Full Length Research Paper
ABSTRACT
There was an accidental death of BALB/c mice in the course of the experiment about hepatitis B immunization. The reasons of the mice breeding failure were analyzed in order to provide some experience for future animal preparation about hepatitis B immunization. Items of all mice including sex, age, dose of hepatitis B vaccine (HepBVac), dose of hepatitis B immunoglobulin (HBIG), immunization route, vaccination schedule and the acclimatization period were recorded before the first immunization. Among 334 mice included, the survival rates in different groups of initial ages were 45.9% in 3-week-old, 49.3% in 4-week-old, 51.9% in 5-week-old, 84.8% in 7-week-old and 96.6% in 8-week-old; the survival rate in seven days acclimatization group was 96.6% and 53.3% in one day acclimatization group. Multivariate logistic regression analysis indicated that smaller ages (3 weeks, 4 weeks) and acclimatization for only one day were the independent risk factors affecting the survival of mice. Multivariate logistic regression analysis showed that injection with HBIG, lower dose of HepBVac and hypodermic injection were independent risk factors for low-and non-response to HepB Vac. It is suggested that the mice should be more than five-week-old and must have acclimated to the environment for one week before the experiment begins. The initial ages of the mice have no impact on their immune efficacy.
Key words: BALB/c mice, age, acclimatization, hepatitis B immunization.
INTRODUCTION
Experimental animal science, an important technical means and a necessary condition of life science research, affects the achievements and levels of scientific research in many fields directly.
Meanwhile, its development and improvement will take scientific research into a new frontier and promote the development of life science. BALB/c mice are distributed globally, and are among the most widely used inbred strains in animal experiment because they have good breeding performance, long reproductive life-span and low cost of feeding and management (Potter, 1985). In particular, BALB/c mice are widely used for the production of monoclonal antibodies and immunological studies (Potter, 1985; Zhao et al., 2016; Latha et al., 2017; Aerts et al., 2015).
More than 300 BALB/c mice were bred for the study of active and passive hepatitis B immunization, which was designed to explore the influence of maternally derived antibody against hepatitis B surface antigen (anti-HBs) on infants’ immune response to hepatitis B vaccine. We originally designed to mimic the fact that neonates got anti-HBs from their mothers by injection of HBIG to BABL/c mice, and three separate doses of HepBVac were given to each mouse at 0, 4 and 8 weeks or 0, 1 and 2 weeks successively. Unfortunately, there was an accidental death of the mice during the course of the experiment. Therefore, the reasons for the mice bred failure are analyzed in order to provide some experience for the researchers who are new to conduct animal experiments. Moreover, we also analyzed whether the reasons for an accidental death of the mice had an effect on the immune efficacy in the experiment of hepatitis B immunization.
MATERIALS AND METHODS
Experimental animals
From 2017 to 2018, 334 BALB/c mice (212 male and 122 female) of SPF grade aged three to eight weeks, with median (25 to 75%, interquartile range, IQR) age of 5 (3, 8) weeks, were bred, and they were housed at animal experiment center of medical college of Qingdao university. Among all mice, 216 were raised in 2017 and 118 in 2018. The mice were allocated to different cages (M1 type, specification 290×178×160 mm) and five to six mice were fed in each cage. Treated wood shavings were used as bedding. All mice were allowed to acclimate to the environment at the beginning of the study, were fed with standard feedstuff and drunk purified water freely. All mice were recorded sex, age, dose of HepBVac, dose of HBIG, immunization route and vaccination schedule before the first immunization. The mice and standard feedstuff were purchased from Qingdao Daren FuchengGraziery co. LTD in China.
Reagents
Recombinant HepBVac (HansenulaPolymorpha): Specification - 10 μg/0.5 ml; Approval number: S20040016, produced by Dalian Hanxin Biological Pharmaceutical Co., LTD in China.
HBIG: Specification - 200 IU/2 ml, Approval number: S10930001, produced by Shandong Taibang Biological Products Co., LTD in China. ARCHITECT Anti-HBs Reagent Kit: produced by Abbott Ireland Diagnostics Division.
Laboratory methods
Anti-HBs were performed by a Chemiluminescent Microparticle immunoassay using ARCHITECT i 2000 full-automatic immune analysis system. The normal reference value was set as 0-10 mIU/ml.
For the specimens of anti-HBs titer >1000 mIU/ml, they were diluted to 10, 20 or 30 times …… until anti-HBs levels fell below the upper limit of detection (1000mIU/ml).
Immunization schedule
All mice were divided into two groups (Group A and B) according to different doses of HepBVac (2 and 5 μg) injected. Each group was sub-divided into four subgroups. The time when the experiment was started was defined as 0 week (w), the next as 1 w, and so on. HepBVac was injected through two routes: intramuscular injection (quadriceps femoris) and hypodermic injection (scruff). HBIG was administrated via intraperitoneal injection.
Subgroups A1, A2, A3, A4
A1: 25 IU HBIG and 2μg HepBVac were administrated at 0 w, and then the second and third HepBVac of the same dose were administrated at 4 and 8 w respectively.
A2: 50 IU HBIG and 2 μg HepBVac were administrated at 0 w, and then the second and third HepBVac of the same dose were administrated at 4 and 8 w respectively.
A3: Three doses HepBVac of 2 μg were administrated at 0, 4 and 8 w (0-4-8 w) respectively.
A4: Three doses HepBVac of 2 μg were administrated at 0, 1 and 2 w (0-1-2 w) respectively.
Subgroups B1, B2, B3, B4
B1: 25 IU HBIG and 5μg HepBVac were administrated at 0 w, and then the second and third HepBVac of the same dose were administrated at 4 and 8 w respectively.
B2: 50 IU HBIG and 5μg HepBVac were administrated at 0 w, and then the second and third HepBVac of the same dose were administrated at 4 and 8 w respectively.
B3: Three doses HepBVac of 5 μg were administrated at 0-4-8 w respectively.
B4: Three doses HepBVac of 5 μg were administrated at 0-1-2 w respectively.
Definition of immunization outcome
Anti-HBs<10 mIU/ml was considered as non-response; anti-HBs from 10 to 100mIU/mL was considered as low-response; anti-HBs>100 mIU/ml was considered as protective response (Wang et al., 2016; Lu et al., 2016).
Sample collection in mice
Blood was collected from the mice eye socket by vacuum tubes (no additives) four weeks after the third dose of HepB Vac (Parasuraman et al., 2010). Mice were broken necks to death immediately after blood drawn. The serum was separated within one hour, transferred into a 1.5 centrifuge tube and stored in a -70°C deep freezer for anti-HBs testing.
Statistical analysis
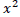 or Fisher’s exact tests were used for categorical variables. For There are 10 mice with age of 6 weeks. Among them, for male, the mice whose weight ≥ 18.0 g were divided into group of 7 weeks and whose weight < 18.0 g were divided into group of 5 weeks; for female, the mice whose weight ≥ 15.85 g were divided into group of 7 weeks and whose weight < 15.85 g were divided into group of 5 weeks.
or Fisher’s exact tests were used for categorical variables. For There are 10 mice with age of 6 weeks. Among them, for male, the mice whose weight ≥ 18.0 g were divided into group of 7 weeks and whose weight < 18.0 g were divided into group of 5 weeks; for female, the mice whose weight ≥ 15.85 g were divided into group of 7 weeks and whose weight < 15.85 g were divided into group of 5 weeks.
Source: Qin (2010). Laboratory Animal Science. People’s Medical Publishing House, Beijing, China, p. 38 (in Chinese). measurement data, normal distribution was first tested. If the result was of non-normal distribution, it was expressed in terms of median (IQR). Logistic regression analysis was used to identify the risk factors. And categorical variables were transformed into dummy variables. Grouping and assignment are shown in Table 1. Variables with p value <0.1 in the univariate analysis were included in a multivariate logistic regression. Statistical calculations were performed using SPSS 22.0 software package with a p value <0.05 considered significantly.
Research ethics
The study was approved by the Ethics Committee of Qingdao Municipal Hospital affiliated with Qingdao University. All procedures followed were in accordance with the Animals (Scientific Procedures) Act (Amendment) Order 1993. Animals’ care was in accordance with institutional guidelines.
RESULTS
Summary of the mice survival
216 mice of the first group, with median (25%, 75% IQR) age of 4 (3, 5) weeks were bred in 2017, among whom there were 101 deaths. These mice were allowed to acclimate to the environment for one day at the beginning of the study. 118 mice of the second group with median (IQR) age of 8 (8, 8) weeks were bred in 2018, among whom there were four deaths. These mice were allowed to acclimate to the environment for seven days at the beginning of the study. There was statistically significant difference in survival between the two groups: 53.2 vs 96.6%,
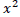 =66.592, p=0.000. In the group of the schedule of 0-1-2 weeks, and survival rates were 88.7, 72.2, 72.2 and 72.2% at week 1, 2, 4, and 6, respectively; in the group of the schedule of 0-4-8 weeks, survival rates were 88.6, 68.7, 68.2, 67.2, 67.2 and 66.2% at week 1, 2, 4, 6, 8 and week 12, respectively.
=66.592, p=0.000. In the group of the schedule of 0-1-2 weeks, and survival rates were 88.7, 72.2, 72.2 and 72.2% at week 1, 2, 4, and 6, respectively; in the group of the schedule of 0-4-8 weeks, survival rates were 88.6, 68.7, 68.2, 67.2, 67.2 and 66.2% at week 1, 2, 4, 6, 8 and week 12, respectively.
Risk factors affecting the survival of mice
Multivariate logistic regression analysis showed that the smaller age of weeks was the risk factor affecting the survival of mice, and further analysis of the results among subgroups showed that the death rate was significantly higher in three weeks- four weeks old mice as compared to eight weeks old mice. There were no significantly differences in the group of five weeks old as compared with the eight weeks old group. The survival rate was much higher in the group of seven days acclimatization than that for one day, but there was no significantly difference between the two groups in the multivariate analysis (Table 2), which did not seem to be consistent with the fact. It was considered to be related to the application of dummy variables which could result in a smaller sample size in each category, so the result with no statistical difference could be obtained. When multivariable analysis was performed without the analysis of dummy variables, it showed that RR value (95%CI)=1.470 (1.179, 1.832), p=0.001, in the group of initial ages and RR value (95%CI)=5.779 (1.511, 22.109), p=0.010, in the group of days for acclimatization. So it is indicated that acclimatization for only one day is also the risk factor affecting the survival among mice (Table 2).
Risk factors for low- and non-response to HepBVacamong mice
There were 229 mice survivors among the two groups, and 23 were of low- and non-response to HepBVac among whom 10 were of non-response to HepBVac and 13 were of low-response to HepB Vac. Multivariate logistic regression analysis showed that injection with HBIG, lower dose of HepBVac and hypodermic injection were independent risk factors for low-and non-response to HepB Vac (Table 3). When the risk factors for low- and non-response to HepBVac were analyzed, the variable intervals among the three doses of HepBVac was eliminated to remove the effect of multicollinearity among variables in the regression model. Statistical results showed that the variable interval among the three doses of HepBVac was not the risk factor for low- and non-response to HepB Vac (Table 4).
DISCUSSION
Due to lack of experience, we had only focused on the implementation of the experimental scheme, and neglected the mice weight which might actually be estimated by their size firstly, when we began the experiment with the first group. Mice in the first group were administered with hepBVac with or without HBIG when they adapted to their environment after one day. Subsequently, large numbers of deaths were found the next day, and number of deaths began to go down two weeks after the start of the experiment.
We discussed and analyzed the possible causes of the death immediately. Firstly, we ruled out environmental factors: the mice were exposed to a 12 h light – 12 h dark cycle at 22 ± 2°C with relative humidity of 60 ± 5%, which is conformance to standardized breeding requirements (National Research Council, 2011; Lu et al., 2017). Secondly, we confirmed that the vaccines were transported by cold-chain logistics and they were still under guarantee. Thirdly, all vaccines in this study were expressed in Hansenulapolymorpha, which has been routinely administered to infants at birth, and the Global Advisory Committee on Vaccine Safety has also confirmed its safety (Das et al., 2019; Yang et al., 2015). Finally, we came to the factor of weight. After the mice were weighted, we found that the weight of these mice was lower than those of the 6-8 weeks old. In fact, when we ordered these mice from the company, we stressed that we needed mice aged six to eight weeks old that were commonly used in studies (Fuller et al., 2001). And the company has admitted that their staffs had made a mistake when they processed our order.
When we ruled out the influence of environment and reaffirmed the safety of reagents, it is suggested that smaller age of weeks (median age of four weeks) estimated by the weight was most likely to be related with the phenomenon of death. It is also indicated that increasing of the adaptive duration before the experiment might also reduce mortality among those mice. Based on the experiences and lessons learned from the first group, mice of the second group were arranged to acclimate the environment for the first seven days that was decided based on reported literatures and weighted to estimate the age of weeks at the beginning of the experiment (Mahdavi et al., 2017; Hogan et al., 2017; Choi et al., 2017). It is shown that the initial median age of the mice was of eight weeks and there were only four mice died during the whole experiment among mice of the second group.
With respect to the classification method of initial ages, for the first study, they were estimated at the second day when the death occurred, and the initiative's aim of doing that was to investigate these deaths. For the second study, the initial ages were estimated at the beginning of the experiment for the purpose of avoiding the deaths due to the smaller ages of mice. The data of estimated initial ages of the first group were obtained one day later than the second group. Since the time interval between the two groups was relative close, we took data of the two groups as initial ages in the statistical process.
Multivariate logistic regression analysis showed that small initial ages (range from three weeks to four weeks) and only one day for acclimation were the independent risk factors resulted in death. Coincidentally, the two risk factors are all in the first group, so it is difficult to make a clear distinction whether the small initial age or the fewer days for acclimation or both the two factors play a leading role in the death event.
In studies about hepatitis B immunization, it is reported that different age groups of BALB/c mice were included such as age of 4-6 weeks (Granovski et al., 2017; Wang et al., 2017), 6-8 weeks (Gui et al., 2017; Kapadia et al., 2017; Skrastina et al., 2014; Fakharzadeh et al., 2013), 8-10 weeks (McCluskie et al., 2002; Abdalla et al., 2014), 11 weeks (Perez et al., 2018), or 1,3,7 days (Weeratna et al., 2001; Ahmadian et al., 2016), among which ages of 6-8 weeks were in the majority. So it is suggested that mice of a certain age group can be selected as flexible according to different experimental requirements.
We did not pay enough attention on acclimatization due to lack of experience on mice experiment. In fact, an appropriate acclimation period can bring down stress, which should be considered as an important part of the experiment design (Schunk and Macallum, 2005). And in this study, it showed that it was especially important for younger mice (less than five weeks as it showed in this study) to have an appropriate acclimation period at the start of each experiment. In addition, the smaller the age, the less resistant to the stress among mice, so it is indicated that both smaller age of weeks and acclimatization for only one day contributed to the death event among mice in this study jointly.
In addition, results in this study showed that the two risk factors mentioned above had no impact on the immune efficacy of HepB Vac. The reasons for low- and non-response to HepBVac might be as follows: (i) administration with HBIG, HBIG may have a negative effect on immune response to HepB Vac (Yang et al., 2003; Pongpipat et al., 1989; Hu et al., 2008; Zhang et al., 2014); (ii) lower dose of HepBVac(2μg), increasing the dose of HepBVac can reduce the inhibition of HBIGon immune response (Hu et al., 2008; Junqueira et al., 2011); (iii) hypodermic injection, the results may provide evidence that muscle injection is the priority method for the hepatitis B vaccination in mice. An interesting phenomenon is that smaller ages (3-4 weeks old) had no significant impact on immune response to HepBVac in this study, and the interpretation may be that when mice reach the age of 3-4 weeks, they already meet the condition of adolescents in human, and the immune system can generate an adequate immune response to HepB Vac.
CONCLUSIONS
We draw an important lesson in this study: short acclimation period and smaller ages can lead to a large number of deaths among BALB/c mice, and it is important to make sure that the age of mice are above five weeks and the acclimatization is seven days or so at the beginning of the experiment, which are all important factors guaranteeing the higher survival rate as well as quality of their life. Fortunately, results in this study indicated that the age of weeks had no impact on the immune effect of HepBVac, which may provide an evidence of a wider range of ages for studies on mice immunization.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors express their sincere appreciations to Professor Xi’enGui from Zhongnan Hospital of Wuhan University for his assistance in preparation and English translation of this article.
FUNDING
This work was supported by National Nature Science Foundation of China [grant number 81502900].
REFERENCES
|
Abdalla DR, Aleixo AA, Murta EF, Michelin MA (2014). Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncology Letters 7(3):886-890. |
|
|
Aerts L, Rhéaume C, Carbonneau J, Lavigne S, Couture C, Hamelin MÈ, Boivin G (2015). Adjuvant effect of the human metapneumovirus (HMPV) matrix protein in HMPV subunit vaccines. Journal of General Virology 96:767-774. |
|
|
Ahmadian N, Pashaei-Asl R, Ahmadian M, Rahmati-Yamchi M, Shahabi S, Vazini H (2016). Immune response of newborn BALB/c mice to Cryptosporidium infection. Journal of Parasitic Diseases 40(3):1066-1070. |
|
|
Choi YY, Kim MH, Ahn KS, Um JY, Lee SG, Yang WM (2017). Immunomodulatory effects of Pseudostellaria heterophylla (Miquel) Pax on regulation of Th1/Th2 levels in mice with atopic dermatitis. Molecular Medicine Reports 15(2):649-656. |
|
|
Das S, Ramakrishnan K, Behera SK, Ganesapandian M, Xavier AS, Selvarajan S (2019). Hepatitis B Vaccine and Immunoglobulin: Key Concepts. Journal of Clinical and Translational Hepatology: 7(2):165-171. |
|
|
Fakharzadeh S, Kalanaky S, Hafizi M, Goya MM, Masoumi Z, Namaki S, Shakeri N, Abbasi M, Mahdavi M, Nazaran MH (2013). The new nano-complex, Hep-c, improves the immunogenicity of the hepatitis B vaccine. Vaccine 31(22):2591-2597. |
|
|
Fuller SA, Takahashi M, Hurrell JG (2001). Immunization of mice. Current protocols in molecular biology. Chapter 11: Unit 11.14. |
|
|
Granovski V, Yokosawa J, Rodrigues-Granovski V, Castro CC, Urbina AC, Granovski N (2017). Humoral Immune Response in Mice Elicited by Combined Yeast-Derived Hepatitis B Virus Core, Surface, and Mosaic Surface Antigens. Intervirology 60(3):90-101. |
|
|
Gui B, Chen Q, Hu C, Zhu C, He G (2017). Effects of calcitriol (1, 25-dihydroxy-vitamin D3) on the inflammatory response induced by H9N2 influenza virus infection in human lung A549 epithelial cells and in mice. Virology Journal 14(1):10. |
|
|
Hogan NC, Anahtar MN, Taberner AJ, Hunter IW (2017). Delivery of immunoreactive antigen using a controllable needle-free jet injector. Journal of Controlled Release 258:73-80. |
|
|
Hu Y, Wu Q, Xu B, Zhou Z, Wang Z, Zhou YH (2008). Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine 26(48):6064-6067. |
|
|
Junqueira AL, Tavares VR, Martins RM, Frauzino KV, Silva AM, Rodrigues IM, Minamisava R, Teles SA (2011). Presence of maternal anti-HBs antibodies does not influence hepatitis B vaccine response in Brazilian neonates. Memoria do Instituto Oswaldo Cruz 106(1):113-116. |
|
|
Kapadia GJ, Soares IAO, Rao GS, Badoco FR, Furtado RA, Correa MB, Tavares DC, Cunha WR, Magalhaes LG (2017). Antiparasitic activity of menadione (vitamin K3) against Schistosoma mansoni in BABL/c mice. Acta Tropica 167:163-173. |
|
|
Latha TS, Reddy MC, R Durbaka PV, Muthukonda SV, Lomada D (2017). Immunomodulatory properties of titanium dioxide nanostructural materials. The Indian Journal of Pharmacology 49(6):458-464. |
|
|
Lu IC, Jean MC, Lin CW, Chen WH, Perng DS, Lin CW, Chuang HY (2016). Predictive factors for anti-HBs status after 1 booster dose of hepatitis B vaccine. Medicine 95(39):e5023. |
|
|
Lu W, Lu C, Zhang C, Zhang C (2017). One mechanism of glucocorticoid action in asthma may involve the inhibition of IL-25 expression. Experimental and Therapeutic Medicine 13(2):657-661. |
|
|
Mahdavi M, Mavandadnejad F, Yazdi MH, Faghfuri E, Hashemi H, Homayouni-Oreh S, Farhoudi R, Shahverdi AR (2017). Oral administration of synthetic selenium nanoparticles induced robust Th1 cytokine pattern after HBs antigen vaccination in mouse model. Journal of Infection and Public Health 10(1):102-109. |
|
|
McCluskie MJ, Weeratna RD, Payette PJ, Davis HL (2002). Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunology and Medical Microbiology 32(3):179-185. |
|
|
National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011). The National Academies Collection: Reports funded by National Institutes of Health. In: Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US). |
|
|
Parasuraman S, Raveendran R, Kesavan R (2010). Blood sample collection in small laboratory animals. Journal of Pharmacology and Pharmacotherapeutics 1(2):87-93. |
|
|
Perez CMB, Kodani M, Choi Y, Joyce J, O'Connor SM, Kamili S, Prausnitz MR (2018). Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioengineering and Translational Medicine 3(3):186- 196. |
|
|
Pongpipat D, Suvatte V, Assateerawatts A (1989). Hepatitis B immunization in high risk neonates born from HBsAg positive mothers: Comparison between plasma derived and recombinant DNA vaccine. Asian Pacific Journal of Allergy and Immunology 7(1):37-40. |
|
|
Potter M (1985). The BALB/c Mouse. Current Topics in Microbiology and Immunology 122 Springer-Varlag, Berlin, New York, Tokyo. |
|
|
Schunk MK, Macallum GE (2005). Applications and optimization of immunization procedures. ILAR Journal 46(3):241-257. |
|
|
Skrastina D, Petrovskis I, Lieknina I, Bogans J, Renhofa R, Ose V, Dishlers A, Dekhtyar Y, Pumpens P (2014). Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B core virus-like particles. PloS ONE 9(12):e114006. |
|
|
Wang C, Wang C, Jia ZF, Wu X, Wen SM, Kong F, Hu KQ, Li J, Jiang J, Niu JQ (2016). Protective effect of an improved immunization practice of mother-to-infant transmission of hepatitis B virus and risk factors associated with immunoprophylaxis failure. Medicine 95(34):e4390. |
|
|
Wang J, Liu R, Liu B, Yang Y, Xie J, Zhu N (2017). Systems Pharmacology-based strategy to screen new adjuvant for hepatitis B vaccine from Traditional Chinese Medicine Ophiocordyceps sinensis. Scientific Reports 7:44788. |
|
|
Weeratna RD, Brazolot Millan CL, McCluskie MJ, Siegrist CA, Davis HL (2001). Priming of immune responses to hepatitis B surface antigen in young mice immunized in the presence of maternally derived antibodies. FEMS Immunology and Medical Microbiology 30(3):241-247. |
|
|
Yang S, Ma X, Ni H, Zhou S, Hu D, Shi H, Chen X, Dong H, Xu G (2015). Safety, immunization coverage and determinants of a new kind of Hepatitis B vaccine firstly applied in Ningbo, China. Human Vaccines and Immunotherapeutics 1(12):2819-2826. |
|
|
Yang YJ, Liu CC, Chen TJ, Lee MF, Chen SH, Shih HH, Chang MH (2003). Role of hepatitis B immunoglobulin in infants born to hepatitis B e antigen-negative carrier mothers in Taiwan. The Pediatric Infectious Disease Journal 22(7):584-588. |
|
|
Zhang L, Gui XE, Teter C, Zhong H, Pang Z, Ding L, Li F, Zhou Y, Zhang L (2014). Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 32(46):6091-6097. |
|
|
Zhao M, Li M, Zhang Z, Gong T, Sun X (2016). Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag m RNA. Drug Delivery 23(7):2596-2607. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0