the second vaccination, all the birds except the negative controls were challenged with NDV Kudu 113 strain. Each bird received a dose of 0.2 ml through the oculonasal route. After challenge, the birds were observed for two weeks for clinical signs, gross lesions and death.
Data analysis
The mean HI antibody titre and percentage of birds with detectable ND antibody were calculated. Data collected were analyzed using Statistical Package for Social Sciences (SPSS) version 17 program. One way analysis of variance (ANOVA) was performed with Tukey post hoc multiple comparison, which determined statistical significant difference between subgroups at 95% confidence interval with p<0.05 considered as significant. The correlation coefficients were calculated to compare the mean HI ND antibody titres between serum and feather samples in the different groups. Mortality and protection rates were also calculated.
Antibody titre using serum
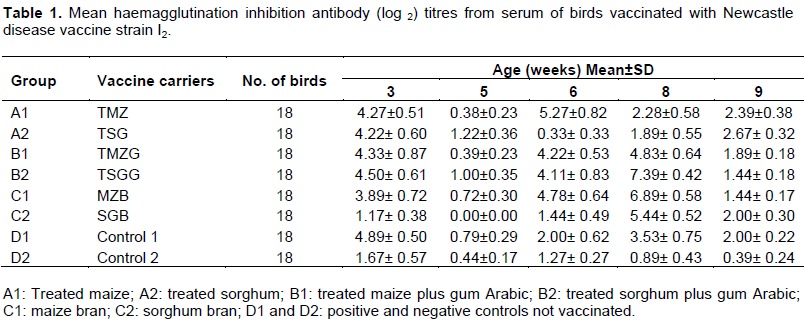
At three weeks of age the mean HI antibody titre was ≥ 3 log2 in all birds except those in group C2 (1.17 ± 0.38 log2) and D2 (1.67± 0.56 log2). Two weeks after primary vaccination (at five weeks of age), the HI ND antibody titre dropped in all groups with group A1 and C2 having the lowest mean HI ND antibody titre of 0.38 ± 0.23 log2. At six weeks of age, the lowest mean HI ND antibody titre was recorded in group A2 (0.33 ± 0.33 log2), while at eight weeks of age after secondary vaccination the highest mean HI ND antibody titre of 7.39± 0.42 log2 was recorded in groups B2 (Table 1).
Antibody titre using feather pulp
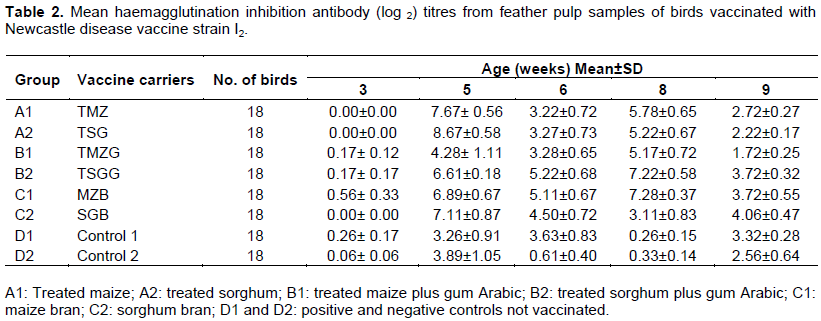
All the birds had low HI mean antibody titre before vaccination at three weeks of age with no detectable antibody titre in groups A1, A2 and C2 (Table 2). The response of birds to primary vaccination was high at five weeks of age with the highest mean HI antibody titre of 8.67 ± 0.58 log2 recorded in group A2; all other groups had mean HI antibody titre ≥ 3 log2. At six weeks of age the mean HI antibody titre in all the groups were ≥ 3 log2 except in group D2 which had the lowest HI antibody titre of 0.61 ± 0.39 log2. Antibody titre of birds in all the groups increased two weeks after booster vaccination (eight weeks of age) with the highest mean HI ND antibody titre recorded in group B2 (7.22 ± 0.58 log2) and 7.28 ± 0.37 log2 in group C, while the control groups had the lowest HI antibody titre of 0.26 ± 0.15 log2 in group D1 and 0.33 ± 0.14 log2 in group D2. At nine weeks of age, the mean HI antibody titre dropped again in all the groups except in groups B2,C1, C2 and D1 which had mean HI antibody titre ≥ 3 log2 (Table 2).
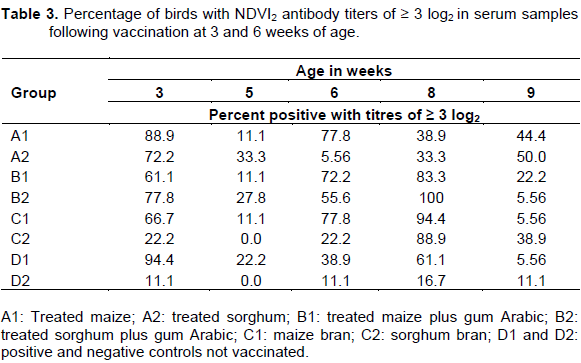
Percentage of birds with ND antibody HI titres of ≥ 3 log2 (serum)
At three weeks of age prior to primary vaccination, 88% of birds in group A1 and 94% of birds in group D1 had the highest HI antibody titre of ≥ 3log2 (Table 3). At five weeks of age, 33% of birds in group A2 and 27% in group B2 had HI antibody titre of ≥ 3log2. At six weeks of age, groups A1 and C1 had 77% of birds with HI antibody titre ≥ 3 log2. At eight weeks of age, 100% of birds in group B1 had HI antibody titre ≥ 3 log2. Prior to challenge at nine weeks of age, 44% of the birds in groups A1 and 50% in group A2 had HI antibody titres ≥ 3 log2 (Table 3).
Percentage of birds with ND antibody HI titres of ≥ 3 log2 (feather pulp)
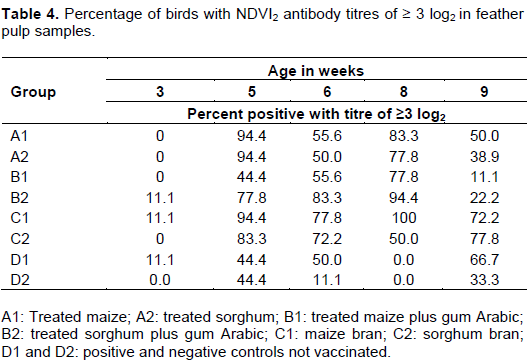
At five weeks of age, 94% of the birds in groups A1, A2, and C1 had HI antibody titres of ≥ 3log2, while group C2 had 83% of birds with ≥ 3 log2 at five weeks of age. The highest percentage of birds (83%) with titres ≥ 3 log2 at six weeks of age was recorded in group B2. All the birds (100%) in group C1 had HI antibody titres of ≥ 3 log2 followed by 94% of the birds in group B2 and 83% of the birds in group A1 at eight weeks of age. At nine weeks of age, 77% of birds in group C2, 66% in group D1, and 11% in group B1 had HI antibody titres of ≥ 3 log2 (Table 4).
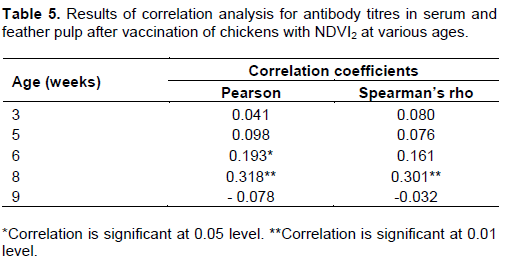
Correlation analysis
The results of Pearson’s and Spearman’s rho correlation to compare the mean ND HI antibody titre between feather and serum are shown in Table 5. There was a correlation only at eight weeks of age (two weeks after second vaccination).b
Mortality rate and protection rate
The mortality and protection rates of birds challenged with NDV Kudu 113 are presented in Figure 1. The highest mortality rate (100%) was recorded in groups B1, C2 and the control group, while the lowest (78%) was recorded in groups A1 and B2. Groups A2 and C1 had 89% mortality rate. Protection rate after challenge was low for all the groups; A1 had (14%), A2 (1.2%), B2 (14%), C1 (1.2%), while B1, C2 and D1 were not protected (Figure 1).
The reported protective antibody titre for ND vaccines are HI ≥ 4 log2 (OIE, 2000) with reference to conventional ND vaccine designed for intensively reared commercial chickens. However, HI ND antibody titre of ³ 3log2 was considered to be adequate for food-based vaccines orally administered to scavenging chickens (Echeonwu et al., 2007). The mean HI antibody titre at three weeks of age was low in all the groups with feather pulp samples in contrast to the high antibody titre recorded at same age in serum. The observed low antibody titre in the feather pulp might be due to movement of antibodies which was more in the central circulation than peripheral at three weeks of age and more in the peripheral circulation at five weeks of age. However, since the birds had no previous vaccination before the primary vaccination, the high antibody titre detected in serum at three weeks of age could be due to the presence of maternal antibody which may also be responsible for the low antibody titre recorded in serum two weeks after primary vaccination at five weeks of age. It has been established that chicks from immunized parents possess high level of maternal antibody which protect the chicks against virulent virus and interferes with vaccine antigens (Saeed et al., 1988; Rahman et al., 2002). The percentage of vaccinated birds with HI antibody titres ³ 3log2 showed a marked increase at six and eight weeks of age in both serum and feather pulp samples. Flock immunity reported by Boven et al. (2008) as the only means to prevent the transmission of NDV can only be achieved when ³ 85% of vaccinated birds have antibody titres of ³ 3log2. In the present study, this was achieved in groups B2, C1 and C2 for serum and groups B2 and C1 for feathers at eight weeks of age. However, prior to challenge at nine week of age, the percentage dropped in both serum and feather with none of the groups having percentage mean HI antibody titre sufficient to protect the birds from challenge. However, it was observed that some birds with low or undetectable ND HI antibody titres survived after challenge. This observation has been reported by Ibrahim et al. (1981) who concluded that low HI antibody titre following NDV4 vaccination were not indicative of susceptibility to challenge, an observation also confirmed by other researchers (Bell et al., 1995; Wambura et al., 2000; Tu et al., 1998). In addition to serum antibody, secretory antibody (IgA) at mucosal surfaces and cell mediated immunity are thought to play a role in resistance to challenge (Alexander, 2003).
There was a general increase in the HI ND antibody titre two weeks after secondary vaccination in serum and feather. Similar results were reported by other workers (Ideris et al., 1990; Spradbrow 1993) who stated that protective immunity is often not apparent until after the second oral vaccination. Similar findings were reported by Baba et al. (2006) and Nasser et al. (2000) that titres among vaccinated birds generally peaked by day 21 post vaccination and declined subsequently. The results of the current study show that administration of 2nd booster vaccination significantly and progressively increased HI antibody titer in all the treatment groups.
The duration of active protective immunity varies with the immune status of the bird and nature of the immune stimulus, which depends on the dose and strain of NDV vaccine and route of administration of the vaccine (Ibrahim et al., 1981; Westbury, 1984). Previous studies found that drinking water induced acceptable immune response and protection, but it is inappropriate particularly in cold weather because vaccination had to be conducted in the mornings, and not all chicken drink water in the mornings even after feeding (Mogoje, 2006). In another study, the administration of a partially thermostable ND vaccine via eye drop application gave the best response, while the vaccine administered via cooked maize meal gave the lowest response. Eye drop vaccination is impractical to implement in village environment (Mogoje, 2006).
There was positive correlation between the mean HI ND antibody titre of serum and feather pulp only at eight weeks of age. This is in contrast to the reports of Roy et al. (1998), who recorded consistent high ND HI antibody titre in serum than in feather three weeks after primary vaccination and three weeks after secondary vaccination in an experiment to compare ND vaccines by serology in tears and feather pulp samples. In the present study, fluctuations were observed in the level of ND HI antibody titre in both serum and feather after primary and secondary vaccination with a marked difference observed at three weeks of age in feather with very low antibody titre than serum and at five weeks of age, feather pulp samples had higher ND antibody titre than serum. These fluctuations could be due to the lack of uniformity of feather pulp samples, since the amount of pulp in each feather will produce some variation in results as reported by Garrido et al. (1992). Results from the present study showed high mortality and low protection rate in all the groups. The difference in the protection rate after challenge with virulent NDV may be due to differences in the vehicles used in the administration of the vaccine. The highest mortality rate (100%) in vaccinated birds was recorded in birds vaccinated with TMZG and SGB. The lowest protection rate was also recorded in birds vaccinated via TMZG and SGB. However, birds vaccinated with TMZ and TSGG had the lowest mortality. These findings is similar to what was reported by Nasser et al. (2000) in vaccination trials in Ethiopia, where untreated and parboiled sorghum used as vaccine carriers for NDVI2 gave low protection to vaccinated birds after challenge. Similarly, in Nigeria Musa et al. (2010) reported that untreated sorghum, parboiled sorghum, sorghum coated with gum Arabic and a commercial feed mash used as vaccine feed carriers for NDVI2 gave low antibody titre and low protection following challenge with a velogenic NDV. The results of these investigations are in contrast to the findings of Echeonwu et al. (2007, 2008b) who tested NDVI2 and V4 vaccines on millet, maize and guinea corn grains and bran in Nigeria and the vaccinated birds were protected after challenge.
Results from their study indicated that the vaccines could be effective for protection of village chickens as food-borne vaccines provided the carriers are adequately processed. Furthermore, different grains induced different level of HI antibody titer. This implies the presence of inherent variation in virus carrying capacity of different grains (Reta et al., 2016). This is an opportunity to screen grains of different species and varieties. Interestingly, treating grains (either cracking or parboiling) increased their efficacy as vaccine carrier. Similar results have been reported in Nigeria by Olabode (2010) as to the efficacy of treated grain particularly maize compared to untreated grain. Grains have been known to contain tannins, anthraquinone, cardiac glycosides and alkaloids. Some of these chemicals have been shown to have antiviral properties (Oakeley, 2000; Musa et al., 2010). The higher HI titer induced by treated grains than untreated ones could be due to the fact that cracking grains increase the surface area of the grains to adsorb the vaccine virus (Oakeley, 2000; Wambura et al., 2007; Olabode, 2010). Cracked maize and treated sorghum were found to be better vaccine carriers in this study, though the protection rate was low.
This is in contrast with the work of Lawal et al. (2016) in Nigeria using maize grit as vaccine carrier for NDVI2. Their study showed that 94.3% of the vaccinated village chickens seroconverted with protective levels of antibodies against ND virus. However, it should be noted that the protection level of the grain based NDVI2 vaccine varies under laboratory conditions, that is,>90% protection (Aini et al., 1990) and under real village conditions, that is, <60% (Aini et al., 1992) and with vaccine delivered by farmers (Aini et al., 1990). Hence, it is necessary to conduct pilot field trial at village level to evaluate the results of the current study under real village conditions.
The study concluded that treated maize, treated sorghum, untreated maize bran and untreated sorghum bran when used as feed carriers for NDVI2 in this study gave low protection to vaccinated birds after challenge with velogenic NDV. The NDVI2 vaccine could be useful for the protection of village chicken against ND provided the carrier feeds are adequately treated to remove antiviral substances. The use of different processing methods for maize and sorghum should be employed to treat these grains and other locally available feeds such as millet to reduce or eliminate possible antiviral substances in them and to test their suitability as ND vaccine carriers. Correlation was found between the NDV HI antibody titre in serum and feather pulp only at eight weeks of age and thus, feather pulp samples cannot be used as an alternative to serum for seromonitoring of vaccinated birds. However, feathers can be easily collected from live or dead birds, and thus can serve as suitable samples for diagnosis of NDV in chickens. The selection of feather for seromonitoring is important; however, since the amount of pulp in each feather will produce variation in results, further research is therefore necessary.
The authors have not declared any conflict of interests.
REFERENCES
|
Aamir S, Tanveer A, Mohammed U, Abdul R, Zahid H (2014). Prevention and control of Newcastle disease. Int. J. Agric. Innov Res. 3(2):454-460.
|
|
|
|
Abdu PA, Mera UM, Sa'iduL (1992). A study of chicken mortality in Zaria, Nigeria. Proceedings of the National Workshop on Livestock and Veterinary Services, Held at the National Veterinary Research Institute, Vom, pp. 51-55.
|
|
|
|
|
Abdu PA, Musa U, Joannis TM, Sa'idu L, Mera UM, Salami-Shinaba, JO, Haruna ES (2012). Vaccination of chickens against Newcastle disease with Lasota and V4 vaccines using brans, ground grains and water as vehicles. Vom. J. Vet. Sci. 9:1-10.
|
|
|
|
|
Aini I, Ibrahim AL, Spradbrow PB (1990). Field trials of a food-based vaccine to protect village chickens against Newcastle disease. Res. Vet. Sci. 49:216-219.
|
|
|
|
|
Aini I, Ibrahim AL, Spradbrow PB (1992). Efficacy of food pellet Newcastle disease vaccine: simulated village experiments. J. Vet. Med. Malaysia 4:81-86.
|
|
|
|
|
Alders R, Spradbrow PB (2001).Controlling Newcastle Disease in Village Chicken. A field Manual. Australian centre for International Agricultural Research. Monograph 821:19.
|
|
|
|
|
Alexander DJ (2003). Newcastle disease and other Avian Paramixoviruses. In: Sairf YM, editor. Disease of poultry. 11th ed. Ames, Iowa: Iowa State press. pp. 63-87.
|
|
|
|
|
Alexander DJ, Bell JG, Alders RG (2004). Technology Review: Newcastle Disease. Rome: FAO Animal Production and Health Paper. pp. 1-26.
|
|
|
|
|
Ameh JA, Mailafia S, Olatunde H. Olabode BJA, God'spower RO, Martha EO, Dolapo IA (2016). Sero-prevalence of Newcastle disease virus antibodies in local and exotic chickens in Gwagwalada, Nigeria. J. Vet. Med. Anim. Health 8(11):193-198.
|
|
|
|
|
Baba SS, Heanacho CC, Ideris JM, El- Yugyda AD (2006). Food-based Newcastle disease vaccine in guinea fowl (Numida Meleagris Galenta Pallas) in Nigeria. Trop. Vet. 22(3):37-45.
|
|
|
|
|
Bell KG, Fotzo TM, Amara A, Agbebe G (1995). A field trial of the heat resistant V4 vaccine against Newcastle disease by eye drop inoculation in village poultry in Cameroon. Prev. Vet. Med. 25:19-25.
Crossref
|
|
|
|
|
Boven Van M, Annemarie B, Teun HF, FabriElly K, Leo H, Guus K (2008). Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Pathol. 37(1):1-5.
Crossref
|
|
|
|
|
Cumming RB (1992). Newcastle disease research at the University of New England. In: P.B. Spradbrow (ed), Newcastle Disease in Village Chickens, Control with Thermostable Oral Vaccines. Proceedings no 39. Canberra: Australian Centre for International Agricultural Research. pp. 48-85.
|
|
|
|
|
Davidson I (2009). Diverse uses of feathers with emphasis on diagnosis of avian viral infections and vaccine virus monitoring. Br. J. Poult. Sci.11 (3):139-148.
Crossref
|
|
|
|
|
Dong-Hun L, Jung-Hoon K, Jin-Yong N, Jae-Keun P, Seong-Su Y, Tseren-Ochir Erdene-Ochir, Sang-Soep N, Yong-Kuk K, Sang-Won L, Chang-Seon S (2016). Viscerotropic velogenic Newcastle disease virus replication in feathers of infected chickens. J. Vet. Sci. 17(1):115-117.
Crossref
|
|
|
|
|
Echeonwu GON, Iroegbu CU, Ngene A, Junaid SA, Ndako J, Echeonwu IE, Okoye JOA (2008a). Survival of Newcastle disease virus (NDV) strain V4-UPM coated on three grains and exposed to room temperature. Afr. J. Biotechnol. 15:2688-2692.
|
|
|
|
|
Echeonwu BC, Ngele MB, Echeonwu GON, Joannis TM, Onovoh EM, Paul G (2008b). Response of chickens to oral vaccination with Newcastle disease virus vaccine strain I2 coated on maize. Afr. J. Biotechnol. 7(10):1594-1599.
|
|
|
|
|
Echeonwu GON, Iroegbu CU, Echeonwu BC, Ngene A, Olabode AO, Okeke OI, Ndako J, Paul G, Onovoh EM, Junaid SA, Nwankiti O (2007). Delivery of thermostable Newcastle disease (ND) vaccine to chickens with broken millet grains as the vehicle. Afr. J. Biotechnol. 6(23):2694-2699.
Crossref
|
|
|
|
|
Echeonwu GON, Iroegbu CW, Emeruwa AC (1993). Recovery of velogenic Newcastle disease virus from dead and healthy free roaming birds in Nigeria. Avian Pathol. 22:383-387.
Crossref
|
|
|
|
|
Garrido MF, Spencer JL, Chambers JR (1992). Feather pulp as a source of antibody to avain viruses. Avian Pathol. 21:333-337.
Crossref
|
|
|
|
|
Guoyuan W, Chen C, Jing G, Zhenyu Z, Yu S, Huabin S, Qingping L, Jun Y, Hongling W, Hongcai W, Tengfei Z, Rongrong Z, GuofuC, Qingzhong Y (2015). Development of a novel thermostable Newcastle disease virus vaccine vector for expression of a heterologous gene. J. Gen. Virol. 96:1219-1228
Crossref
|
|
|
|
|
Ibrahim AI, Ideris A, Babjee AM (1992). An overview of the use of food based Newcastle disease vaccine in Malaysia. In: Spradbrow PB (ed). Newcastle in Village Chickens Control with Thermostable Oral Vaccine. Australian Centre for International Agricultural Research Canberra, 1992.
|
|
|
|
|
Ibrahim AL, Chulan U, Babjee AM (1981). An assessment of the Australian V4 strains of Newcastle disease virus as a vaccine by spray, aerosol and drinking water administration. Austr. Vet. J. 57:227-280.
Crossref
|
|
|
|
|
Ideris A, Ibrahim AL, Spradbrow PB (1990). Vaccination of chickens against Newcastle disease with a food pellet vaccine. Avian Pathol.19:371-384.
Crossref
|
|
|
|
|
Jagne J, Aini I, Schat KA, Fennel A, Touray O (1991). Vaccination of village chickens in the Gambia against Newcastle Disease using heat-resistant, food pelleted V4 vaccine. Avian Pathol. 20:721-724.
Crossref
|
|
|
|
|
Jayawardane GWL, de Alwis MCL, Dawwda B (1990). Oral vaccination of chickens against Newcastle disease with V4 vaccine delivered on processed rice grains. Austr. Vet. J. 67(10):364-366.
Crossref
|
|
|
|
|
Kaleta EF, Baldouf C (1988). Newcastle Disease, Boston/Dordrect/London: Kluwer Academic Publishers. pp. 197-246.
|
|
|
|
|
Khan A, Ikhwan A, Muhammad A, Mushtaq A, Hamidullah A (2000). Prevalence of poultry diseases in Kohat. J. Sci. Tech. 24:25-28.
|
|
|
|
|
Lawal JR, El-Yuguda AD, Ibrahim UI (2016). Efficacy of feed coated Newcastle disease I2 vaccine in Village Chickens in Gombe State, Nigeria. J. Vet. Sci. Technol. 7:349.
|
|
|
|
|
Mogoje BL (2006). Evaluation of a thermostable Newcastle disease vaccine in free range chickens. Magister Technologiae Agriculture. Department of Animal Sciences, Tshwane University of Technology, South Africa. P 122.
|
|
|
|
|
Musa U, Abdu PA, Mera UM, Emmenna PE, Ahmed MS (2010). Vaccination with Newcastle disease vaccines strain I2 and LaSota in commercial and local chickens in Plateau state Nigeria. Nig. Vet. J. 31(1):46-55.
|
|
|
|
|
Nasser M, Lobr J, Mebratu Y, Zessein KH, Ademe Z (1998). Oral feed-based Newcastle disease vaccination trials in Ethiopia with the Australia V4 vaccine strain. Proceedings, Fourth Asian Pacific Poultry Health Conference, 22-26 November, 1998. Melbourne, Australia. P 129.
|
|
|
|
|
Nasser M, Lohr JE, Mebratu GY, Zessin KH, Baumann MPO, Ademe Z (2000). Oral Newcastle disease vaccination trials in Ethiopia. Avian Pathol. 29:27-34.
Crossref
|
|
|
|
|
Oakeley RD (2000). The limitation of a feed/water based heat-stable vaccine delivery system for Newcastle disease control strategies for backyard poultry flocks in sub-Saharan Africa. Prev. Vet Med. 47:271-279.
Crossref
|
|
|
|
|
Office International Des Epizootics (2000). Newcastle disease, Manual of Standards for Diagnostics Tests and Vaccines. pp. 104-124.
|
|
|
|
|
Office International Des Epizootics (OIE) (2004). Newcastle disease. In: OIE manual for diagnostic tests and vaccines for terrestrial animals. 5th ed. 1:270-283.
|
|
|
|
|
Olabode AO, James A, Ndako GON, Echeonwu GON, Anthony AC (2010). Use of cracked maize as a carrier for NDV4 vaccine in experimental vaccination of chickens. Virol. J. 7(67):1-5.
Crossref
|
|
|
|
|
Oladipo EO (1985). Characteristics of thunderstorms in Zaria, Nigeria. Weather 40:316.
Crossref
|
|
|
|
|
Otchere EO, Adeoye AT, Gefu JO, Adewuyi AA (1990). Preliminary observations on village poultry production in North-Central Nigeria. In: Proceedings of an International Workshop on Rural Poultry Development in Africa (Ed. EB Sonaiya), Ile-Ife, Nigeria, Thelia Publishers pp. 196-200
|
|
|
|
|
Pangani P (1999). Stock breeding support programme in Gaza and Inhambane Provinces, Draft Activity Report by the Animal Health Advisor, October, 1999.
|
|
|
|
|
Philemon N, Wambura J, Meers P,Spradbrow PB (2007). Survival of avirulent thermostable Newcastle disease virus (strain I-2) in raw, baked, oiled and cooked white rice at ambient temperatures. J. Vet. Sci. 8:303-305.
Crossref
|
|
|
|
|
Rahman MM, Bari ASM, Giasudin MJ, Islam RM, Sil AC (2002). Evaluation of maternal and humoral immunity against Newcastle disease virus in chicken. Int. J. Poult. Sci. 1:161-163.
Crossref
|
|
|
|
|
Reta DA, Kasahun A, Olana M, Yilkal A, Eseyas G, Marta Y, Teshale Sori (2016). Serological response and protection level evaluation in chickens exposed to grains coated with I2 Newcastle disease virus for effective oral vaccination of village chickens. BMC Vet. Res. 8:12, 279.
|
|
|
|
|
Roy P, Koteeswaran A, Stridevi P, Venugopalan AT (1998). Comparison of Newcastle disease vaccines by serology using serum, tears and feather pulp samples. Trop. Anim. Health Prod. 30(1):31-35.
Crossref
|
|
|
|
|
Saeed Z, Ahmad S, Rizvi AR, Ajmal M (1988). Role of maternal antibody in determination of an effective Newcastle disease vaccination programme. Pak. J. Vet. Res. 1:18-20.
|
|
|
|
|
Spradbrow PB (1993). Newcastle disease in village chickens. Poult. Sci. Rev. 5(2):57-96.
|
|
|
|
|
Tu TD, Phuc KV, Dinh NTK, Quoc DN, Spradbrow PB (1998). Vietnamese trial with a thermostable Newcastle disease (Strain I-2) in experimental and village chickens. Prev. Vet. Med. 34:205-214.
Crossref
|
|
|
|
|
Wambura PN, Kapaga AM, Hyera JMK (2000). Experimental trials with a thermostable Newcastle disease virus (Strain I-2) in commercial and village chickens in Tanzania. Prev. Vet. Med. 43:75-83.
Crossref
|
|
|
|
|
Wambura PN, Meers J, Spradbrow PB (2007). Survival of avirulent thermostable Newcastle disease virus (strain I-2) in raw, baked, oiled, and cooked white rice at ambient temperatures. J. Vet. Sci. 8:303-305.
Crossref
|
|
|
|
|
Westbury HA (1984). Comparison of the immunogenicity of Newcastle disease virus strain V4, B1 and La Sota in chickens. Tests in susceptible chickens. Aust. Vet. J. 61:5-9.
Crossref
|
|