ABSTRACT
We have successfully synthesized Samarium oxide (Sm2O3) doped borate glasses by conventional rapid melt quench method. The prepared batch was melted at suitable transition temperature and to improve the crystalline phases, glass was kept at 500°C for 7 h. The X-ray diffraction (XRD) pattern identified the amorphous nature of glass and photoluminescence emission properties proved the optical properties of Sm2O3 doped borate glass. Fourier transform infrared spectroscopy (FTIR) and FT-Raman studies suggested that Sm2O3 could modify the properties of glass
Key words: Rare earth element, X-ray diffraction (XRD), photoluminescence, Fourier transform infrared spectroscopy (FTIR) and FT-Raman studies.
In the earlier decades, borate glass with its huge variability in composition, structure and properties, promised a bright future in the field of linear and non-linear optics and related fields (Sasaki et al., 2000). Owing to some unique properties of glass like the transparency at room temperature, high corrosion resistance etc., it becomes a suitable alternative for ordinary concrete (Eugen, 2011). The optical research on rare earth (RE) doped glasses draws great consideration due to their broad applications in optical areas like optical switches for laser and sensors and optical communications (Hajer et al., 2014). The significant concerns in rare earth doped glasses are to identify the dopant effect to the host materials. Glasses include some unique properties such as high hardness and transparency at room temperature, along with enough strength and excellent corrosion resistance (Dag and Annette, 2007). Glassy materials have agreeable advantages, like physical isotropy, the absence of grain boundaries, continuous variable composition and hence they are practically useful for optical applications (Joseph et al., 2001). The structure and luminescent properties of rare earth doped materials have been extensively studied because of their applications in optical communications, sensors, lasers, etc (Sobczyk et al., 2008; Li, et al., 2005; Baykal et al., 2000). Borate oxide is one of the most excellent materials which help to solidify the glass and improve the glass quality with amelioration in transparency, refractive index and rare earth ion solubility and hardness. The borate matrix have well defined gathering of BO3 triangles and BO4 tetrahedra to form stable borate groups such as diborate, triborate and tetraborate (Maheshvaran et al., 2013). The rare earth ions like samarium (Sm) can be used as a dopant in diverse crystal hosts and also glass hosts for intense emissions in the visible region (Sailaja et al., 2013). Especially, reddish orange emission region from Sm-doped materials possesses strong luminescence intensity, large stimulated emission cross section, and high quantum efficiency, which could be suitable for laser applications. Samarium oxide doped glasses exhibit inelastic properties due to their valence instability. Unlike other rare earth elements, samarium contains large number of closely placed energy levels and makes it more difficult to determine the spectral properties than others. These types of glasses can be implemented in high density optical memory applications such as coastguard communication, colour display, solar cells etc (Suresh and Edison, 2014). In the present work, we report the structural properties of the prepared glasses by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and FT-Raman analysis.
Preparation of glass samples
Alkaline earth borate glasses of composition 60H3BO3+XZnO+XLi2CO3+X1 Sr2O3+X2 Y2O3 + 0.5 Sm2O3 (X ranges from 10 to 50, X 1 from 5 to 10, X2 from 0 to 2 in molecular % ) were prepared by melt quenching method. All the chemicals taken in powdered form from Aldrich with 99.995% purity. All appropriate weighed chemicals were finely grinded and mixed together using mortar for 10 min. The ground raw material placed in a platinum crucible was then exported to an electric furnace and heated to 1300°C for 7 h. Under the continuous examination of 4 h, the melted sample was immediately transferred to a muffle furnace regulated at 500°C for annealing to prevent breakage during processing. The sample kept inside furnace for 7 h and it was gradually cooled down to room temperature. To get the smoothened surface, the prepared glass was polished and subjected to various characterization techniques for defining structural and optical properties.
XRD characterization
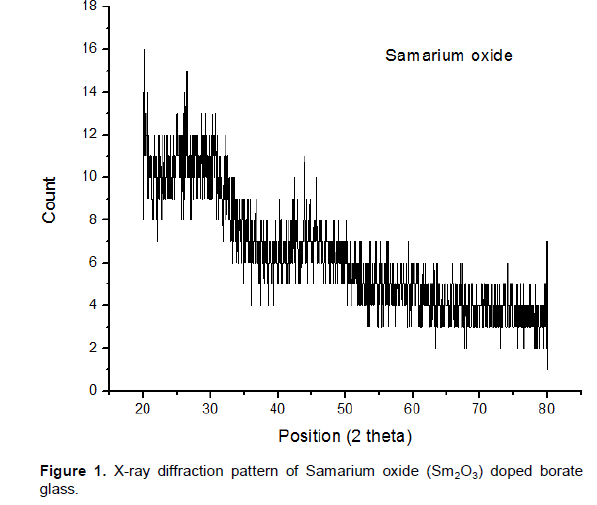
The XRD analysis was used to confirm the amorphous state of the materials. The X-ray diffraction pattern of 0.5 Samarium Oxide doped borate glasses was recorded in the range of 20° and 80°. The results showed a broad diffusion band at lower scattering angles indicating the presence of long range structural disorder which is characteristic of amorphous nature as shown in Figure 1. The resultant graph did not reveal any sharp or discrete peak which indicates the disordered structure of amorphous materials.
Optical properties
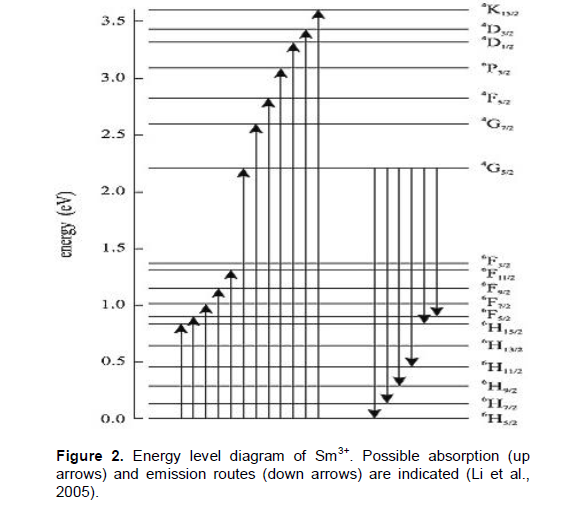
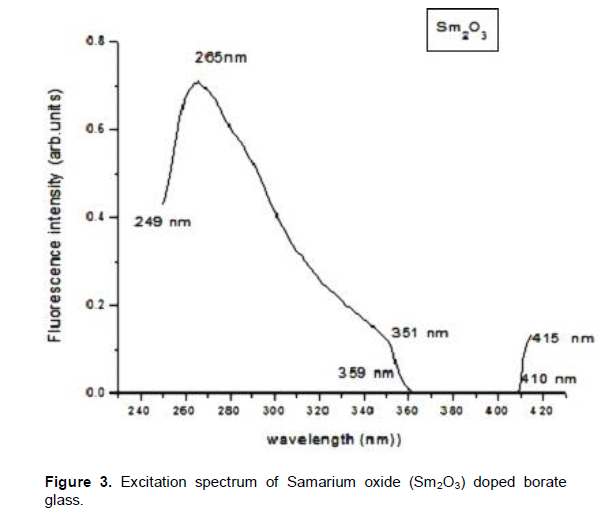
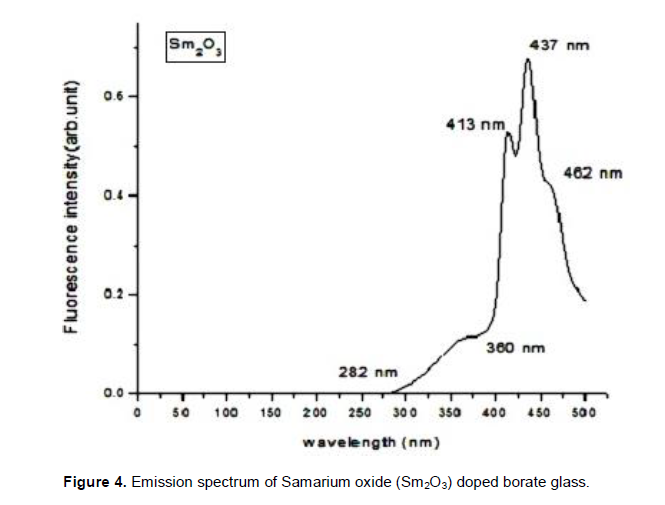
The energy level diagram of samarium oxide is shown in Figure 2 (Binnemans, 1998). Being a member of lanthide series, its oxidation state is +3. Sm3+ ion has the electronic configuration 4f66s2. Since its energy levels are closely packed, it leads to self quenching of fluorescence with a particularly narrow line width of 2.2 nm and absorption from 4G5/2 metastable state. The spectrum of this glass shows strong UV absorption at 265 nm. As in the case of samarium oxide doped borate glass absorption spectrum, absorption bands are observed at 249, 265, 351, 359, 410 and 415 nm. The ions in the ground state 6H5/2 absorb photon of energy and get excited to higher energy levels like 4F5/2, 6F11/2, 6F9/2 , 6H5/2 etc,. The results agree with earlier findings in Sm3+-doped borate glasses (Lin et al., 2005). In addition to these visible emissions, there are further emissions in the near infrared spectral range, that is, the 4G5/2 to 6H15/2 (910 nm) and the 4G5/2 to 6F5/2 (955 nm) transition; the NIR emissions are more than two orders of magnitude less intense. The radioactive lifetime of Sm3+ was recorded at 600 nm (4G5/2 to 6H7/2 transition); the emission was excited at 400 nm (6H5/2 to 6P5/2 absorption). Among all these excitation bands, a band observed at 265 nm was sharper than the others in the spectrum. As per spectroscopic theory, sharp excitation wavelength can give intense emission. Hence, the emission spectrum in the present work was recorded at 260 nm using CW laser source. In this study absorption takes place in energy transition from 6 H5/2 ® 4F5/2 which indicates a wavelength value of 410 nm and is shown in Figure 3. Figure 4 shows the emission spectra of samarium oxide doped borate glass. Five emission bands were observed at peaks 282, 360, 413, 437 and 462 nm. Within the Sm3+ion energy conversion takes place 4G5/2 ® 6H5/2 with the emission of wavelength 462 nm in the green region of ultraviolet spectrum. Also the spectrum exhibits a large number of weak energy transitions.
FT-IR spectral studies
The FTIR spectroscopy offers structural studies to explore the fundamental and functional fractions in crystalline and non-crystalline matrices. The observed broad bands are due to a combination of the higher degeneracy of vibrational states, thermal broadening of the lattice dispersion and mechanical scattering of the powdered samples (Azlan et al., 2013). The FTIR spectrum of the prepared glass samples were recorded in the range of 500 to 4500 cm-1 and are shown in Figure 5. It can be seen that the spectrum consists of several peaks specifying its local structure (Azlan et al., 2013).
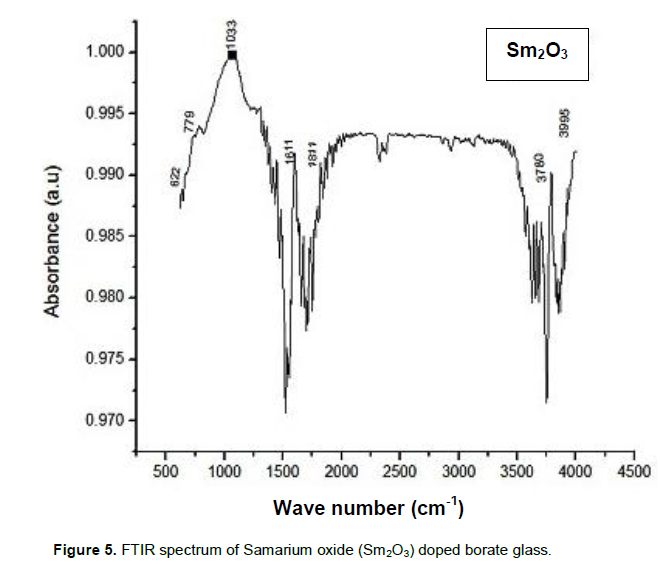
The peak positions and their assignments are presented in Table 1. The transmission spectrums of the glass structure consist of three extensive absorption bands; 660 to 671 cm-1, 1017 to 1239 cm-1, and 1342 to 1346 cm-1. The spectrum has enormous number of weak absorption peaks which indicates weak O-H vibrations and sharp peaks for strong O-H vibrations also. Figure 5 shows likely wide intense band in the region 1150 to 1650 with strong absorption. An intense wide band is observed around 1033 cm-1 due to Sm3+ ions doping in the prepared sample. The presence of samarium oxide in the prepared glass improves the optical properties.
Raman spectrum analysis
The Raman spectrum of the prepared glass exhibited broad peaks in the range studied 100 to 2200 cm-1 as shown in Figure 6. In this spectrum, the broadening of peaks arises due to disorders in the glass matrix. In the present spectrum, the peak at 1249 cm-1 is high intense, due to symmetric stretching mode and while others are less intense compared to this. The less intensity peaks happens due to asymmetric stretching vibration. Hence it can concluded that Samarium oxide contributes broad peaks around 1250 cm -1 due to sm3+- O-sm 3+ vibrations.
The bands in the range of 200 to 900 cm-1 can be attributed to symmetric stretching anion motion (that is, vibration of bridging oxygen). The peak at 950 cm -1 originates from the symmetric breathing vibration of boroxol rings.
Samarium oxide doped borate glasses were prepared by the conventional melt quenching method. The XRD spectrum revealed that the prepared glasses were amorphous in nature. The optical properties of Photoluminescence properties of Samarium oxide doped borate glass were analyzed by using the photoluminescence spectrum. The quantitative analysis of the results, done with the support of FTIR and Raman spectral data, indicated the co-doping of Sm3+ ions mixed glasses. Hence, it was found suitable for wide application in the field of spectral hole burning process, photovoltaic cells and other Laser applications.
The authors have not declared any conflict of interests.
The authors are very grateful to Anna University, Chennai and SREC Coimbatore for providing characterization techniques for various studies. The authors thank BRNS for sanctioning the major research project.
REFERENCES
|
Azlan MN, Halimah MK, Shafinasand SZ, Daud WM (2013). Effect of erbium nanoparticles on optical properties of zinc borotellurite glass system. J. Nanomater. 2013:168.
|
|
|
|
Baykal A, Kiziyalli M, Gozel G, Knep R (2000). Synthesis of Strontium Borophosphate SrBPO5, by solid state and hydrothermal methods and characterization. Cryst. Res.Technol. 35:47-254.
Crossref
|
|
|
|
|
Binnemans K (1998). Spectroscopic properties of trivalent lanthanide ions in fluorophosphate glassesJ Non Cryst. Solids 238:11-29.
|
|
|
|
|
Dag L, Annette M (2007). Advanced Materials and Structures and their Fabrication Processes, Book manuscript. Narvik University College, HiN, pp. 11-15.
|
|
|
|
|
Eugen A (2011). Glasses as engineering materials: A review. Mater. Des. 32:1717-1732.
Crossref
|
|
|
|
|
Hajer SS, Halimah MK, Azmi Z, Azlan MN (2014). Optical properties of Zinc- Borotellurite doped Samarium. Chalcogenide Lett. 11:553-566.
|
|
|
|
|
Joseph CM, Binu PR, Shreekrisnakumar K (2001). Preparation and physical properties of CuPc-substituted borate glass. Mater. Lett. 50:251-253.
Crossref
|
|
|
|
|
Lin H, Yang D, Liu G, Ma T, Zhai B, An Q, Yu J, Wang X, Liu X, Pun E (2005). Optical absorption and photoluminescence in Sm3+- and Eu3+-doped rare-earth borate glasses. J. Luminescence 113:121-128.
Crossref
|
|
|
|
|
Maheshvaran K, Veeran P, Marimuthu K (2013). Structural and optical studies on Eu 3+ doped boro-tellurite glasses. J. Solid State Sci. 17:54-62.
Crossref
|
|
|
|
|
Sailaja S, Raju CN, Reddy CA (2013). Optical properties of Sm 3+-doped cadmium bismuth borate glasses. J. Mol. Struct. 1038:29-34.
Crossref
|
|
|
|
|
Sasaki T, Mori Y, Yoshimura M, Yap YK, Kamimura T (2000). Recent development of nonlinear optical borate crystals: Key materials for generation of visible and UV light. Mat. Sci. Eng. Rep.30:1-54.
Crossref
|
|
|
|
|
Sobczyk M, Straynowitz P, Lisieski R, Ryba-Romanowski W (2008). Effect of crystallization in Sm3+ doped B 2O3-Li 2 O-Nb2O5 glass system. Opt. Mater. 30:1571-1575.
Crossref
|
|
|
|
|
Suresh S, Edison C (2014). Applications of Chalcogenide Glasses: An Overview. Int. J. Chem. Tech. 6(11):4682-4686.
|
|
|
|
|
Yi-Zhong W, Bo-Ping H, Gui-Chuan L, Hong-Shuo L, Xiu-Feng H, Chang-Ping Y (1997). Hard magnetic properties of the novel compound. J. Phy.: Condensed Matter 9:13.
|
|