ABSTRACT
Field experiments were conducted in the Agricultural Research Institute, Uyole, using five susceptible maize varieties during 2013 and 2014 growing seasons. The varieties were used to determine yield losses due to northern leaf blight disease in Mbeya Region of Tanzania. The trials were laid out in two blocks of E. turcicum inoculated and Mancozeb treatment arranged in randomized complete block design in three replicates. Five fungicide sprays were done at weekly interval, starting from 35 days after planting (DAP) while disease inoculation was done twice at 35 and 45 DAP, using whorl placement technique. Data on disease severity index were collected using visual scale of 0-5, and grain yield of each treatment was recorded after harvest and drying. Such data were subjected to analysis of variance, correlation coefficient and coefficient of determination (R²). Means were separated using Turkey’s-Kramer simultaneous test at P≤0.05. Results indicated that Mancozeb sprays enhanced maize grain yield by 30 to 46.6% and 1000-grain weight by 19 to 24%. Disease severity index range of 78.7 to 95.7% indicated that Bora, Kilima, Situka-1, Staha and TMV-1 varieties were more susceptible to Northern leaf blight (NLB) disease. Yield losses ranged from 46 to 62.8% in grain yield (tons/ha) and 31.9 to 38.9% (g/plot) in 1000-grain weight. Disease severity index assessed at silking dry stage had a strong relationship to yield in all the varieties, but varied from Kilima (r = - 0.7617, R² = 0.580, P ≤ 0.078) in 2014 to Staha (r = - 0.9901, R² = 0.9803, P ≤ 0.001) in 2013 in grain yield. The minimum relationship between1000-grain weight and severity index of NLB was recorded in Staha (r = - 0.9300, R² = 0.8649, P ≤ 0.007) in 2013. The grain yield was enhanced and crop loss models indicated good fitness with strong and reliable validity in all the varieties, and as such can be used to estimate potential losses of maize caused by NLB disease in Mbeya, Tanzania.
Key words: Zea mays, northern leaf blight, yield loss, Tanzania.
Maize is an important food crop extensively grown in both developed and developing countries of the world (International Maize and Wheat Improvement Centre (CIMMYT) and IITA, 2011). About 100 million hectares are under cultivation in 125 developing countries (FAOSTAT, 2010), with an annual worldwide production of 822 and 817 million tons in 2008 and 2009, respectively (Food and Agriculture Organization (FAO),
2009, 2010). The demand for maize is estimated to double by 2050 (Rosegrant et al., 2008; Yan et al., 2011). Northern leaf blight of maize (Zea mays L.) caused by Exserohilum turcicum (Pass.) Leonard and Suggs (syn. Helminthosporium turcicum (Pass.) is almost ubiquitous in all the countries where maize is grown and is a threat to maize production in many areas of the world (Pandurangegowda et al., 1993; Carlos, 1997; Harlapur et al., 2000; Muiru et al. 2010).
In East Africa, the disease has been reported as an important foliar fungal disease of maize, resulting to substantial yield losses (Nkonya et al., 1998; Muriithi and Mutinda, 2001; Pratt et al., 2003; Ramathani et al., 2011). The disease epidemics commonly occurs in cool humid regions characterized by heavy dew during the growing season (Jordan et al. 1983; Dorothea et al. 1998; Juliana et al., 2005), temperature range of 20-27°C, relative humidity from 90-100% and low luminosity and the presence of large amount of inocula (Ullstrup, 1970; Shurtleff, 1980; Hennessy et al., 1990; Bentolila et al., 1991; Khatri, 1993; Gregory, 2004; Levicet al., 2008).
Northern leaf blight causes premature death of blighted leaves and results in significant yield reductions due to loss of photosynthetic leaf areas to blighting (De Vries and Toenniessen, 2001; Veerabhadraswamy et al., 2014). Severity of 40-70% on susceptible variety and yield loss of 60% have been reported in Tanzania neighbouring countries of Zambia, Uganda, Kenya, South Africa and Ethiopia (Simelane, 2007). High infection of E. turcicum diverted sugar from the stalks for grain filling resulting to crop lodging (Ferguson and Carson, 2004).When the disease is established before silking and spreads to upper leaves during grain filling, severe yield losses can occur (Ullstrup and Miles, 1957; Raymundo and Hooker, 1981). Yield losses of as high as 98% have been reported (Chenulu and Hora, 1962; Kachapur and Hegde, 1988).On average, grain yield losses of maize due to NLB ranged from 15-50% (Perkins and Hooker, 1981; CIMMYT, 1985; Nwanosike et al., 2015a) however varies based on the plant stage when infection occurred, severity of disease and the resistance of the maize genotype (Jha, 1993).
Leaf position in maize and other cereals contributed significantly to yield. Reports have shown that, top, middle and bottom leaves contributed approximately 10:5:1%, respectively to grain yield(Hooker, 1979; Bowen et al., 1991). The first and second leaves above the ear contributed significantly to yield and their mechanical removal reduced yield by 32% (Levy and Leonard, 1990). Based on entire leaf canopy and leaf positions, several models have been reported (Raymundo and Hooker, 1981; Perkins and Pedersen, 1987; Campbell and Madden, 1990; Harlapur et al., 2005) for predicting and estimating NLB disease. However, Perkins and Pedersen (1987) reported that critical point (CP) and area under disease progress curve (AUDPC) models gave relatively good fit of r² = 0.68 and r² = 0.66, respectively.
Seed treatment and application of fungicides (Raid, 1990, 1991; Patilet al., 2000; Wise and Muelle, 2011; Reddy et al., 2013; Veerabhadraswamy et al., 2014; Wathaneeyawech et al., 2015), host plant resistance and tolerant genotypes (Degefu, 2003; Harlapur, 2005; Ramathani et al., 2011), field sanitation and conventional tillage (de Nazareno et al., 1993), sowing date (Ngugi et al., 2000; Fininsa and Yuen, 2001; Rai et al., 2002) and crop rotation (Pataky and Ledencan, 2006; Lipps and Mills, 2011) have been recommended for management of NLB. Despite these control measures, NLB continues to be a major constraint in maize production worldwide. Previous reports have shown variations among genetic background of different maize varieties and cultural practices in different regions and countries. This spatial and temporal variation makes it difficult to develop common strategies to combat northern leaf blight disease.
In Tanzania, among the diseases adversely affecting productivity, ubiquitous incidence of maize leaf blight in the pre-harvest stage was prominent particularly in the highlands of Mbeya and Arusha regions (Nwanosike et al., 2015b). Mbeya region is a major maize producing area in the Southern High lands of Tanzania. The climate varies from tropical to temperate, with altitude ranging from 400 and 3,000 masl. Temperatures are warm in the lowlands and cool in the highlands with cumulative rainfall between 750 to 3,500 mm annually (Bisanda et al., 1998). Such environmental conditions according to Nwanosike et al. (2015b) favoured NLB development and may be responsible for relatively low grain yield of 1.3 to 1.5 tons/ha (Rowhaniet al., 2011). The study therefore aimed at determining yield losses associated with northern leaf blight in five commonly grown varieties of maize and to develop yield loss models for estimating potential losses caused by E. turcicumin maize.
Study area and field management
Field experiments were conducted at Agricultural Research Institute, Uyole in Mbeya Region 2013 and 2014 growing seasons using five commonly grown maize varieties under artificial
inoculation. The maize varieties were Bora, Kilima, Situka-1, Staha, and TMV-1. Plots were established and maintained in the same field for the two growing seasons. Field experiments were laid out in Randomized Complete Block Design (RCBD) in three replications. The trial was conducted in paired blocks with E. turcicuminoculated and Mancozeb fungicide treatments (Harlapur, 2005). Four maize seeds were planted per hole and thinned to stand density of about 68,000 ha-1, in a plot size of 3 m × 5 m, separated by 75 cm, 30cm and one meter inter-row, intra-row and inter plot/replicates, respectively. Thereafter, ten stands each were randomly selected at the two middle rows of each plot and tagged. Such stands were used for disease assessment.
The protected block was sprayed with 0.25% Mancozeb (Dithane M45, 80% WP) at 1.68 kg/ha (Patakyet al., 1998;Harlapur, 2005) using 15-L-knapsack sprayer while the unprotected block was inoculated with pure culture of E.turcicumisolates, mass produced in sorghum seedsand used for maize inoculation using the whorl placement technique (Adipalaet al., 1993; Veerabhadraswamyet al., 2014). Plants in the two middle rows were inoculated by placing about 10 infected sorghum seeds on five whorls of each stand (50 seeds per stand). Inoculation was done twice at 35 and 45 DAPat 1600 h and thereafter spread with water using a 15-L-knapsack sprayer (Harlapur, 2005) to disperse conidia for infection.Five fungicide sprays were done, starting from 35 DAP and thereafter, maintained at intervals of 7 days. To avoid inter-plot dispersal of inoculum and drift of the fungicide, three rows of tall and late maturing local variety of maize presumed to be resistant to northern leaf blight were planted between the protected and inoculated blocks. Blanket application of Dimethioate insecticide was applied twice, 30 and 45 days after planting at commercial recommendation in the two blocks to avoid insect damage. Agronomic recommendations for maize production were observed.
Northern leaf blight assessment
Disease severity were recorded based on percent leaf area infected at the silk dry stage using visual scales of 0-5 as described by CIMMYT (1985) and Durrishahwar et al. (2008) with modification. Disease severity rating was as follows; 0 = leaves free from infection, 1 = a few restricted lesions on the lower leaves (≤ 5 %), 2 = several small and large lesions on many leaves (5.1-10%), 3 = numerous small and large lesions on many leaves (10.1-25%), 4 = many enlarged and coalesced lesions on many leaves above the cob (25.1-50%) and 5 = several coalesced lesions, leaf showing wilting, tearing and blotching typical blight symptoms (> 50%). Severity scores were converted to percent disease index as described by Wheeler(1969).After harvesting, grain yield and 1000-grain weight were calculated from weight of hand threshed maize and converted to tons/ha and g/plot after adjusting to 15.5% moisture content with ‟Mini GAC Moisture tester” by Dickey-John Corporation Auburn, Illinois, USA. Such data were used to determine yield losses.
Data analysis
Data were subjected to combined analysis of variance (ANOVA).Means that showed significant differences were compared using Turkey’s-Kramer simultaneous test for data at P≤ 0.05 (Steel et al., 1997). Data on grain yield and 1000-grain weight were used to evaluate grain yield losses and grain yield enhancement of Mancozeb fungicide over the inoculated plots for each variety in the two growing seasons. E. turcicum inoculated treatments were expressed as a percentage of Mancozeb treated plots as described by Harlapur (2005).
Crop loss assessment model
Crop loss assessment models were developed for each of the five varieties using grain yield (tons/ha) and 1000-grain weight (g/plot) in 2013 and 2014. The grain yield, 1000-grain weight values and the percent disease index (PDI) values were used to study the relationship between northern leaf blight severity index and losses in the maize varieties used (Nwanosikeet al., 2015a). Critical point models for northern leaf blight of maize were developed using simple linear regression functions;
Y = a + bx
Where; Y = the yield loss, ‘a’ = constant, ‘b’ slope and ‘x’ = per cent disease index (PDI). Yield expressed as a percentage of the average yield of Mancozeb treated plots were used as the dependent variable. Selection of the best fitting models were based on correlation coefficient (r), which showed the relationship between dependent and independent variables, 2) coefficient of determination (R2), which indicated the proportion of the total variation explained by the model and 3) F-statistics, which tests the significance of the regression model (p < 0.05) as described by Perkins and Pedersen (1987). Genstat 14th edition (PC/window7, 2013), IBM SPSS statistics 20 and XLSTAT 2015 version software statistical packages were used for analysis of data.
Northern leaf blight(NLB) severity index indicated significant differences (p ≤0.05) in Mancozeb treated and E. turcicum inoculated plots with means of 23 and 88.9%, respectively (Table 1). Severity index of NLB ranged from 78.7% in Situka-1to 95.7% (Bora) in the E. turcicum inoculated plots, with relatively low disease severity index in Mancozeb treated plots (23%).The study revealed high E. turcicum pressureattributed tofavourable climatic factors in Mbeya.
In terms of grain yield, Mancozeb significant increase yield (3.31 tons/ha) over the 1.51 tons/ha recorded in E. turcicum inoculated plots (Table 1). When yield was measured in1000 grain weight,there were no significant differences (P ≤ 0.05) in both Mancozeb treated and E.turcicum inoculated plots.However, Mancozeb sprays indicatedhigh grain weights (340.3 g/plot) compared toE.turcicuminoculated plots (219.8 g/plot). Such results indicated grain yield enhancement of 30% in TMV-1 and 46.6% in Staha. Similarly, yield was also enhanced with the 1000-grain weight and ranged from 19% (Situka-1) to 24% in the variety Bora (Figure 1). Although all the varieties were susceptible to NLB disease under artificial inoculation, Bora and Staha consistently recorded high disease index and high grain yield enhancement when Mancozeb fungicicde was used.
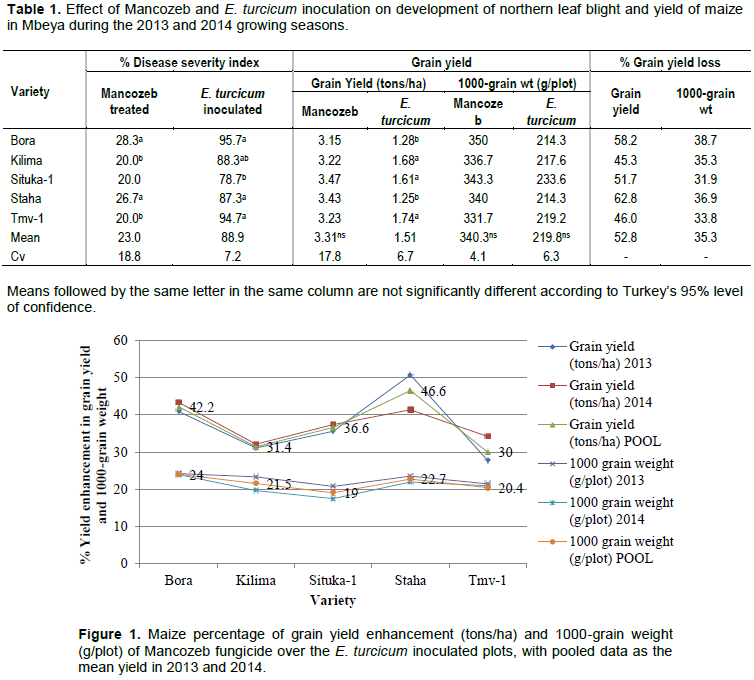
Northern leaf blight disease significantly (P ≤ 0.05) and adversely reduced grain yield of the maize varieties used.Results (Table 1) showed grain yield losses of 46 to 62.8% with an average loss of 52.8 and 31.9% (Situka-1) to 38.7% (Bora)with mean loss of 35.3% when yield was measured 1000-grain weight. The variety Staha recorded 62.8 grain yield loss followed by the variety Bora which gave 58.2%. Kilima recorded the least yield loss of 45.3% in 2013 and 2014 maize growing season in Tanzania. It was similarly found that, the variety Bora showed yield loss of 38.7% followed by Staha (36.9%) while Situka-1 recorded lowest loss of 31.9%, considering the 1000-grain weight. Statistically, there were no significant differences (P ≤ 0.05) in NLB severity index of the varieties Bora, Staha and TMV-1 in the E. turcicum inoculated plots, but there were variationsin yield losses both in grain yield and in 1000-grain weight of the varieties. Such inconsistenciessuggested that grain losses of maize due to NLB do not only depend on the level of susceptibility but also on variety tolerance to the disease.
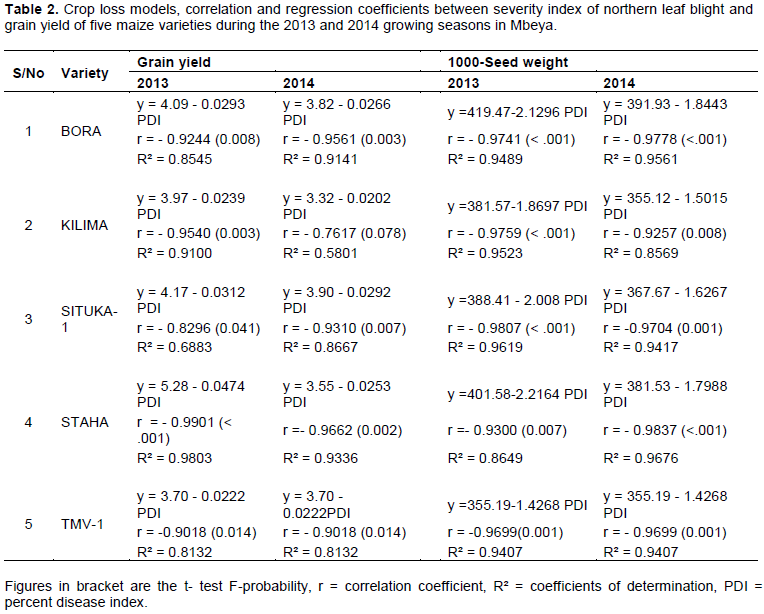
Crop loss assessment model for each of the five varieties using grain yield, 1000 grain weight and the percent NLB disease index (PDI) values were used to determine the relationship between NLB severity index and losses in the maize varieties. The cumulative effect of the disease epidemics indicated high negative significant correlation coefficients (P≤ 0.05) between NLB severity index and grain yield (tons/ha and 1000-grain weight). Correlation coefficients ranged from - 0.83 (Situka-1) to 0.99 (Staha) in 2013 and - 0.76 (Kilima) to - 0.97 (Staha) in 2014in grain yield (tons/ha). In the 1000-grain weight, correlation coefficients range of -0.83 (Situka-1) to -0.99 (Staha) in 2013 and -0.76 (Kilima) to -0.97 in Staha (Table 2) were also observed. The results confirmed that increase in the NLB severity index reduced grain yield in the five varieties of maize used in the study.
Coefficient of determination varied from R² = 0.69 - 0.99 in 2013 to R² = 0.58 - 0.96 in 2014 in grain yield (tons/ha) and ≥ 0.86in 1000-grain weight for the two growing seasons. The study confirmed that 69-99% and 58-96% variation in grain yield were attributed to NLB in 2013 and 2014, respectively and more than 86% in 1000-grain weight. It is therefore, clear that critical point models using percentage leaf area affected by NLB indicated good fit in the five varieties. Therefore, the validity of the relationship between grain yield and severity index at silk dry stage showed strong evidence and varied from variety Kilima (r = - 0.7617, R² = 0.580, P ≤ 0.078) in 2014 to Staha (r = - 0.9901, R² = 0.9803, P ≤ 0.001) in 2013. Similarly, observation was made in terms of yield measured in 1000-grain weight. However, the minimum relationship was very strong in variety Staha (r = - 0.9300, R² = 0.8649, P ≤ 0.007) in 2013 (Table 2). The study confirmed that the predicted grain yield (tons/ha and 1000 grain weigh) loss values in varieties due to NLB indicated good fit.
The linear regression coefficients indicated negative slopes, - 0.02 to -0.04 and - 0.02 to - 0.3 in 2013 and 2014, respectively (Table 2). The varieties Kilima and TMV-1 consistently showed - 0.02 slope coefficients. Bora, Situka-1 and Staha differed and ranged from - 0.03 to - 0.04, particularly in 2013 grain yield. In slope coefficients using 1000-grain weight, variety TMV-1 recorded -1.4 while Staha, Bora, Kilima and Situka-1 ranged from -1.5 to -2.2. Such indicated that grain yield loss in every unit increase in NLB intensity. However, losses varied among the varieties due to different levels of tolerance to NLB.
Northern leaf blight (NLB) disease progressed faster and was very high (88.9%) in the maize inoculated with E. turcicum, than in Mancozeb treated plots. This was evident inthe low severity index (23.0%) in Mancozeb treated plots. The study agreed with those of Kachapur and Hegde (1988), Raid (1990), Pandurangegowda et al.(1993), Harlapur et al. (2007) and Wathaneeyawech et al. (2015) who reported that Mancozeb sprays at 0.25% significantly reduced NLB and increased grain yield of maize. The high disease index was associated with favourable climatic conditions that prevailed during the growing seasons (Raid, 1991; Nwanosike et al., 2015b). Pataky (1992) also reported that under favourable environmental conditions, maize hybrids reacted differently to NLB.
Timely application of Mancozeb also increased grain yield by 30 to 42.2% in grain yield and 20 to 24% in 1000-grain weight over the inoculated plots. However, the consistently high disease index and grain yield enhancementobserved in the varieties Bora and Staha indicated that even among susceptible maize varieties, NLB reacted differently. Raid (1991) earlier reported low NLB disease development in maize due to application of Mancozeb, such management approach should be prompt and not when NLB had reached epiphytotic level before initiation of fungicide control particularly for susceptible hybrids. This is because increase in corn residues serves as a source of primary inoculum (Wise and Mueller, 2011), and the disease severity has a profound effect on yield losses of maize (Veerabhadraswamy et al., 2014).
Previous reports have shown that NLB significantly and adversely reduced grain yield of the maize varieties and hybrids (Pataky, 1992; Solomonovish et al., 1992; Adipala et al., 1993; Harlapur, 2005). Yield losses of 45.3% (Kilima) to 62.8% (Staha) in grain yield and 33.8 to 38.7% in 1000-grain weight suggested differences in the genetic background and levels tolerance among the maize varieties to NLB disease. Earlier, reports have shown that yield losses due to NLB in maize varied among genotypes based on resistance or susceptibility of hybrids (Pataky et al., 1998; Shivankar and Shivankar, 2000; Babuet al., 2004). Nwanosike et al. (2015a) reported grain losses of 23.9-40.4% in grain yield and 11.2-36.1% in 1000-grain weight in Morogoro, Tanzania. Perkins and Pedersen (1987) reported that reduction in 500-grain weights of maize affected yield losses due to loss of active leaf area.
The highly significant (P ≤ 0.05) negative correlations between NLB severity index and grain yield (r = -0.76 to -0.99) and in 1000-grain weight (r = -0.92 to -0.98) indicated that increase in severity index of NLB reduced yield in the five varieties of maize. Harlapur (2005) also reported high negative correlation (-0.97) between two susceptible maize genotypes (CM-202 and Deccan-103). Similar high negative correlation of -0.59 to -0.98 between NLB and five susceptible varieties of maize were also reportedin Morogoro, Tanzania (Nwanosikeet al., 2015a). Pataky et al. (1998) reported that yield measured as weight of ears and number of marketable ears decreased with increase in NLB disease severity index. Highly positive significant correlation (r = 0.76 - 0.94) have been reported between NLB and percentage of unmarketable ears of susceptible maize genotypes (Campbell and Madden, 1990; Raid, 1991; Pataky et al., 1998).
Significant coefficient of determination (R² = 0.58 to 0.98) in grain yield and 1000-grain weight (R² = 0.85 to 0.96) suggested that 58-98% and 85-96% variation in yield could be attributed to NLB severity index at harvest. Hence the study confirmed that the predicted grain yield losses in the varieties Bora, Kilima, Situka-1, Staha and TMV-1 indicated a good fit. This was also evident in the negative slope coefficients (Table 2), which indicated considerable yield loss per every unit increase in NLB disease severity index. Reports have shown good predictions on grain yield reduction of susceptible maize genotypes due to NLB disease, using critical point model. (Perkins and Pedersen, 1987; Bowen and Pedersen, 1988; Adipalaet al., 1993; Patakyet al., 1998; Nwanosikeet al., 2015). Negative slope coefficient observed between NLB severity index and yield of maize have been documented (Chenulu and Hora, 1962; Fisher et al., 1976; Pataky, 1987, 1992; Harlapur, 2005).
The study therefore, reveals that Mancozeb fungicide sprays against NLB disease enhanced grain yield of the maize varieties particular in high susceptible varieties (Bora and Staha). For cultivation of such highly susceptible varieties, fields must be adequately scouted for NLB, and initiation of Mancozeb sprays must be routinely timed prior or as soon the disease is detected in the field to suppress the disease. It was also established that, under artificial inoculation of E.turcicum, the maize varieties Bora, Kilima, Situka-1, Staha and TMV-1 were highly susceptible and resulted to grain yield losses. The crop loss models for individual variety indicated good fitness with strong and reliable validity. Such models can be used to estimate potential losses of maize caused by NLB disease in Mbeya Region. However, being the first report on the relationship between maize grain yield and northern leaf blight disease in Mbeya Region of Tanzania, there is a need for further studies, mostly using leaf position to confirm the developed crop yield loss models for sustainable management of northern leaf blight in such endemic and high maize production region.
The authors have not declared any conflict of interests.
REFERENCES
|
Adipala E, Lipps PE,Madden LV (1993). Use of disease assessment methods in predicting yield loss due to northern leaf blight of maize. African Crop Science Journal 1(2):159-173.
Crossref
|
|
|
|
Babu R, Mani VP, Pandey AK, Pant SK, Rajeshsingh KS.Gupta HS (2004). Maize Research at Vivekan and ParvatiyaKrishiAnus and hanSans than -An Overview. Technical Bulletin, Vivekan and ParvatiyaKrishiAnus and hanSansthan, Almora 21:31.
|
|
|
|
|
Bentolila S, Guitton C, Bouvet N, Sailand A, Nykaza S, FreyssinetG (1991). Identification of an RFLP markertightly linked to the Ht1 gene in maize. Theoretical and Applied Genetics 82:393.
Crossref
|
|
|
|
|
Bisanda S, Mwangi W, Verkuijl H, Moshi AJ, Anandajayasekeram P (1998). Adoption of maize production technologies in the southern highlands of Tanzania. Mexico D.F: International maize and wheat improvement centre (CIMMYT), The United Republic of Tanzania, Southern African center for cooperation in Agricultural Research (SACCAR). ISBN: 970-648-013-7.
|
|
|
|
|
Bowen KL, Everts KL,Leath S (1991).Reduction in winter wheat in North Carolina due to powdery mildew and leaf rust. Phytopathology 81:503-511.
Crossref
|
|
|
|
|
Bowen KL, Pedersen WL (1988).Effects of northern leaf blight and detasselling on yield on yields and yield components of corn inbred. Plant Disease72:952-956.
Crossref
|
|
|
|
|
Campbell CL, Madden LV (1990). Introduction to plant disease epidemiology. John Willey and Sons, NY, 532 pp.
|
|
|
|
|
Carlos DL (1997). Diseases of maize in south-east Asia, relevance and management. Abstract of the Symposium 'Indian Phytopath. Soc. Golden Jubilee. Paper presented in International Conference on Integrated Plant Disease Management for Sustainable Agriculture, New Delhi, p.22.
|
|
|
|
|
Chenulu VV,Hora TS (1962). Studies on losses due to Helminthosporium blight of maize. Indian Phytopathology15:235-237.
|
|
|
|
|
CIMMYT, IITA (2011). Maize - Global Alliance for Improving Food Security and the Livelihoods of the Resourceâ€poor in the Developing World.The proposal submitted by CIMMYT and IITA to the CGIAR Consortium Board, pp.1-184.
|
|
|
|
|
CIMMYT (1985).Managing trails and reporting data for CIMMYT's International Maize Testing Programme. CIMMYT, El Batan, Mexico.
|
|
|
|
|
De Nazareno NRX, Lipps PE, Madden LV (1993). Effects of levels of corn residue on the epidemiology of gray leaf spot of corn in Ohio. Plant Disease77:67-70.
Crossref
|
|
|
|
|
De Vries J, Toenniessen G (2001).Securing the harvest, biotechnology and breeding methods for African crops. Wallingford: CABI publication.
Crossref
|
|
|
|
|
Degefu Y (2003). Cloning and characterisation of xylanase genes from phytopathogenic fungi with a special reference to Helminthosporium turcicum, the cause of northern leaf blight of maize. Academic Dissertation, Faculty of Agriculture and Forestry, University of Helsinki, September 19, 2003, Helsinki, Finland 85p
|
|
|
|
|
Dorothea SB, Hunter HW,Hartwig HG (1998).Molecular marker analysis of European Setosphaeriaturcica population. European Journal of Plant Pathology104:611-617.
Crossref
|
|
|
|
|
Durrishahwar H, Rehman SMA, Shah IAK,Ali F (2008). Recurrent selection for yield and yield associated traits under leaf blight (Helminthosporiummaydis) stress in maize. Sarhad Journal of Agriculture24(4):599-605.
|
|
|
|
|
FergusonLM,Carson ML (2004).Spatial diversity of Setosphaeriaturcica sampled from Eastern United states.Phytopathology 94:892-900.
Crossref
|
|
|
|
|
Fininsa C,Yuen J (2001). Association of maize rust and leaf blightepidemic with different cropping systems in Hararghe highlands,eastern Ethiopia. Crop Protection 20:669-678.
Crossref
|
|
|
|
|
Fisher DE, Hooker AL, Lim SM, Smith DR (1976).Leaf infection andyield loss caused by four Helminthosporium leaf diseases of corn. Phytopathology 66:942-944.
Crossref
|
|
|
|
|
Food and Agricultural Organisation of the United Nations (FAO) (2010). Rome, Italy.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2009). Global agriculture towards 2050.Briefing paper for FAO high-level expert forum on 'How to feed the world 2050.
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAOSTAT) (2010). Statistical databases and dataâ€sets of the Food and Agriculture Organization of the United Nations.
|
|
|
|
|
Gregory S (2004). Northern Corn Leaf Blight on Corn.Plant Disease: Pests and Crop23:4.
|
|
|
|
|
Harlapur SI (2005). Epidemiology and management of turcicum leaf blight of maize caused by Exserohilumturcicum(Pass.) Leonard and Suggs.Ph.D. Thesis, Univ. Agric.Sci.,Dharwad, India.
|
|
|
|
|
Harlapur SI, Wali MC, Anahosur KH, Muralikrishna S (2000). A report on survey and surveillance of maize diseases in northern Karnataka. Karnataka Journal of Agricultural Sciences13:750-751.
|
|
|
|
|
Hennessy GG, De Milliano WAJ, McLaren CG (1990). Influence of primary weather variables on sorghum leaf blight in southern Africa. Phytopathology 80:943-945.
Crossref
|
|
|
|
|
Hooker AL (1979). Estimating disease losses based on the amount of healthy tissues during the plant reproductive period. Genetika 11:181-192.
|
|
|
|
|
Jha MM (1993). Assessment of losses due to maize diseases in widely grown maize cultivars At Dholi.18 the Annual Progress Report on Rabi Maize, AICMIP, Indian Agricultural Research Institute, New Delhi, p. 138.
|
|
|
|
|
Jordan EG, Perkins JM, Schall RA,Pedersen WL (1983).Occurrence of race 2 of Exserohilum turcicumon corn in the central United States. Plant Disease 67:1163-1165.
Crossref
|
|
|
|
|
Juliana BO, Marco OG,Luis EAC (2005).New resistance gene inZea mays- Exserohilum turcicum pathosystem. Genetics and Molecular Biology 28(3):435-439.
Crossref
|
|
|
|
|
Kachapur MR,Hegde RK (1988). Studies on turcicum blight of maize (Zea mays L.) caused by Exserohilumturcicum(Pass) Leonard &Suggs with special reference to crop loss assessment. Plant pathology - News 6:33-35.
|
|
|
|
|
Khatri NK (1993). Influence of temperature and relative humidity on the development of Helminthosporium turcicum on maize in western Georgia. Indian Journal of Mycology and Plant Pathology23:35-37.
|
|
|
|
|
Levic J, Stankovic S,Petrovic T (2008). The determination of Exserohilum turcicum virulence factors in Serbia. Genetika 40(3):271-281.
Crossref
|
|
|
|
|
Levy Y,Leonard KJ (1990).Yield loss in sweet corn in response to deforliation or infection by Exserohilum turcicum. Journal of Phytopathology 128:161-171.
Crossref
|
|
|
|
|
Lipps P,Mills D (2011).Northern corn leaf blight. Ohio state university extension fact sheet, plant pathology, AC-20-02 Coffey Road, Columbus, OH 43210-1087.
|
|
|
|
|
Muiru WM, Koopmann B, Tiedemann AV, Mutitu EW, Kimenju JW (2010).Race typing and evaluation of aggressiveness of E.turcicum isolates of Kenya, German and Austrian origin. World Journal of Agricultural Sciences 6(3):277-284.
|
|
|
|
|
Muriithi LM, Mutinda CJM (2001).Genetic variability of maize genotype for resistance to Exserohilum turcicum in Kenya. 7thEastern and Southern Africa Regional maize Conference, 11-15th February, pp. 106-109.
|
|
|
|
|
Ngugi HK, Julian AM, King SB, PeacockeBJ (2000).Epidemiology of sorghum anthracnose (Colletotrichumsublineolum) and leaf blight (Exserohilumturcicum) in Kenya.Plant Pathology49:129-140.
Crossref
|
|
|
|
|
Nkonya E, Xavery P, Akonaay H, Mwangi W, Anandajayasekeram P, Verkuijl H, Martella D,Moshi A (1998). Adoption of maize production technologies in Northern Tanzania. Mexico D.F: International maize and wheat improvement centre(CIMMYT), I(SACCAR). ISBN: 970-648-003-X.
|
|
|
|
|
Nwanosike MRO, Mabagala RB,Kusolwa PM (2015a). Effect of northern leaf bight (Exserohilumturcicum) severity on yield of maize (Zea mays L.) in Morogoro, Tanzania. International Journal of Science and Research 4(9):466-475.
|
|
|
|
|
Nwanosike MRO, Mabagala RB, Kusolwa PM (2015b). Disease intensity and distribution of Exserohilum turcicumincitant of northern leaf blight of maize in Tanzania. International Journal of Pure & Applied Bioscience 3(5):1-3.
Crossref
|
|
|
|
|
Pandurangegowda KT, Shetty HS, Jayaramegowda G, Sangamlal F (1993). Incidence of turcicum leaf blight of maize in southern Karnataka. Current Research 22:100-101.
|
|
|
|
|
Pataky JK (1992). Relationship between yield of sweet corn and northern leaf blight caused by Exserohilum turcicum. Phytopathology 82:370-375.
Crossref
|
|
|
|
|
Pataky JK (1987). Quantitative relationships between sweet corn yield and common rust, Pucciniasorghi. Phytopathology 77:1066-1071.
Crossref
|
|
|
|
|
Pataky JK,Ledencan T (2006). Resistance conferred by the Ht1 gene in sweet corn infected by mixture of virulent Exserohilum turcicum. Plant Disease 90:771-776.
Crossref
|
|
|
|
|
Pataky JK, Raid RN, du Toit LJ,Schueneman TJ (1998). Disease severity and yield of sweet corn hybrids with resistance to Northern leaf blight. Plant Disease 82:57-63.
Crossref
|
|
|
|
|
Patil VS (2000). Epidemiology and management of leaf blight of wheat caused by Exserohilumhawaiienesis(Bugnicourt) Subram and Jain, Ex. Ellis, M.B. Ph.D. Thesis, University of Agricultural Sciences, Dharwad.
|
|
|
|
|
Perkins JM,Hooker AL (1981).Reactions of eighty-four sources of chlorotic lesion resistance in corn to three biotypes of Helminthosporiumturcicum. Plant Disease 65:502-504.
Crossref
|
|
|
|
|
Perkins JM, Pedersen WL (1987). Disease development and yield losses associated with northern leaf blight on corn. Plant Disease 71:940-943.
Crossref
|
|
|
|
|
Pratt R, Gordon S, Lipps P, Asea G, Bigirwa G, Pixley K (2003). Use of IPM in the control of multiple disease in maize: strategy for selection of host resistance. African Crop Science Journal 2(3):189-198.
Crossref
|
|
|
|
|
Rai B, Kumar S, Kumar B (2002).Effect of environmental parameters on the development of turcicum leaf blight of maize. Annals of Biology 18:153-155.
|
|
|
|
|
Raid RN (1990).Evaluation of fungicides for control of northern corn leaf blight and common rust on sweet corn.APS Fungicide and Nematicde Tests 45:14.
|
|
|
|
|
Raid RN (1991).Fungicide control of foliar sweet corn diseases in the presences of high inoculums levels. Proceedings of Florida Horticultural Society 104:267-270.
|
|
|
|
|
Ramathani I, Biruma M, MartinT, Dixelius C,Okori P (2011). Disease severity, incidence and races of Setosphaeriaturcica on Sorghum in Uganda. European Journal of Plant Pathology 131:383-392.
Crossref
|
|
|
|
|
Raymundo AD, Hooker AL (1981). Measuring the relationship between northern corn leaf blight and yield losses. Plant Disease 65:325-327.
Crossref
|
|
|
|
|
Reddy TR, Reddy PN, Reddy RR (2013).Turcicum leaf blight of maize incited by Exserohilum turcicum: A review. International Journal of Applied Biology and Pharmaceutical Technology 5(1):54-59.
|
|
|
|
|
Rosegrant MW, Msangi S, Ringler C, Sulser TB, Zhu T, Cline SA (2008). International Model for Policy Analysis of Agricultural Commodities and Trade (IMPACT): Model Description. International Food Policy Research Institute: Washington, D. C.
|
|
|
|
|
Rowhani P, Lobell DB, Linderman M,Ramankutty N (2011).Climate variability and crop production in Tanzania. Agricultural and Forest Meteorology151:449-460.
Crossref
|
|
|
|
|
Shivankar SK, Shivankar RS (2000). Losses in grain yield due toturcicum leaf blight disease in maize. Agricultural Science Digest 20:201-202.
|
|
|
|
|
Shurtleff MC (1980). Compedium of corn diseases. American phytopathology society, St, paul, MN.
|
|
|
|
|
Simelane VB (2007). Assessment and genetic characterization of maize (Zea mays L.) germplasm for leaf blight (Helminthosporium turcicum Pass.). MSc Thesis, University of Zambia, Lusaka, 85p.
|
|
|
|
|
Solomonovish S, Levy Y,Pataky JK (1992). Yield losses in sweet corn hybrids in response to defoliation and to infection by Exserohilum turcicum. Phytoparacitica 20(2):133-121.
Crossref
|
|
|
|
|
Steel RGD, Torrie JH, Dickey DA (1997).Principles and procedures of statistics: A biometrical approach. New York: McGraw-Hill.
|
|
|
|
|
Ullstrup AJ (1970). A comparison of monogenic and polygenic resistance to Helminthosporiumturcicum.Phytopathology60:1597-1599.
Crossref
|
|
|
|
|
Ullstrup AJ,Miles SR (1957). The effect of some leaf blights of corn on grain yield. Phytopathology 47:331-336.
|
|
|
|
|
Veerabhadraswamy AL, Pandurangegowda KT,PrasannaKumar MK (2014). Efficacy of strobilurin group fungicides against turcicum leafblight and Polysora rust in maize hybrids.International journal of agriculture and crop sciences7(3):100-106.
|
|
|
|
|
Wathaneeyawech S, Kirdsiri K, Sirithunya P, Smitamana P (2015). Efficacies of Some Fungicides and Antagonists in Controlling Northern Corn Leaf Blight Disease.Journal of Agricultural Technology11(4):925-936.
|
|
|
|
|
Wheeler BEJ (1969). An Introduction to plant diseases. John Wiley and Sons Ltd, London.
|
|
|
|
|
Wise K,Muelle D (2011). Are fungicides no longer just forfungi? An analysis of foliar fungicides use in corn.
Crossref
|
|
|
|
|
Yan JB, Warburton M,Crouch J (2011). Association Mapping for Enhancing Maize (Zea mays L.)Genetic Improvement.Crop Science51:433-449.
Crossref
|
|