ABSTRACT
This study was conducted to determine tick burden and immunological parameters of resistance to East Coast fever (ECF) in Tarime and Sukuma cattle. Tick load, packed cell volume (PCV), Theileria parva (T. parva) specific antibody percent positivity (PP), and prevalence of T. parva parasites were studied in relation to dipping regime, strains, and season. A total of 50 experimental cattle were included in this study. Tick load was determined by whole body counts, antibody percent positivity was determined by the polymorphic immunodominant molecule (PIM)-based T. parva enzyme-linked immunosorbent assay (ELISA), and prevalence of T. parva parasites was detected by a nested polymerase chain reaction (PCR) based on the p104 gene. Dipping frequency on tick burden showed no statistically significant differences when cattle of either strain were dipped either once every 2 or 3 weeks in the dry and wet seasons. However, Tarime cattle had higher (p<0.05) tick count than Sukuma cattle and non dipped groups maintained high tick infestation throughout the experimental period. The PCV values were within the physiological range, although this parameter was lower in Tarime cattle (p<0.05). All cattle regardless of strain were seropositive, although Tarime cattle maintained higher PP compared to Sukuma by 15%. Conversely, the prevalence of T. parva parasites was lower in Tarime (38%) compared to Sukuma cattle (38.5%), but the difference was not significant (p>0.05). During the study period, 20% (5/25) of Sukuma cattle contracted ECF, but none of the Tarime cattle showed clinical signs for the disease. The differences between the two strains shown in terms of PP and T. parva parasite prevalence may indicate the ability of individual cattle to resist tick infestation and ECF infection under natural challenge. Higher antibody levels but lower parasite prevalence attained by Tarime cattle, suggests inherent ability of Tarime cattle to resist clinical development of ECF infection, but to remain as T. parva carriers.
Key words: Carrier state, Theileria parva, ticks, seropositivity.
Tanzania is endowed with valuable indigenous strains of cattle (Das and Mkonyi, 2003). These livestock resources, apart from offering direct food products like meat and milk, also provide draught animal power as labor saving technologies; manure for fertilizing crop fields and biogas for electrification or cooking fuel which has a potential for reducing deforestation (MLFD, 2015). About 80% of the indigenous cattle strains in Tanzania are exposed to vector-borne infections, among which is East Coast fever (ECF) which is a major killer disease (Kivaria, 2006) causing substantial losses in terms of morbidity in adult cattle of improved breeds and calf mortality, and therefore, hinders development of the livestock sector (Swai et al., 2007; Chenyambuga et al., 2008). Control strategies for ECF are based on the use of acaricides to control the vector ticks, chemotherapy of sick animals as well as immunization of cattle by the infection and treatment method (ITM) (Norval et al., 1992; Musoke et al., 2004; Oura et al., 2004). Acaricide use and chemo-therapy are often limited by high costs, development of resistance by the vector ticks, and the parasites as well as environmental impacts (Mugisha et al., 2005; Kivaria, 2006; deCastro, 1997; George et al., 2004; Ministry of Water and Livestock Development, 2004). On the other hand, ITM offers a valuable alternative for ECF control (Oura et al., 2004); however, its widespread application has faced many challenges. These include the requirement of cold chain mode of delivery to remote areas and high cost of the vaccine (up to US$10 per animal), which is unaffordable to most smallholder herders (Di Giulio et al., 2009). Furthermore, ITM does not completely eliminate the need for acaricide application due to the potential existence of other tick-borne diseases.
Indigenous strains of cattle found in ECF endemic areas around the Lake zone of Tanzania (that is, areas around Lake Victoria), such as the Tarime cattle are reported to be more tolerant to ECF than exotic cattle and other zebu in the same region (Paling et al., 1991; Chenyambuga et al., 2008). This is probably due to natural selection, because of the long-time exposure to the disease in the region. However, much of available evidence is based on anecdotal information regarding tolerance of Tarime cattle to ECF (Chenyambuga et al., 2008).
The aim of present study was to assess tick burden and immunological parameters of resistance to ECF in Tarime in comparison to Sukuma cattle found in the same zone. Cattle from the two strains were subjected to acaricide application using two different frequencies, either once every 2 or 3 weeks. The findings from this study will make it possible to make strong recom-mendation about the potential of indigenous strains for rational utilization in ECF endemic areas.
Study area
This study was conducted at Tanzania Livestock Research Institute (TALIRI), Mabuki centre in Mwanza region, Lake Zone of Tanzania. The centre is located between latitudes 2° 58' 84" South and longitudes 33° 58' 12" East at an altitude of 1174 m above sea level. Temperatures at the centre range between 25 and 35°C and rainfall ranges from 600 to 800 mm per annum. The rainfall pattern is bimodal, with short rains starting in November and ending in February and long rains starting in mid March and ending in May.
Study design and animals
A total of fifty cattle (25 Tarime and 25 Sukuma), aged 9 to 12 months were randomly selected from a cohort of 110 cattle, which were previously purchased from farmers around the Lake Zone of Tanzania, where the animals have been kept for many years under smallholder management systems with poor tick control practices. Farmers around the Lake Zone believe that the Tarime and Sukuma strains possess resistance to tick infestation and/or ECF infection. The original cohort of 110 cattle had been kept at Mabuki Research centre in Mwanza, Tanzania for a yearlong monitoring of tick-borne diseases, during which time the cattle were kept under similar management conditions including weekly dipping. At the onset of this study, cattle from each type, shown to be free of ECF (by absence of blood piroplasms) were divided into groups of eight, eight, and nine. The cattle were individually identified by different colored ear tags and allocated to experimental groups, which were distinguished by different dipping regimes. Cattle in group 1 were dipped once after two weeks while those in group 2 were dipped once every three weeks. The third group, comprising 9 cattle served as control group without dipping throughout the study period. Dipping was done using alphacypermethrin (Dominex®) with an initial dip filling of 1 L of Dominex® for 2000 L of water, and dip replenishing by adding 1 L of Dominex® for 1250 L of water. The experiment was conducted during the dry season (mid-August to mid-November 2014) and the wet season (mid-November 2014 to mid-February 2015).
The three cattle groups were grazed together and monitored throughout the study period for tick counts and any ECF clinical signs (fever; that is body temperature above 39.5°C for more than 3 consecutive days; swelling of parotid and prescapular lymph nodes; presence of a macroschizont index ≥5% on collected smears and acute respiratory distress). Any diseased animal was promptly treated using buparvaquone (2.5 mg/kg) and all animal biodata was recorded on field sheets.
Sampling
Whole body tick counts were done once every week just before dipping. Only adult ticks were counted and identified to genus level in situ. Once every three weeks blood samples were collected by jugular vein-puncture into plain vacutainer tubes for serology and into EDTA-containing vacutainer tubes for whole blood (Poly Medicure Ltd, Faridabad, India) (for packed cell volume [PCV] and DNA extraction). A total of 400 blood samples were, therefore, collected during 6 months in 8 sampling periods at three week intervals. The blood samples were transiently stored in cool boxes containing ice packs and then transferred to Tanzania Veterinary Investigation and Laboratory Agency (TVLA) in Mwanza for initial processing (PCV and serum extraction) and then transferred to the Faculty of Veterinary Medicine laboratories at Sokoine University of Agriculture, Morogoro for further analyses.
Tick identification and counts
Tick counts were done according to Londt et al. (1979). Visible adult ticks were counted from whole animal body and identified using the keys of Mathysse and Colbo (1987). The ticks were identified to genus level and recorded for each animal in relation to frequency of dipping season and strain.
Genomic DNA extraction and detection of Theileria parva
Genomic DNA was extracted from 400 whole blood samples using the Pure Gene Blood Core (QIAGEN) Kit (Minnesota, USA) according to the manufacturer’s instructions. The nested p104 polymerase chain reaction (nPCR) was used to screen all field samples for the presence of T. parva. Primers derived from the T. parva–specific 104-KDa antigen (p104) gene were used in the PCR amplification as previously described by Odongo et al. (2010) and Iams et al. (1990). The sequences of the forward and reverse primers were 5’ATT TAA GGA ACC TGA CGT GAC TGC 3’ and 5’ TAA GAT GCC GAC TAT TAA TGA CAC C 3’, respectively, for first the round and 5’ GGC CAA GGT CTC CTT CAG AAT ACG3’ and 5’TGG GTG TGT TTC CTC GTC ATC TGC 3’, respectively, for second the round. The nPCR amplifications were performed in a total volume of 20 μl containing 14 µl nuclease-free water, 0.5 μl (10 pmol) of each of forward and reverse primers and 5 μl of genomic DNA (20 ng/μl) template added into the lyophilized pellet (Bioneer PCR Pre-Mix - Korea), followed by vortexing and brief spin down to dissolve the pellet. Reaction conditions for the primary PCR included initial denaturation at 94°C for 5 min, denaturation at 94°C for 60 s, annealing at 60°C for 60 s and extension at 72°C for 60 s and the amplification was done in 30 cycles. For a second round, amount of water was 18.5 and 0.5 μl of the primary PCR product was used as a template. The cycling profile condition for the second PCR was the same as the primary amplification, except for the annealing temperature which was 50°C. The nPCR reactions were carried out in a thermocycler (TAKARA Bio Inc., Japan). The nPCR products were separated on 1.5% agarose gels and visualized on ultra violet (UV) trans-illuminator.
Packed cell volume (PCV)
The PCV was determined using whole blood drawn from the jugular vein into EDTA-containing vacutainer tubes. Blood was drawn from the tubes using capillary tubes filled to ¾ of its length. One end of the tube was sealed with cristaseal and then placed into a micro-haematocrit centrifuge (ex UK with 9 cm rotor radius) for 5 min at 12,000 rpm (RCF; 14,489.28 g). Reading of the PCV was performed on the Micro-haematocrit Reader Scale. The PCV was determined from the reading expressed as percentage of packed red cells in the total volume of blood.
Indirect ELISA for T. parva antibodies
The PIM-based enzyme-linked-immunosorbent assay (ELISA) described by Katende et al. (1998) was used to measure specific antibodies to T. parva (sensitivity > 99%, specificity 94 to 98%). Optical density (OD) of each sample was measured at 405 nm using an Erba Lisascan II ELISA reader (ERBA diagnostics, Mannheim GmbH, Germany). The OD readings were used to compute antibody percent positivity (PP) for each sample using the formula:
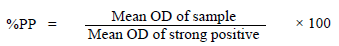
The PP of 20% or higher was considered positive.
Data analysis
The data obtained from tick count, PCV, ELISA and PCR were coded and analysed using the statistical package for social sciences (SPSS) research software version 16 (SPSS, 2008). The percent of tick species, PCV, PP, and the prevalence of T. parva were compared using a chi-square to test the significance of the differences in tick infestation and prevalence of T. parva between strains, dipping regimes, and seasons. Analysis of variance (ANOVA) was used to analyze the data on tick count to assess the statistical significance of differences. The fixed effects assessed were strain, dipping regime and season. All results were considered significant at p ≤ 0.05.
Ticks infestation among Sukuma and Tarime cattle strains
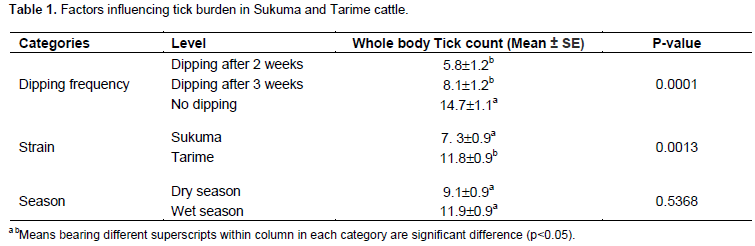
Three tick genera, Amblyomma genus, Boophilus sub-genus of Rhipicephalus genus and other species of Rhipicephalus were identified in the study area (Tables 1 and 2). Rhipicephalus genus accounted for 68.2% of the total ticks whereas Boophilus sub-genus of Rhipicephalus genus and Amblyomma constituted 16.3 and 15.5%, respectively. Significant differences were observed between Tarime and Sukuma cattle in terms of tick counts (p<0.05). Tarime cattle had relatively higher (11.8 ± 0.9) number of ticks per animal compared to Sukuma cattle (7.3 ± 0.9). However, dipping frequency of once every 2 or 3 weeks did not reveal significant differences in tick burden in both strains in both seasons. As expected non-dipped cattle had the highest (14.7 ± 1.1) tick burden compared to cattle dipped at either intervals (p<0.05). When tick infestations on the two cattle strains were compared during between the seasons, higher tick counts were observed during the wet season but the difference between wet and dry seasons was not statistically significant (p> 0.05).
Clinical ECF manifestation and PCV among Sukuma and Tarime cattle strains
During the entire experimental period, animals were monitored for clinical signs of ECF. Although no mortality was recorded, however, five animals of Sukuma cattle (20%) showed clinical signs of ECF infection as confirmed by microscopy of blood and lymph smears. All the ECF cases occurred in group 3 animals (Sukuma control group) during the wet season and the sick animals recovered after treatment with buparvaquone (2.5 mg/kg). No cattle from the Tarime strain suffered from ECF during the study period (Table 2).

Prevalence of antibody percentage positivity in Sukuma and Tarime cattle strains
Levels of specific antibodies to T. parva detected in the Tarime and Sukuma cattle strains are shown in Table 4.
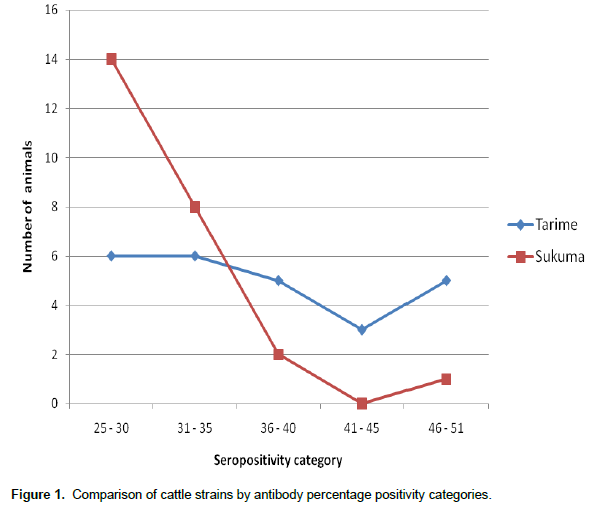
All cattle regardless of strain were positive for T. parva antibodies. Tarime cattle maintained signify-cantly higher antibody percent positivity compared to Sukuma cattle by 15% (p<0.05). This was further demonstrated when cattle from the two strains were clustered into seropositivity categories (Figure 1). Thus, higher proportion of Sukuma strain cattle clustered to lower antibody PP (20 to 40) whereas most Tarime strain cattle fell into the higher antibody cluster PP (41 to 60). None of the cattle displayed antibody PP above 60%. Figure 1 depicts the distribution of cattle by strain across seropositivity categories. Interestingly, only a few Sukuma cattle showed antibody PP above 41% whereas Tarime cattle were evenly distributed across the seropositivity categories.
This study confirms anecdotal information regarding tolerance of Tarime cattle to ECF as compared to Sukuma cattle kept in the same lake zone of Tanzania. This conclusion is based on three clear findings emanating from this study. Firstly, our study clearly indicated that Tarime cattle carried significantly higher tick burdens than Sukuma cattle. Most (> 68%) of the ticks found on the cattle of both strains were of Rhipicephalus appendiculatus species, vectors of T. parva, the causative agent of ECF. Furthermore, none of the Tarime cattle showed signs of clinical ECF whereas signs were evidenced in 20% of the Sukuma cattle. These findings provide the first testimony to hitherto farmers’ assertions that Tarime cattle possess tolerance to both ticks and clinical ECF. Farmers keeping Tarime cattle do not dip their cattle frequently; however, animals of this strain rarely show clinical ECF signs as compared to cattle of other strains communally grazing in the same area (Laisser et al., 2014). Ability of the Tarime cattle to carry more ticks and suffer less ECF may, therefore, be ascribed to an inherent feature of this strain of cattle, which has a small body size and seem to tolerate harsh local environmental conditions, including pressure from tick infestation. Our result on tick burdens and incidence of ECF in Tarime cattle concur with the findings by Taylor (2006), who reported a significant negative correlation between animal body weight and tick counts. Animals with an average body weight below 250 kg had 42% more ticks compared to animals with higher body weight. Also the high tick infestation observed on non- dipped cattle agrees with the report by Mathee et al. (1997) who showed that regardless of breed, if ticks are not controlled, animals will always carry more ticks throughout the year and if the animals are not tolerant, there will be high mortality. However, the number of ticks per animal observed in this study differs from that observed by Laisser et al. (2014) in Serengeti and Tarime districts of Mara region. The difference observed may be attributed by several factors, which include management practices and animal factors in these ecological areas. Generally, our findings demonstrate that animals which are adapted to a given environment usually carry fewer ticks as previously reported by Wambura et al. (1998).
Secondly, when we compared Tarime and Sukuma cattle in terms of prevalence of T. parva parasites, our study has revealed that Tarime cattle had slightly lower parasite prevalence, although the difference from Sukuma cattle was not statistically significant. These results agree with previous findings by Gachohi et al. (2012) and Marufu (2008) who reported that when different breeds of cattle are kept together in T. parva endemic area, less resistant cattle tend to acquire more parasites. Lower T. parva parasite prevalence observed in Tarime cattle may indicate a higher ability of these animals to clear T. parva parasites compared to more susceptible cattle breeds. Our results have further shown that parasitemia increased gradually in both strains from the beginning of the study (period 1) to the end of the study (period 8) by almost 150 to 180%. These data provide an indication of inherent T. parva carrier state of cattle of both strains and that the carrier state increased incrementally throughout the study period. Our data also show that cattle of both strains picked up the infection gradually since they co-grazed on same pastures, however, it is interesting to point out that Tarime cattle did not develop clinical signs to the extent shown by Sukuma cattle. The Lake zone in Tanzania is an endemic area for ECF and as such it would be expected that cattle kept in this area are under constant exposure to the T. parva parasites. Zebu cattle usually acquire immunity to T. parva following recovery after primary ECF infection. It is also evidently reported that natural tick challenge incrementally boosts the immunity acquired by zebu cattle kept in endemic areas (Kazungu et al., 2015), therefore, resistant individuals or breeds are likely to attain higher immunity compared to susceptible breeds. Our result on the differences between Tarime and Sukuma cattle strains further confirms the preference of farmers in the Lake Zone for Tarime cattle than other strains of cattle (Laisser et al., 2015). As such, farmers’ knowledge on unique attributes of their cattle breeds is usually passed on through generations (FAO/links, 2000) and thus our result has demonstrated evidence to farmers’ beliefs and paves way to designing improvement programmes for Tarime cattle for not only ECF tolerance but also in terms of productivity Thirdly, this study has revealed a broad range of antibody percent positivity levels for both strains, but the majority of the Tarime cattle clustered to higher antibody PP categories. Besides being an epidemiological indicator, the level of antibodies in animals in an endemic area may also indicate a measure of resistance to infection pressure in the study area. It is possible that the higher level of specific antibodies reflects the response of resistant individuals in a T. parva endemic area under constant natural tick challenge. Probably, these findings could also suggest the ability of Tarime cattle to mount a stronger antibody response, which primarily play a crucial role in neutralization of T. parva sporozoites in early stages before ECF infection. This is also supported by our finding of lesser clinical ECF infections in Tarime compared to Sukuma cattle. The findings of lower parasite prevalence, but higher antibody levels shown by Tarime cattle suggest a potential state of endemic stability, whereby cattle develop a carrier state of T. parva parasites without clinical disease (Norval et al., 1992; Deem et al., 1993; Perry and Young, 1995; Matovelo et al., 2003). Our result concurs with Martins et al. (2010) who reported a significant negative association between antibody percent positivity levels in cattle and incidences of ECF cases.
This study has revealed, high seroprevalence which was indicated for both, Sukuma and Tarime cattle strains. The 100% seroprevalence indicates that all study animals had previous exposure to T. parva, although differences existed between the strains and individual cattle in terms of level of antibodies produced. The high seroprevalence obtained in this study area further demonstrates the potential development of a state of endemic stability in the Lake zone, whereby the majority of cattle are expected to be carriers as previously shown by Kazungu et al. (2015). Dipping regime did not show any influence on seroprevalence results.
The present study has demonstrated differences in terms of tick burdens between Tarime and Sukuma cattle, whereby Tarime cattle carried significantly higher tick load. Tarime strain of cattle was also different from Sukuma cattle in terms of T. parva parasite prevalence and antibody percent positivity. The higher antibody levels but lower parasite prevalence shown by Tarime cattle suggests a higher proportion of resistant individuals in this strain, which may potentially support development of a T. parva carrier state. Our study provides evidence to farmers’ beliefs in the Lake zone of Tanzania regarding resistance of Tarime compared to Sukuma cattle. This paves a way to designing improvement programmes for the Tarime cattle for rational utilization.
The authors have not declared any conflict of interest.
The authors are very grateful for the financial support from the Norwegian Agency for International Development (NORAD) through the programme for Enhancing Pro-poor Innovation in Natural Resources and Agricultural Value Chains (EPINAV) at Sokoine University of Agriculture. This financial support enabled us to accomplish this work. They would like to thank the staff of TALIRI, Mabuki and TVLA, Mwanza for their kind support in data handling and initial processing and storage of samples before being transported to SUA. They also thank Mr. Edson Rugaimukamu and Ms Yvette Kazungu for laboratory technical support. They are also grateful to the Livestock herdsmen who devoted their time in looking after the experimental animals.
REFERENCES
|
Chenyambuga SW, Waiswa C, Saimo M, Ngumi P, Gwakisa PS (2008). Knowledge and perceptions of traditional livestock keepers on tick-borne diseases and sero-prevalence of Theileria parva around Lake Victoria Basin. Livest. Res. Rural Dev. 22(7).
|
|
|
|
Das SM, Mkonyi JI (2003). Important aspects of conservation of indigenous cattle in Tanzania: A review. In: Tanzania Society of Animal Production Conference series. 30:59-70.
|
|
|
|
deCastro JJ (1997). Sustainable tick and tick-borne disease control in Livestock improvement in developing countries. Vet. Parasitol. 71:77-97.
crossref
|
|
|
|
Deem SL, Perry BD, Katende JM, McDermott JJ, Mahan SM, Maloo SH, Morzaria SP, Musoke AJ, Rowlands GJ (1993). Variations in prevalence of tick-borne diseases in Zebu cattle by agro-ecological zone: implications for East Coast fever immunisation. Prev. Vet. Med. 16:171-187.
crossref
|
|
|
|
Di Giulio G, Lynen G, Morzaria S, Oura C, Bishop R (2009). Live immunization against East Coast fever – current status. Trends Parasitol. 25:85-92.
crossref
|
|
|
|
FAO/LinKS (2000). Benefits and Risks of Sharing Local Knowledge. LinKS Technical Report No 3, FAO LinKS Project. Dar-es-Salaam.
|
|
|
|
Gachohi J, Skilton R, Hansen F, Ngumi P, Kitala P (2012). Epidemiology of East Coast fever (Theileria parva infection) in Kenya, past, present and the future. Parasit. Vect. 5:194.
crossref
|
|
|
|
George JE, Pound JM, Davey RB (2004). Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 129(7):353-366.
crossref
|
|
|
|
Iams KP Young JR, Nene V (1990). Characterisation of the gene encoding a 104-kilodalton micronemerhoptry protein of Theileria parva. Mol. Biochem. Parasitol. 39:47-60.
crossref
|
|
|
|
Katende JM, Toye P, Skilton RA, Nene V, Morzaria SP, Musoke AJ (1998). An ELISA for detection of Theileria parva antibodies in cattle using a recombinant polymorphic immunodominant molecule. Parasitol. Res. 84:408-416.
crossref
|
|
|
|
Kazungu YEM, Mwega E, Ole Neselle M, Sallu R, Kimera SI, Gwakisa P (2015). Incremental effect of natural tick challenge on the infection and treatment method-induced immunity against T. parva in cattle under agro-pastoral systems in Northern Tanzania. Ticks and Tick-borne Diseases 6:587-591.
crossref
|
|
|
|
Kivaria FM (2006). Estimated direct costs associated with tick-borne diseases on cattle in Tanzania, Trop. Anim. Health Prod. 38:291-299.
crossref
|
|
|
|
Laisser ELK, Chenyambuga SW, Msalya G, Kipanyula MJ, Mdegela RH, Karimuribo ED, Mwilawa AJ, Kusiluka LJK (2015). Knowledge and perception on ticks, tick-borne diseases and indigenous cattle tolerance to East Coast fever in agro pastoral communities of Lake Zone in Tanzania. Livest. Res. Rural Dev. 27(4). 1st April, 2015.
|
|
|
|
Laisser ELK, Kipanyula MJ, Msalya G, Mwega ED, Mdegela RH, Karimuribo ED, Kusiluka LJK, Mwilawa AJ, Chenyambuga SW (2014). Tick burden and prevalence of Theileria parva infection in Tarime zebu cattle in the lake zone of Tanzania. Trop. Anim. Health Prod. 46(8):1397-1406.
crossref
|
|
|
|
Londt JGH, Horak IG, de Villiers IL (1979). Parasite of domestic and wild animal in South Africa, XIII, The seasonal incidence of adult ticks (Acarina: Ixodidae) on cattle in the northern Transvaal. Onderpoort J. Vet. Res. 46:31-39.
|
|
|
|
Martins SB, Di Giulio G, Lynen G, Peters A, Rushton J (2010). Assessing the impact of East Coast Fever immunisation by the infection and treatment method in Tanzanian pastoralist systems. Prev. Vet. Med. 97:175-182.
crossref
|
|
|
|
Marufu MC (2008). Prevalence of ticks and tick borne diseases in cattle on communal rangelands in the highland areas of the Eastern Cape Province, South Africa, MSc. dissertation, University of Fort Hare.
|
|
|
|
Mathee S, Meltzer DGA, Horak IG (1997). Sites of attachment and density assessment of ixodid ticks (Acari: Ixodidae) on impala (Aepyceros melampus). Exp Appl. Acarol. 21:179-192.
|
|
|
|
Mathysse JG, Colbo MH (1987). The Ixodid Ticks of Uganda, Entomological Society of America. Collage Park. Maryland. USA.
|
|
|
|
Matovelo JA, Gwakisa PS, Gwamaka M (2003). Induction of acquired immunity in pastoral zebu cattle against East Coast Fever after natural infection by early diagnosis and early treatment. J. Applied Res. Vet. Med. 1(2):136-142.
|
|
|
|
Ministry of Water and Livestock Development, UROT (2004). National Tick and Tick-borne Disease Control Strategy. Directorate of Veterinary Services, Dar Es Salaam.
|
|
|
|
MLFD (2015). Ministry of Livestock and Fisheries Development. Tanzania Livestock Modernization Initiatives (TLMI) for the period 2015/2016 - 2020/2021. Statement by the President of United Republic of Tanzania. July 2015.
|
|
|
|
Mugisha A, McLeod A, Percy R, Kyewalabye E (2005). Strategies, effectiveness and rationale of vector-borne disease control in the pastoralist system of south-west Uganda. Trop. Anim. Health Prod. 37:479-489.
crossref
|
|
|
|
Musoke RA, Tweyongyere R, Bizimenyere E, Waiswa C, Mugisha A, Biryomumaisho S, MacHardy N (2004). Teatment of East Coast fever of Cattle with a Combination of Parvaquone and Frusemide. Trop. Anim. Health Prod. 36(3):233-245.
crossref
|
|
|
|
Norval RAI, Perry BD, Young AS (1992). The epidemiology of theileriosis in Africa. Academic Press, London. UK. P 481.
|
|
|
|
Odongo D O, Sunter JD, Kiara HK, Skilton RA, Bishop RP (2010). A nested PCR assay exhibits enhanced sensitivity for detection of Theileria parva infections in bovine blood samples from carrier animals. Parasitol. Res. 106 (2):357-65.
crossref
|
|
|
|
Oura CAL, Bishop R, Wampande EM, Lubega GW, Tait A (2004). The persistence of component Theileria parva stocks in cattle immunized with the live vaccine against East Coast fever in Uganda. Parasitology 129:27-42.
crossref
|
|
|
|
Paling RW, Mpangala C, Littikhuizen B, Sibomana G (1991). Exposure of Ankole and crossbred cattle to Theileriosis in Rwanda. Trop. Anim. Health Prod. 23:203-214.
crossref
|
|
|
|
Perry BD, Young AS (1995). The past and future roles of epidemiology and economics in the control of tick-borne diseases of livestock in Africa: the case of theileriosis. Prev. Vet. Med. 25:107-120.
crossref
|
|
|
|
SPSS (Statistical Packages for Social Science) (2008). SPSS for Windows, Release 16.0, User Manual, SPSS Inc. Chicago, USA.
|
|
|
|
Swai ES, Karimuribo ED, Kambarage DM, Moshy WE, Mbise AN (2007). A comparison of seroprevalence and risk factors for Theileria parva and Theileria mutans in smallholder dairy cattle in the Tanga and Iringa regions of Tanzania. Vet. J. 174:390-396.
crossref
|
|
|
|
Taylor GJ (2006). Factors affecting the production and reproduction performance of adapted beef cattle in southern africa. PhD thesis. University of Pretoria.
|
|
|
|
Wambura PN, Gwakisa PS, Silayo RS, Rugaimukamu EA (1998). Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos Taurus. Vet. Parasitol. 77:63-70.
crossref
|