Full Length Research Paper
ABSTRACT
Magnetic nanoparticles (MNPs) consisting of magnetite (Fe3O4) are promising as nanodiagnostic tools in plant pathology. Magnetic nanoparticles (MNPs) were produced using hydrothermal protocols. MNPs size diameter and size distribution were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). Fe3O4 nanoparticles are monodispersible and spherical with an average diameter of 82 nm. Dynamic light scattering analysis of the same samples revealed that the synthesized MNPs were highly monodispersed and had a hydrodynamic diameter ranging from 100 to 201. Fungal DNA was extracted using MNPs in comparison with the conventional sodium dodecyl sulfate (SDS) method in the context of quality, quantity and timing process. The quality and yields of the isolated DNA from all Cladosporium strains using magnetic nanoparticles were higher compared to the DNA isolation method via sodium dodecyl sulfate (SDS). PCR using specific primers targeting ITS and Actin genes were amplified 100% of varying sized gene fragments, verifying the high quality of the isolated DNA. Forty seven fungal isolates belonging to the Cladosporium cladosporioides complex were observed and phylogenetically evaluated on the basis of DNA sequences of the internal transcribed spacer regions ITS1 and ITS2, in addition to partial actin and translation elongation factor 1-α gene sequences. Maximum likelihood phylogenetic analyses were performed for the combined data set (ITS + ACT + TEF) using RAxML. The three Cladosporium strains were isolated from tomato in Saudi and Egypt was identified very similar to Cladosporium asperulatum and Cladosporium myrtacearum based on their molecular phylogenetic characteristics. DNA isolation using magnetic nanoparticles will expectedly be used commonly both in plant pathology laboratories and in the nanobiotechnology industry.
Key words: Cladosporium, magnetic nanoparticle, DNA recovery.
INTRODUCTION
MATERIALS AND METHODS
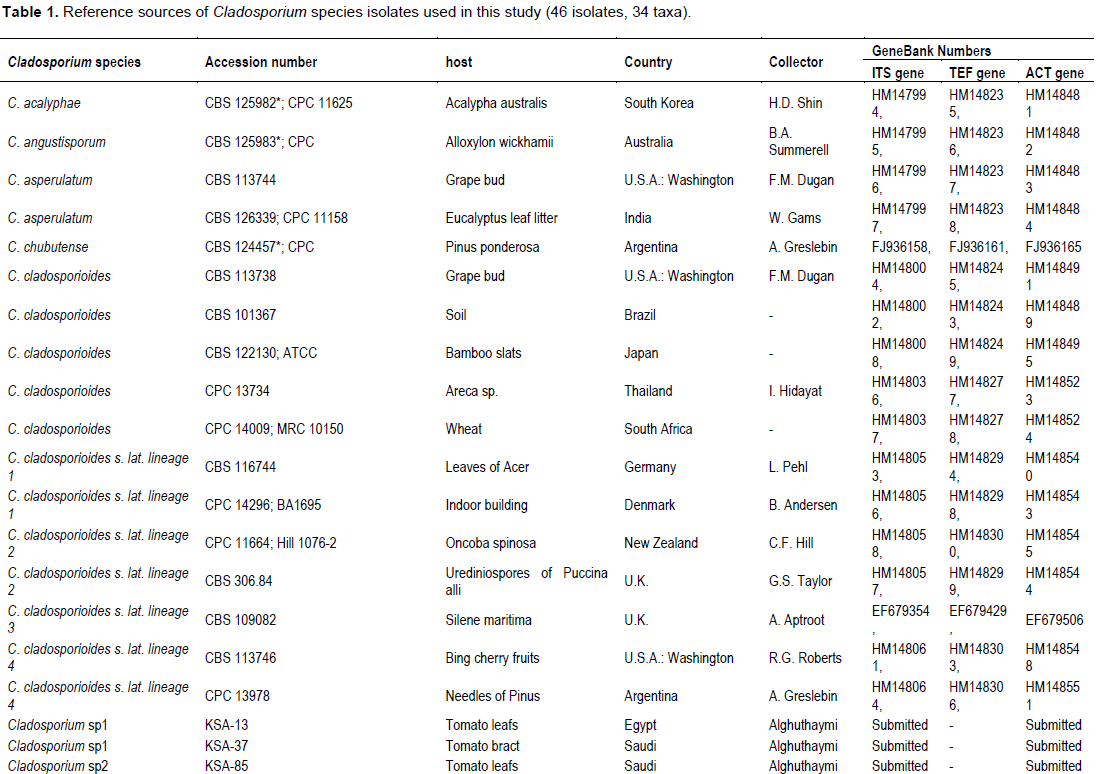
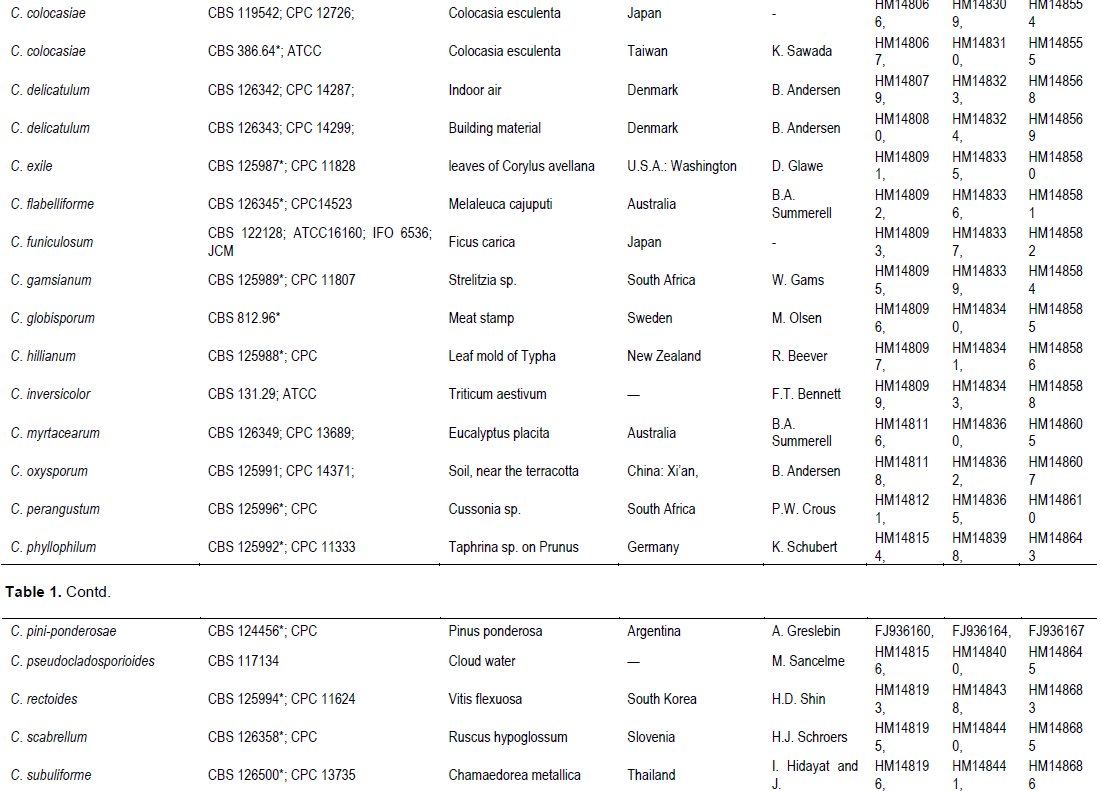
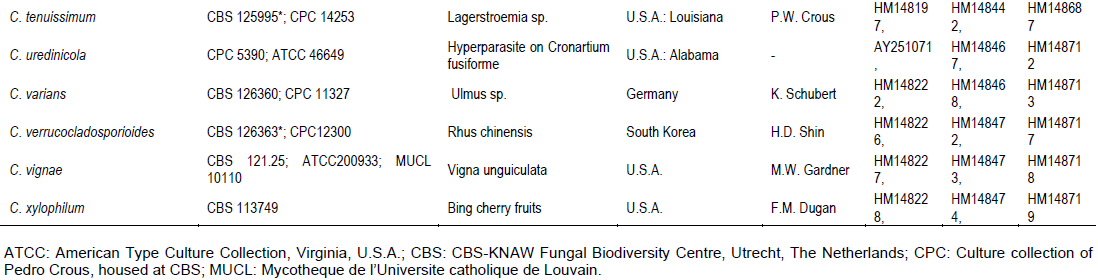
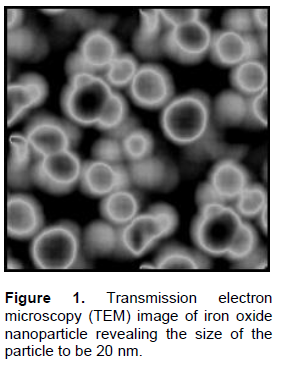
RESULTS
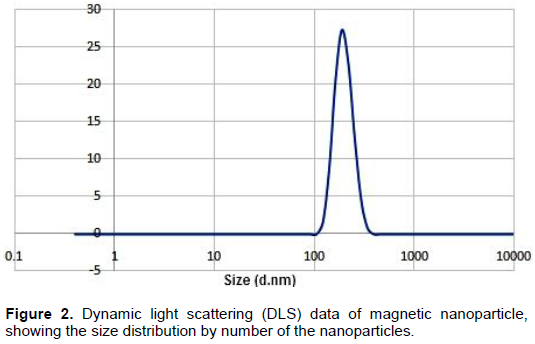
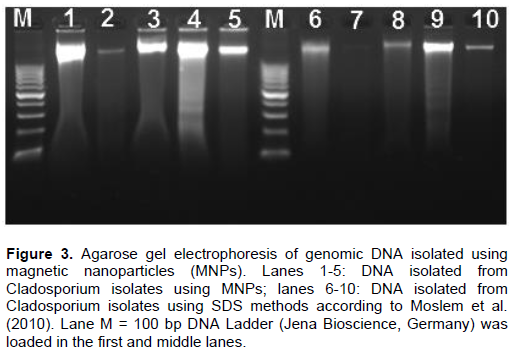
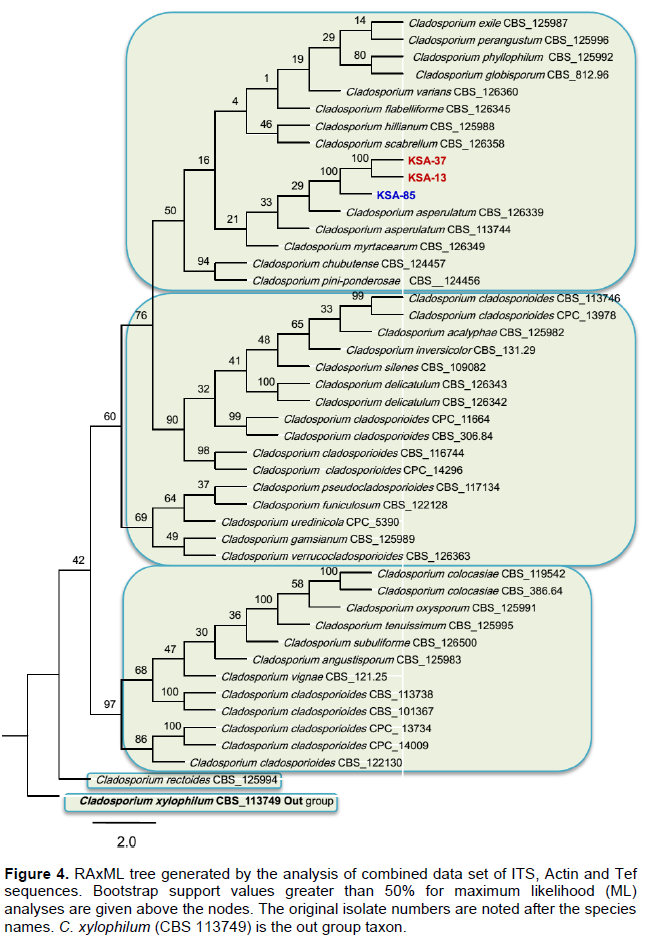
DISCUSSION
CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Agrios GN (2005). Plant Pathology. 5th ed. Academic Press, New York. Pp. 922. |
|
|
Alghuthaymi MA, Ali AA, Hashim AF, Abd-Elsalam KA (2016). A Rapid Method for the Detection of Ralstonia solanacearum by Isolation DNA from Infested Potato Tubers Based on Magnetic Nanotools. Philipp. Agric. Sci. 99:113-118. |
|
|
Bandyopadhyay A, Chatterjee S, Sarkar K (2011). Rapid isolation of genomic DNA from E. coli XL1 Blue s train approaching bare magnetic nanoparticles. Curr. Sci. 101:210-214. |
|
|
Basu S, Chatterjee S, Bandyopadhyay A, Sarkar K (2013). Potential application of superparamagnetic nanoparticles for extraction of bacterial genomic DNA from contaminated food and environmental samples. J. Sci. Food Agric. 93:788-793. |
|
|
Beenish S, Paul PK (2016). Microbial Colonization of Tomato Phylloplane is influenced by Leaf Age. J. Funct. Environ. Bot. 6:8-15. |
|
|
Bensch K, Braun U, Groenewald JZ, Crous PW (2012). The genus Cladosporium. Stud. Mycol. 72:1-401. |
|
|
Bensch K, Groenewald JZ, Braun U, Dijksterhuis J, de Jesús Yá-ez-Morales M, Crous PW (2015). Common but different: The expanding realm of Cladosporium. Stud. Mycol. 82:23-74. |
|
|
Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin H-D, Dugan FM, Schroers HJ, Braun U, Crous PW (2010). Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 67:1-94. |
|
|
Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553-556. |
|
|
Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir. 21:1-170. |
|
|
Crous PW, Shivas RG, Quaedvlieg W, Van der Bank M, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, Braun U (2014). Fungal Planet description sheets: 214–280. Persoonia 32: 184-306. |
|
|
Deng M, Jiang C, Jia L (2013). N-methylimidazolium modified magnetic particles as adsorbents for solid phase extraction of genomic deoxyribonucleic acid from genetically modified soybeans. Anal. Chim. Acta 771:31-36. |
|
|
El-Morsy EM (2000). Fungi isolated from the endorhizosphere of halophytic plants from the Red Sea Coast of Egypt. Fungal Divers. 5:43-54. |
|
|
Frasz SL, Miller JD (2015). Fungi in Ontario maple syrup and some factors that determine the presence of mold damage. Int. J. Food Sci. Technol. 207:66-70. |
|
|
Hall T (2004). Bioedit version 6.0.7. Department of Microbiology, North Carolina State University. |
|
|
Islam M, Hasin F (2000). Studies on phylloplane mycoflra of Amaranthus viridis L. Natl. Acad. Sci. Lett. 23:121-123. |
|
|
Katoh K, Standley DM (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30:772-780. |
|
|
Khiyami MA, Almoammar H, Awad YM, Alghuthaymi MA, Abd-Elsalam KA (2014). Plant pathogen nanodiagnostic techniques: forthcoming changes? Biotechnol. Biotechnol. Equip. 28(5):775-785. |
|
|
Köhl J, Scheer C, Holb IJ, Masny S, Molhoek W (2015). Toward an integrated use of biological control by Cladosporium cladosporioides H39 in apple scab (Venturia inaequalis) management. Plant Dis. 99:535-543. |
|
|
Kulik T, Treder K, Załuski D (2014). Quantification of Alternaria, Cladosporium, Fusarium and Penicillium verrucosum in conventional and organic grains by qPCR. J. Phytopathol. 163:522-528. |
|
|
Levetin E, Dorseys K (2006). Contribution to leaf surface fungi to the air spora. Aerobiologia 22:2-12. |
|
|
Lindow SE, Brandl MT (2003). Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. |
|
|
Maeda Y, Toyoda T, Mogi T, Taguchi T, Tanaami T, Yoshino T, Matsunaga T, Tanak T (2016). DNA recovery from a single bacterial cell using charge-reversible magnetic nanoparticles. Colloids Surf. B Biointerfaces 139:117-122. |
|
|
Moslem MA, Abd-Elsalam KA, Bahkali AH, Pierre JGM de Wit (2010). An efficient method for DNA extraction from Cladosporioid fungi. Genet. Mol. Res. 9(4):2283-2291. |
|
|
Pershina AG, Sazonov AE, Filimonov VD (2014). Magnetic nanoparticles-DNA interactions: Design and applications of nanobiohybrid systems. Russ. Chem. Rev. 83:299-322. |
|
|
Rambaut A (2012). FigTree version 1.4.0. Available at http://tree.bio.ed.ac.uk/software/figtree/ |
|
|
Riesen T, Sieber T (1985). Endophytic fungi in winter wheat (Triticum aestivum L.). Swiss Federal Institute of Technology, Zürich. |
|
|
Saiyed ZM, Bochiwal C, Gorasia H, Telang SD, Ramchand CN (2006). Application of magnetic particles (Fe3O4) for isolation of genomic DNA from mammalian cells. Anal. Biochem. 356:306-308. |
|
|
Sandoval-Denis M, Gené J, Sutton DA, Wiederhold NP, Cano-Lira JF, Guarro J (2016). New species of Cladosporium associated with human and animal infections. Persoonia 36:281-298. |
|
|
Schubert K, Greslebin A, Groenewald JZ, Crous PW (2009). New foliicolous species of Cladosporium from South America. Persoonia 22:111-122. |
|
|
Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink MS, Hill CF, Zalar P, Hoog GS de, Crous PW (2007). Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Stud. Mycol. 58:105-156. |
|
|
Sharon M, Choudhary AK, Kumar RJ (2010). Nanotechnology in agricultural diseases and food safety. J. Phytol. 2(4):83-92. |
|
|
Silvestro D, Michalak I (2012). raxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12:335-337. |
|
|
Stamatakis A (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688-2690. |
|
|
White TJ, Bruns T, Lee SJ, Taylor JW (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In. PCR Protocols: A Guide to Methods and Applications, eds. Innis, M.A., D.H. Gelfand, J.J. Sninsky, and T.J. White. Academic Press, Inc., New York. Pp. 315-322. |
|
|
Zahid M, Kim B, Hussain R, Amin R, Park Sh (2013). DNA nanotechnology: a future perspective. Nanoscale Res. Lett. 8(1):119. |
|
|
Zalar P, De Hoog GS, Schroers KJ (2007). Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Stud. Mycol. 58:157-183. |
|
|
Zhao Y, Hao C, Yong Q, Qu C, Chen W, Peng C, Kuang H, Zhou H, Wang L, Xu C (2012). Systematic comparisons of genetically modified organism DNA separation and purification by various functional magnetic nanoparticles. Int. J. Food Sci. Technol. 47:910-917. |
|
|
Zhou Z, Kadam U, Irudayaraj J (2013). One-stop Genomic DNA Extraction by Salicylic Acid Coated Magnetic Nanoparticles. Anal. Biochem. 442(2):249-252. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0