Full Length Research Paper
ABSTRACT
In Senegal, studies regarding the distribution of Candida species involved in vulvovaginal candidiasis (VVC) and their antifungal susceptibility are scarce. Here we reported the frequency of Candida species isolated in VVC and their antifungal susceptibility in Dakar. A cross-sectional study included women referred to the laboratory of the Military Hospital of Ouakam for vaginal discharge was carried out. From each patient, two vaginal swabs and socio-demographic parameters were collected. The automated Vitek® 2 compact system determined species identification and antifungal susceptibility testing. Overall, 119 patients were enrolled. The mean age was 34.8 ± 10 years. The overall VVC prevalence was 31.93%, with the predominant Candida albicans (72%) followed by Candida glabrata (6.7%), Candida tropicalis (1.7%), and Candida kefyr (0.8%). C. albicans strains showed 100% susceptibility regarding the different antifungals except for flucytosine, in which two cases of resistance (8.34%) were noted. The observed susceptibility rates of C. glabrata to fluconazole, voriconazole, caspofungin, micafungin, amphotericin B, and flucytosine were 0, 100, 14.29, 100, 100 and 100%. C. tropicalis antifungal susceptibility was 100% for all tested drugs, while C. kefyr susceptibility was only noted for voriconazole and flucytosine. This study provides an overview of the distribution of Candida species involved in VVC in Dakar with C. albicans which showed a high susceptibility rate to the different antifungals.
Key words: Vulvovaginal candidiasis, antifungal susceptibility, Dakar.
INTRODUCTION
Vulvovaginal candidiasis (VVC) is a fungal infection caused by yeasts belonging to the genus Candida which includes around 200 species of non-pigmented, non-capsulated, multilateral budding yeasts that produce filaments, except for Candida glabrata (Achkar and Fries, 2010). Candida albicans, the primary yeast involved in candidiasis, is a polymorphic fungus that can rapidly alternate between round cells (yeasts) and, pseudomycelian filaments or hyphae (Berman and Sudbery, 2002). Hyphae are resistant to phagocytosis more adherent to host surfaces, and can invade epithelial cell layers resulting in tissue damage (Nicholls et al., 2011). VVC is one of the most common gynecological disorders, with symptoms such as leukorrhea, pruritus, and burning frequently identified as causes of consultation (Barajas et al., 2019; Sobel, 2007). Approximately 70 to 75% of women have at least one episode of VVC during their lifetime (Sobel, 2007; Yano et al., 2019; Gonçalves et al., 2016). Although C. albicans is commonly reported to be the pathogenic agent, species known as non-albicans Candida (NAC), such as C. glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei, have recently emerged (Denning et al., 2018; Felix et al., 2019; Amouri et al., 2010). In Senegal, previous studies regarding VVC caused by C. albicans showed a prevalence range of 24 to 34% (Boye et al., 2022; Seck et al., 2015; Sylla, 2018). However, Sylla (2018) found in their study carried out at Fann Teaching Hospital of Dakar a prevalence rate of 27.96% of VVC due to NAC species. While both clinical and diagnosis of VVC are well-known and performed routinely, therapeutic management raises challenging concerns regarding the choice of appropriate antifungal drugs. Thus, despite the availability of effective antifungals, vulvovaginal candidiasis remains a complicated disorder to manage because of the drug resistance of some strains and the recurrent character of the diseases (Sobel, 2016). Also, antifungal susceptibility testing is not performed in most mycology laboratories in Africa. This study aimed to determine the frequency of Candida species involved in VVC and their antifungal susceptibility among patients referred to the microbiology laboratory of the Military Hospital of Ouakam, Dakar.
METHODOLOGY
Study site and population
This study was carried out from April to June 2022 at the microbiology laboratory of the Military Hospital of Ouakam. The study population included women referred to the laboratory for vaginal discharge during the study period. The Military Hospital of Ouakam is a level 3 public hospital located in Dakar, the capital city of Senegal.
Sample and data collection
From each patient, two vaginal swabs (VS) and socio-demographic parameters such as age, symptoms, marital status, education level, and address were collected. One VS was used to perform a wet smear and a thin smear for Gram Stain. The second VS was used for inoculation of Sabouraud Dextrose Agar (SDA). The culture medium was incubated at 35 to 37°C during 18 to 24 h under aerobic conditions. Plates without growth after 24 h were re-incubated for a further 24 h. Preparation and performance evaluation of the culture media was performed according to the manufacturer's instructions.
Identification and antifungal susceptibility of Candida species
Species identification and antifungal susceptibility testing of yeasts were determined by the automated Vitek® 2 compact system (bioMe?rieux, France) using YST-21343 and AST-YS01 cards. The VITEK 2 compact is a computerized microbiology system using growth-based technology using colorimetric reagent cards that are automatically incubated and interpreted. The YST-21343 identification card is based on established biochemical methods and newly developed substrates. The AST-YS07 Vitek® 2 Antifungal Susceptibility Card is intended for use with Vitek® 2 systems in clinical laboratories as an in vitro test to determine the susceptibility of Candida. Briefly, isolated yeast colonies were selected on Sabouraud agar, then transferred aseptically 3.0 ml of sterile saline (0.45 to 0.50% aqueous NaCl, pH 4.5 to 7.0) into a clear plastic test tube (12 mm × 75 mm). Next, a sterile swab transfers a sufficient number of morphologically similar colonies into the saline tube. A homogeneous suspension of organisms with a density equivalent to a McFarland No. 1.80 to 2.20 using the Vitek® 2 DensiCHEKTM Plus was prepared. Finally, the suspension tube and the YST card were placed into the cassette. The filled cassette was inserted manually into Vitek® 2 compact reader-incubator module. The final identification of excellent, good, acceptable, or low discrimination was considered correct.
Statistical analysis
Statistical analyses were performed using R software. Continuous variables were described as mean (standard deviation). Normally distributed variables were compared with a t-test. Categorical variables were presented as percent, and Fisher exact or Chi-squared tests were used for proportional assessments. Univariate logistic regression analysis assessed the association between the related risk factors and positivity. Four age groups were defined for analysis, that is, 17 to 25 years; 26 to 35 years; 36 to 45 years, and over 45 years. For all statistical tests, we accepted that a two-sided level of significance was set at p≤ 0.05.
Ethical considerations
This study was hospital-based research conducted in normal conditions under the Declaration of Helsinki. Ethical permission was obtained from the hospital authorities, and the patient's consent was also received before specimen collection. Information collected during the study was analyzed using the participant's identification code to ensure confidentiality.
RESULTS
Socio-demographic characteristics of the study population
Over the course of the study period, 119 patients were enrolled. The mean age was 34.8 years (Standard Deviation +/- 10 years). The age group 26 - 35 years was most represented [44.54% (95% CI: 40 - 59)]. According to marital status, 87.4% (n =104) were married. By educational level, patients who completed their secondary school level were most represented with 43.70% (n = 52), followed by university and primary level with 29.41 and 11.76%, respectively. The patient's most common symptoms were grouped as follows: discharge and irritation [37% (95% CI: 28 - 46)], discharge only [28.6% (95% CI: 21 - 38)], discharge and irritation and burning sensation [17.6% (95% CI: 11 - 26)]. Detailed characteristics of the study population are presented in Table 1.
Prevalence of VVC and distribution of Candida spp.
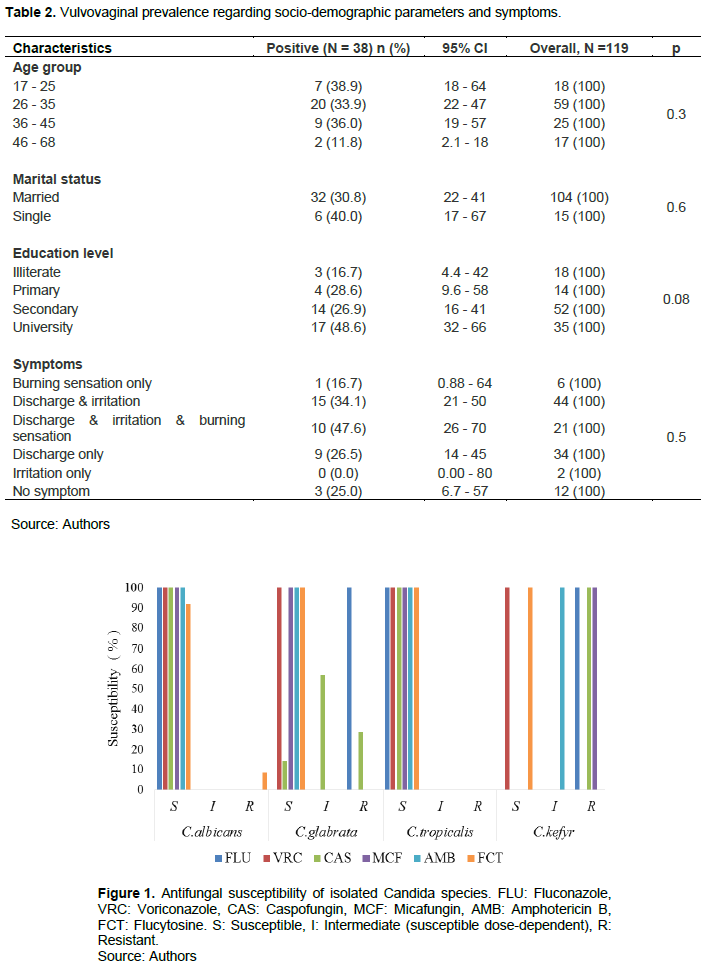
The overall VVC prevalence was 31.93% (95% CI: 24 - 41). Four Candida spp. were isolated with the predominant C. albicans [72% (n=27)] followed by C. glabrata [6.7% (n=8)], C. tropicalis [1.7% (n=2)], and C. kefyr [0.8% (n=1)]. According to age group, the highest prevalence was recorded in patients aged between 36 and 45 years with 36.0% (95% CI: 19 -57). Those over 45 years were less affected, with 11.11% (95% CI: 2.1 - 18). By marital status, single patients were more concerned with 40.0% (95% CI: 17 - 67) than married ones with no significant difference (p = 0.55). The distribution of VVC prevalence by education level showed that the highest rate was recorded [48.57% (95% CI 32- 66)] in patients with university level and the lower one in the illiterate group [16.7% (95% CI: 4.4 - 42)] with no significant difference (p = 0.079). According to symptomatologic status, the highest prevalence rate [47.6% (95% CI: 26 - 70)] was noted in patients with discharge, irritation, and burning sensation, followed by women with discharge and irritation [34.1% (95% CI: 21 -50)] and discharge only with 26.5% (95% CI: 14 - 45). Any VVC was recorded in patients who presented irritation only as a symptom. The other details regarding VVC prevalence are shown in Table 2.
Antifungal susceptibility
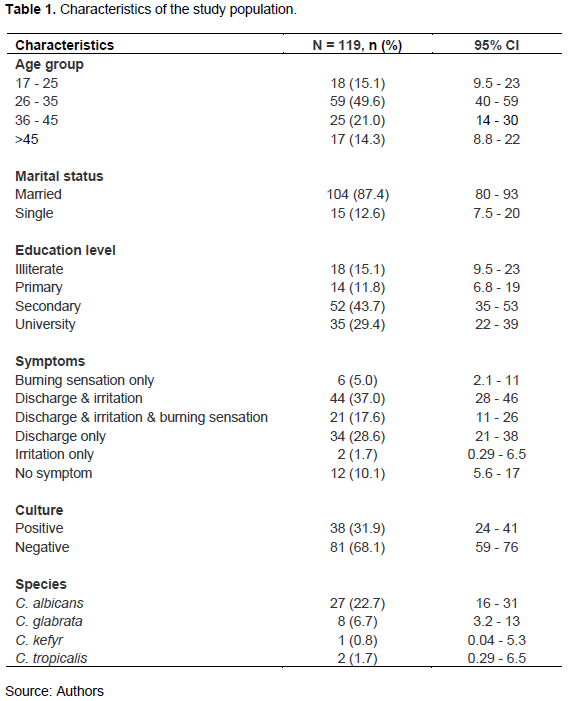
Antifungal susceptibility was tested for six drugs: Amphotericin B (AMB), Flucytosine (FCT), Fluconazole (FLU), Caspofungin (CAS), Micafungin (MCF), and Voriconazole (VRC)). From the 38 Candida strains isolated, 34 antifungal susceptibilities were successfully performed as follows: C. albicans (n=24), C. glabrata (n =7), C. tropicalis (n=2), and C. kefyr (n=1). C. albicans strains showed 100% susceptibility regarding the different antifungals except for Flucytosine, in which two cases of resistance (8.34%) were noted. The observed susceptibility rates of C. glabrata to FLU, VRC, CAS, MCF, AMB, and FCT were 0, 100, 14.29, 100, 100, and 100%. C. tropicalis antifungal susceptibility was 100% for all tested drugs, while C. kefyr susceptibility was only noted for Voriconazole and Flucytosine (Figure 1).
DISCUSSION
This study aimed to determine the frequency of Candida spp involved in vulvovaginal candidiasis and their antifungal susceptibility among patients referred to the microbiology laboratory of the Military Hospital of Ouakam using the automated Vitek® system.
Frequency of vulvovaginal candidiasis
In our study, the frequency of vulvovaginal candidiasis was 31.93%. This finding is in line with those previously reported in the same region of Dakar by Sylla (2018) who found a prevalence rate of 32.6%. Comparable prevalence rates were reported in Ghana and Iran by Waikhom and Matehkolaei with 30.7 and 28.3%, respectively (Waikhom et al., 2020; Rezaei-Matehkolaei et al., 2016). However, in Lebanon, Ghaddar et al. (2020) registered a higher frequency rate of 39%. Similarly, a prevalence of VVC of 51.3% was reported in Vietnam by Anh et al. (2021). These findings show that the frequency of vulvovaginal candidiasis is relatively high and differs from country to country.
In our series, patients aged between 26 and 35 years were the most represented. Still, the highest VVC prevalence was recorded in patients aged between 36 and 45 years, with 36.0%, indicating that this age group is most concerned by vaginal disorders in Dakar. Contrary to the findings of Sylla (2018), VVC was more prevalent among women aged 20-35 years. Likewise, in Ghana, Waikhon et al. (2020) noted that the 20-29 age group was the most affected by VVC. In another study carried out in Iran and involving 120 patients, Rezaei-Matehkolaei et al. (2016) reported that the 41-50 age group was the most affected.
Also, in our study, patients with university degrees were the most affected by VVC (48.57%), while illiterate patients were the least likely to be involved. Our finding is in line with the conclusion reached by Rathod et al.
(2012) but in contradiction with the results of Yadav and Prakash (2016) and Bitew and Abebaw (2018), who reported that illiterate women were more affected than those patients with primary school education and above. These findings suggest that the hypothesis that improved personal hygiene resulting from schooling can reduce the risk of vaginal infection may not always be verified. According to marital status, singles were more affected than married patients in our study, but the association was not statistically significant. The same trend was previously observed by Sylla (2018) in Dakar. Similarly, in Ethiopia, Bitew and Abebaw (2018) reported that vulvovaginal candidiasis was higher among divorced study subjects (52.6%) compared to unmarried (41.5%) or married (37.4%) study subjects. Contrary to the report of Waikhom et al. (2020) in Accra, most of the VVC were noted as married subjects.
Prevalence of different isolated Candida spp.
In this report, cultures were positive for 38 strains (31.93%), with four Candida species identified: C. albicans (72%), C. glabrata (19%), C. tropicalis (6%) and C. kefyr (3%). The most frequently isolated species was C. albicans. The non-albicans species (NAS) were observed in 28%. These findings align with those reported by Sylla (2018) who estimated the prevalence of C. albicans at 71.51% and that of NAS at 27.96%. Also, the predominance of C. albicans was noted in the series of Shi et al. (2020) 76.9% and in Ethiopia by Bitew and Abebaw (2018) at 58.6%. However, in Accra, C. glabrata was identified as the main species causing vulvovaginal candidiasis with 57.4% (Waikhom et al., 2020), while this species ranked in the second position in our series with 19%. In Ivory Coast (Kpongbo et al., 2018) and Iran (Rezaei-Matehkolaei et al., 2016), C. glabrata ranks second in species causing vaginal candidiasis with 19.2 and 8.8%, respectively. In Ethiopia, Bitew and Abebaw (2018) reported that C. krusei was the most isolated species after C. albicans in vaginal candidiasis. Regarding the frequency of C. tropicalis (6%) and C. kefyr (3%), our results are in line with those found in Iran and Vietnam by Anh et al. (2021) and Nejat et al. (2018) who reported a prevalence of 4.31 and 5.7%, respectively.
Antifungal susceptibility
In our study, antifungal susceptibility was assessed for Fluconazole, Voriconazole, Caspofungin, Micafungin, Amphotericin B, and Flucytosine.
Regarding the Azoles antifungal group (Fluconazole and Voriconazole), all isolated Candida strains were 100% susceptible to Voriconazole. C. kefyr and C. glabrata were not tested with Fluconazole. On the other hand, no resistance to Fluconazole was found for C. albicans and C. kefyr strains, that is, 100% susceptibility
of both species to Fluconazole. The same trend of antifungal susceptibility of azoles was reported by Bitew and Abebaw (2018). However, Sharifynia et al. (2017) suggested a high resistance of C. albicans to Fluconazole than in non-albicans Candida spp.
The antifungal susceptibility test to the Echinocandins group (Caspofungin and Micafungin) showed 100% susceptibility of all isolated Candida spp., except C. glabrata which showed 28.57% of resistance to Caspofungin and 53.14% susceptible dose-dependent (intermediate) to the same drug. Regarding Micafungin, our results are supported by Anh et al. (2021) and Bitew and Abebaw (2018) who reported 100% susceptibility of 133 Candida spp. involved in VVC to Micafungin. However, regarding Caspofungin susceptibility, a resistance rate of 4.7% was reported for C. albicans by Rezaei-Matehkolaei et al. (2016).
Regarding Amphotericin B and Flucytosine susceptibility tests, all isolated Candida strains were 100% susceptible to amphotericin B. This finding agrees with Abidjan's findings by Kpongbo et al. (2018) who showed that all Candida strains isolated in his series were susceptible to amphotericin B.
Also, in our series, all Candida spp. were sensitive to Flucytosine, except for two C. albicans strains suggesting a resistance of 8.34%. Previously in Senegal, Mane et al. (unpublished data) reported a resistance rate of C. albicans to flucytosine of 4%. Similarly, a low resistance rate of 2% of C. albicans to Flucytosine was found by Bitew and Abebaw (2018).
CONCLUSION
This study regarding the frequency of species involved in vulvovaginal candidiasis and their antifungal susceptibility in Dakar reveals a prevalence rate comparable with those previously reported in Dakar. Furthermore, it overviews the distribution of Candida spp. in Dakar. Concerning the antifungal susceptibility, C. albicans, the main species involved in VVC, showed a high susceptibility rate to the different antifungals tested. However, antifungal susceptibility monitoring for early resistance detection should be continued.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors are grateful to the patients who participated in this study. They thank all the microbiology laboratory staff who participated in this study.
REFERENCES
|
Achkar JM, Fries BC (2010). Candida infections of the genitourinary tract. Clinical Microbiology Reviews 23:253-273. |
|
|
Amouri I, Abbes S, Sellami H, Makni F, Sellami A, Ayadi A (2010). La candidose vulvovaginale: revue. Journal de Mycologie Médicale 20:108-115. |
|
|
Anh DN, Hung DN, Tien TV, Dinh VN, Son VT, Luong NV, Van NT, Quynh NT, Van Tuan N, Tuan LQ, Bac ND (2021). Prevalence, species distribution and antifungal susceptibility of Candida albicans causing vaginal discharge among symptomatic non-pregnant women of reproductive age at a tertiary care hospital, Vietnam. BMC Infectious Diseases 21:523. |
|
|
Barajas JF, Wehrs M, To M, Cruickshanks L, Urban R, McKee A, Gladden J, Goh EB, Brown ME, Pierotti D, Carothers JM (2019). Isolation and characterization of bacterial cellulase producers for biomass deconstruction: A microbiology laboratory course. Journal of Microbiology and Biology Education 20(2):50. |
|
|
Berman J, Sudbery PE (2002). Candida albicans: a molecular revolution built on lessons from budding yeast. Nature Reviews Genetics 3:918-930. |
|
|
Bitew A, Abebaw Y (2018). Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health 18:94. |
|
|
Boye CS, Dubrous P, Mahou C, Diallo TA, Koumondji LK, Mangou K, Dieng A, Diouf MD, Diop A, Seck A, Diongue K (2022). Vulvovaginal candidiasis at Institute Pasteur of Dakar, Senegal: Prevalence and associated risk factors. Journal of Advances in Microbiology 113-119. |
|
|
Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R (2018) Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infectious Diseases 18:e339-e347. |
|
|
Felix TC, de Brito Röder DVD, Dos Santos Pedroso R (2019). Alternative and complementary therapies for vulvovaginal candidiasis. Folia Microbiologica (Praha) 64:133-141. |
|
|
Ghaddar N, Anastasiadis E, Halimeh R, Ghaddar A, Dhar R, AlFouzan W, Yusef H, El Chaar M (2020). Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infectious Diseases 20(1):1-9. |
|
|
Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S (2016). Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Critical Reviews in Microbiology 42:905-927. |
|
|
Kpongbo EA, Djohan V, Vanga-Bosson HA, Kassi KF, Konatà A, Kiki-Barro CP, Yavo W (2018). Susceptibility of Candida species isolated from recurrent vulvovaginal candidiasis to antifungal agents among women at Institut Pasteur of Cte dIvoire. Journal of Yeast and Fungal Research 9:21-26. |
|
|
Nejat ZA, Farahyar S, Falahati M, Khozani MA, Hosseini AF, Faiazy A, Ekhtiari M, Hashemi-Hafshenjani S (2018). Molecular identification and antifungal susceptibility pattern of non-albicans candida species isolated from Vulvovaginal candidiasis. Iran Biomedical Journal 22:33-41. |
|
|
Nicholls S, MacCallum DM, Kaffarnik FA, Selway L, Peck SC, Brown AJ (2011). Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genetics and Biology 48:297-305. |
|
|
Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P (2012). Epidemiologic features of Vulvovaginal Candidiasis among reproductive-age women in India. Infectious Diseases in Obstetrics and Gynecology 2012:859071. |
|
|
Rezaei-Matehkolaei A, Shafiei S, Zarei-Mahmoudabadi A (2016). Isolation, molecular identification, and antifungal susceptibility profiles of vaginal isolates of Candida species. Iranian Journal of Microbiology 8:410-417. |
|
|
Seck MC, Faye B, Ndiaye M, Sow A, Lô G, Biadiane A (2015). Prevalence of Trichomonas vaginalis and Candida albicans in women at the laboratory of the military hospital of Ouakam, Dakar, Senegal. Médecine d'Afrique Noire 6201(2015):31-37. |
|
|
Sharifynia S, Falahati M, Akhlaghi L, Foroumadi A, Fateh R (2017). Molecular identification and antifungal susceptibility profile of Candida species isolated from patients with vulvovaginitis in Tehran. Journal of Research in Medical Sciences 22:132. |
|
|
Shi Y, Zhu Y, Fan S, Liu X, Liang Y, Shan Y (2020). Molecular identification and antifungal susceptibility profile of yeast from vulvovaginal candidiasis. BMC Infectious Diseases 20:287. |
|
|
Sobel JD (2007). Vulvovaginal candidosis. Lancet 369(9577):1961-1971. |
|
|
Sobel JD (2016). Recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology 214(1):15-21. |
|
|
Sylla K (2018). Vulvovaginal candidiasis in the laboratory of parasitology-mycology at Fann university hospital, Dakar (Senegal). Revue Africaine et Malgache de Recherche Scientifique/Sciences de la Santé 5(2). |
|
|
Waikhom SD, Afeke I, Kwawu GS, Mbroh HK, Osei GY, Louis B, Deku JG, Kasu ES, Mensah P, Agede CY, Dodoo C (2020). Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth 20:266. |
|
|
Yadav K, Prakash S (2016). Prevalence of vulvovaginal candidiasis in pregnancy. Global Journal of Medicine and Medical Sciences 4(1):108-116. |
|
|
Yano J, Sobel JD, Nyirjesy P, Sobel R, Williams VL, Yu Q, Noverr MC, Fidel PL (2019). Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes. BMC Women's Health 19:1-9. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0