ABSTRACT
The banana weevil, Cosmopolites sordidus Germar mates repeatedly in its lifetime. No elaborate courtship behavior was seen before mating in the laboratory. However, ‘sniffing’ (an activity by which male weevils used their antennae to contact the abdominal tip of the female, as if to perceive some stimulus), often preceded mating. This observation may be an indication of a chemical pheromone involved in the mating behaviour of this insect. Male banana weevils generally displayed aggressive mating behaviour. Matings occurred under both light and dark conditions, but significantly more in the dark. A direct and positive linear correlation was observed between mating and sniffing under both light and dark conditions. Similar trends or correlations were observed between mating and mating duration; mating and sniffing durations respectively.
Key words: Cosmopolites sordidus, courtship, sniffing, pre-copulatory, copulatory, mating behavior.
The banana weevil, Cosmopolites sordidus Germar, is recognized globally as the major insect pest of banana and plantain (Ostmark, 1974; Reddy et al., 2008; Tinzaara et al., 2011). It attacks all species of the genus Musa, and no cultivar in this group is known to have total resistance to the insect’s larva (or borer) (Wolcott, 1933; Simmonds, 1966; Gold et al., 2001). Certain cultivars, however, are known to be more susceptible to the pest than others (Wolcott, 1933; Feakins, 1971; Viswanath, 1981; Mesquita et al., 1984; Gold et al., 2001). The ‘Lacatan’ variety for instance, is less susceptible, while ‘Maduranga’ is more susceptible (Viswanath, 1981). Plantains and highland bananas are also more susceptible to the weevil than desert or brewing bananas (Gold et al., 2001).
Damage from the pest includes eating up of large portions and growing points of the corm, and setting up of secondary rots from which the plant scarcely recovers (Simmonds and Simmonds, 1953); reduced vitality and drought resistance, poor bunch development and ‘snapping’ (pseudostem breakage around the base of the plant) (Harris, 1947). Gold et al. (2001) reported a reduced nutrient uptake, weakened plant stability and crop failures for newly established farms following larval activities; while established farms experienced reduced bunch weights, mat die-out and shortened stand life.
Damage and yield losses were also increased with time. In spite of the economic importance of this pest, detailed information on its reproductive biology is lacking. Pavis (1988) similarly reported that the details of its sexual behaviour are unknown. Up till date, there has been no detailed published work on the subject. This study was therefore conducted to provide information on the mating and sexual behaviour of this pest.
The mating behaviour of C. sordidus was studied in the laboratory using Petri dishes (9.5 cm diameter) and bigger plastic containers (20 cm diameter × 10 cm high) that contained moist sand, because the weevil is known to prefer moist or humid conditions (Roth and Willis, 1963). The Petri dishes contained a pair of weevils (a male and a female), and the larger containers held groups of 5:5 or 10:10 males and females respectively, to tract the behaviour of individual mating pairs in particular and those that were grouped.
Observations were made both under dark* and lighted conditions of the laboratory (that is, about 1000 lux, 24 ± 2°C and r.h. of ≈ 80%) in a 12:12 L:D photoperiod with scotophase starting at 9 am; during the hours of 09.00 and 14.00 hours daily (that is, 5 h of close observation daily). Prior to start of trials (between 7 and 9 am), the weevils were thoroughly washed and sorted according to sex, using the methods of Longoria (1968) (rostrum punctuations) and Roth and Willis (1963) (curvature of the last abdominal sternite); as adopted by other Researchers too (Budenberg et al., 1993a, b; Gold et al., 2001). Freshly collected males from the field were paired with lab-reared females in all trials, except where otherwise stated; as earlier laboratory observations found them to mate more eagerly and readily.
Light and dark conditions represented treatments, and ten (10) trials or observations were made under each condition (light and dark condition) everyday (each trial lasting 30 min (that is, a total of 10 trials per treatment in each day). Each treatment was replicated four (4) times (that is, repeated over 4 days). Data consisted of qualitative descriptions of the weevil’s pre-copulatory and copulatory behaviours; and quantitative accounts on time spent in mating.
The Dark room had a single light fluorescent source covered with a Red filter (Kodak Wratten No. 70, admitting wavelengths > 640 nm). The dim light permitted observations without any apparent disturbance to the insects. The method was adapted from Budenberg et al. (1993a, b).
Statistical analysis
The observed incidences of mating and sniffing; and the durations of mating and sniffing in the light and dark conditions were subjected to simple T-tests to see if differences were statistically different. The hypothesis was that light and dark had no effect on weevil mating. Differences were deemed to be significant at P < 0.05. Correlation analysis was also done to determine the relationships between these observations (the correlation matrix is presented in Tables 7 and 8).
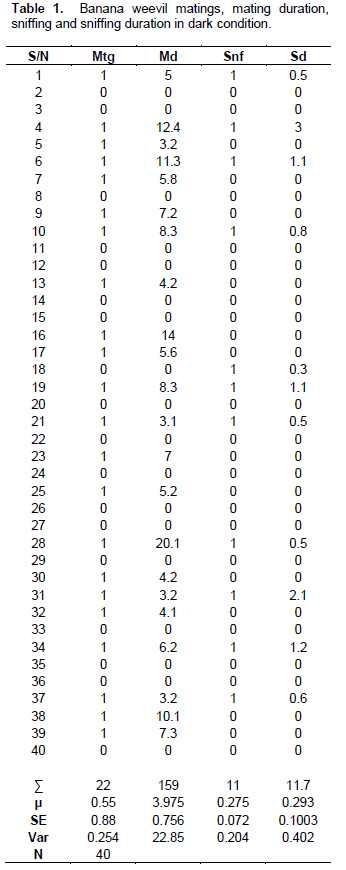
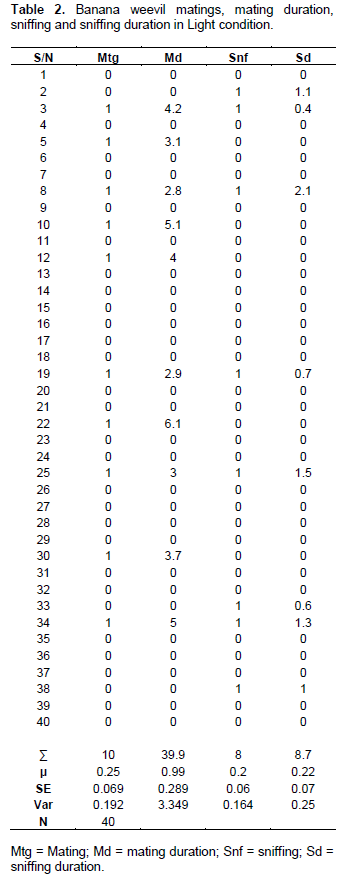
Before mating, males generally displayed no elaborate or detectable courtship behaviour. On coming into contact with each other, the male mounted the female from any angle possible (from the head, rear or sides). If mounting was initiated from the female’s head (Figure 1a), the male usually proceeded to sniff the female’s abdominal end with its antennae (Figure 1c). It periodically raised its antennae up, and soon afterwards resumed the sniffing behaviour, as if perceiving some chemical signal. This activity usually lasted for periods of a few seconds 0.3 to 3 min (µ = 0.3 min ± 0.10 SE, n = 40) in dark conditions (Table 1). In light conditions, 0.6 to 2.1 min (µ = 0.22 min ± 0.07 SE, n = 40) (Table 2). Immediately afterwards, the male usually turned around to adopt a copulatory position, on top of the female and facing in the same direction (Figure 1f). In this position, the male firmly grasped the female and lifted the latter’s abdominal end with its hind legs, curving its abdomen inwardly to insert its aedeagus into the female’s vulva, thus commencing mating.

At other times however, the males first drummed its antennae on the female’s head and pronotal area, and soon after slid backwards to engage genitalia with the female after lifting the females abdominal end with its hind legs. Most males that mounted from the female’s rear usually displayed this behaviour. Sniffing was not common among these males; rather, they usually just stroked the back of the female. Receptive females remained still, and the males immediately slid backwards to commence mating. This antennal stroking on the back of the female, might be the only observable ‘courtship’ behaviour in these insects.
Males that mounted from the side of the female, often quickly maneuvered themselves into appropriate copulatory positions, after first sniffing the abdominal end of the female and stroking the female’s head area, or both; or none of these at other times.
During copulation, the male was observed on top of the female with its legs grasping the female’s sides, and with its head, thorax and the upper half of its abdomen raised from the female’s body; the male’s lower abdomen was tightly engaged with that of the female (Figure 1f) and close observation revealed genital contact. Mating (time spent in copula) usually lasted for a period ranging between 0 to 20.1 min (in dark conditions, µ = 3.98 min ± 0.76 SE, n = 40) (Table 1). In light conditions = 0 to 6.1min (µ = ≈1 min ± 0.29 SE, n = 40) (Table 2). However, it was not unusual to find mating pairs in apparent copulatory positions for periods of >1hr, but without any genital contact. Before and sometimes during mating, certain females tended to walk away or disengage from mounted males. Such males usually responded quickly by stroking the back of the females with their antennae, which often induced them to resume mating. Unreceptive females usually repelled males by running rapidly while mounted, which dislodged them, or by kicking at them with their hind legs.
In the bigger containers with several mating pairs, an aggressive mating behaviour was observed. Most males mounted and attempted to mate with virtually every weevil they contacted (male or female), and disturbed other weevils in copula, by attempting to also mate with them. These intruding males sometimes first sniffed the abdominal end of the female in copula, and then climbed on top of the pair in an attempt to mate. This behaviour often led to an aggregation of weevils around or on top of a mating pair, which resulted in premature disengagements, as the female tried to break free from the crowd of males.
Males that attempted to mate with other males usually faced distinct resistance as the assaulted male often kicked to repel the aggressive male. However, it was not uncommon to find two males mounting each other and adopting copulatory positions. Males mated repeatedly with either the same or other females. Time interval between matings (re-mating time interval) was highly variable and in-fact difficult to track, especially amongst grouped weevils (because of their gregarious and aggressive nature). So amongst the same pairs, it could occur approximately 1 to 2 h after the first successful mating, whilst already mated males could mount another female and attempt to mate with it within approximately 30 min.
Several observations in the laboratory revealed that freshly collected field weevils tended to be more aggressive and eager to mate than those maintained or reared in the laboratory. They mated more readily and also more frequently compared with those from the laboratory colonies. Matings were observed under both light and dark conditions, although significantly more frequent in the dark (T-stat 2.841 > T-tab 1.665; P < 0.005, n = 80 (Table 3).
Mating duration was similarly significantly longer in the dark compared to light conditions (P < 0.001; n = 80) (Table 4). No significant differences were observed in sniffing incidents in the dark or light conditions (T-stat 0.781ns < T-tab 1.665; P > 0.05; n = 80, Table 5). Neither was differences in sniffing duration (comparing light and dark conditions) found to be significant (T-stat 0.588ns < T-tab 1.666; P > 0.05; n = 80, Table 6).
A direct and positive linear correlation was however, observed between mating and sniffing, both under dark and light conditions (r = 0.445**; P < 0.01 1-tailed; n = 40) (r = 0.433**, P < 0.01, 1-tailed, n = 40) (Tables 7 and 8, respectively). Similar trends or correlations followed mating and mating duration; mating and sniffing duration.
Both sexes of the banana weevil, C. sordidus mate repeatedly in their lifetime. Khairmode et al. (2015) also found both sexes of the two known banana weevil pests (that is, C. sordidus and Odoiporus longicollis Oliver, both Coleoptera: Curculionidae) to be polygamous; Martins et al. (2013) also reported re-matings in the rice water weevil, Oryzophagus oryzae. Repeated matings in C. sordidus, involving the same or different partners were a common sight in this study. The significance of this observation is not clear. Cuille (1950) reported that insemination in this insect is needed only once per year, as isolated females were capable of ovipositing continuously for a period of 11 months without further insemination. However, after cessation of oviposition, introduction of males provoked a resumption of egg laying. The fact that these insects are long-lived (4 years, Rukazambuga et al. 1998, Gold et al. 2001) would support the need for females to replenish their sperm reserves. Sperm replenishment after multiple matings is a common phenomenon in some insects such as Drosophila melanogaster (Pyle and Grombo, 1978).
The significance of multiple matings to the banana weevil, could be for increased fecundity. As Hinton (1981) noted, many species, including most Coleoptera, require more than one mating for maximum fecundity, and as a rule, male insects are capable of inseminating females throughout the females’ lifetime. He further stated that species that live for several years are able to inseminate females each season, but that as the male grows older, its capacity declines. Johnson and Hays (1969) observed that females of the plum curculio, Conotracchelus nenuphar, produced more eggs when mated two or three times than when mated only once. Repeated matings in the milkweed beetle, Tetropes tetraopthalmus (Forster) increased female fecundity and fertility (Lawrence, 1990).
The banana weevil, C. sordidus is known to be nocturnal (Cuille, 1950; Reddy et al., 2008; Khairmode et al., 2015); not surprisingly, dark conditions in this study were found to be more favourable for mating incidents and for longer durations too, than the lighted conditions. But contrary to Khairmode et al. (2015) report of matings only at night, the insect was seen mating under both light and dark conditions of the lab. The reason for this behaviour is presently not known but might be associated with its gregarious and polygamous nature..
The sniffing behaviour of male weevils before mating, and the absence of copulatory attempts with freeze-killed weevils by males in the laboratory trials (Uzakah, 1995), showed that olfaction and some form of mechanical stimulus (perhaps movement) seemed to be necessary for mating to occur in these insects. Male weevils apparently are sexually aroused or activated only after some form of movement by the females. Similar observations were made in the tsetse fly, Glossina morsitans orientalis (Dean et al., 1969). These observations were however, contrary to those of Selander (1978) and Tiles et al. (1988) who recorded copulatory attempts with freeze-killed pine weevils, Hylobius abietis (L). The sniffing behaviour of males before mating suggests that females of this insect release a sex pheromone that stimulates the males to mate. Cross and Mitchell (1966) noted that male boll weevils Anthonomus grandis Boheman responded to females at distances not greater than ≈ 5 cm, and they speculated that the females produced a weak secondary pheromone that attracted other males. Ravi and Palaniswami (2002) also reported presence of aggregation and sex pheromone in the closely related banana weevil, Odoiporous longicollis Olivier. The reason for the occasional male-to-male mating attempt in C. sordidus was not clear, but it could be caused by contamination with female emissions or may be simply representative of the aggressive nature of the males. Aggregation of both sexes is a common feature in this insect, following a release of a male produced aggregation pheromone (Budenberg et al., 1993a); but actual mating seems to be precipitated by the release of a female sex pheromone as evidenced from the pre-copulatory sniffing behaviour of the males.
Sex and aggregation pheromones are common amongst weevils (Tinzaara et al., 2002). According to these authors, pheromones and other behaviour modifying chemicals (e.g. kairomones) hold a great potential as tools for pest management; as they could be useful in pest monitoring, and also as control measures through mating disruptions, mass trapping and even as means for aggregating herbivores to delivery sites for biological control agents. They therefore called for further exploits in the synergism between banana plant extracts (kairomones) and the synthetic pheromone in attracting the banana weevil, C. sordidus.
This work was a part of a major study carried out by the Senior Author for his PhD, and was funded by the Royal Norwegian Ministry of Research Cooperation and Development (NORAD). It was conducted at the International Centre of Insect Physiology and Ecology (ICIPE) in Nairobi, using the facilities of the Behavioral and Chemical Ecology Research Unit. The contributions of all my supervisors are highly appreciated.
The authors have not declared any conflict of interest.
REFERENCES
Budenberg WJ, Ndiege IO, Karago FW (1993a). Evidence for male-produced pheromone in banana weevil, Cosmopolites sordidus. J. Chem Ecol. 19:1905-1916.
Crossref |
|
|
Budenberg WJ, Ndiege IO, Karago FW, Hansson BS (1993b). Behavioral and electrophysiological responses of the banana weevil, Cosmopolites sordidus to host plant volatiles. J. Chem. Ecol.19 (2):267-277.
Crossref |
|
|
Cross WH, Mitchell HC (1966). Mating behaviour of the female boll weevil. J. Econ. Entomol. 59 (6):1503-1507.
Crossref |
|
|
|
Cuille J (1950). Recherches sur le charancon du bananier. Inst. Fruits Agrumes Coloniaux, Serie Technique 4, Societe D'editions Techniques Coloniales, Paris. 225P. |
|
|
Dean GJW, Clements SA, Paget J (1969). Observations on sex attraction and mating behaviour of the tsetse fly Glossina morsitans orientalis Vanderplank. Bull. Entomol. Res. 59:355-365.
Crossref |
|
|
|
Feakin S (1971). Pest control in bananas. Manual No.1. PANS Manual No. 1 Completely Revised Edition. Centre for Overseas Pest Research, London. 128P. |
|
|
Gold CS, Pena JE, Karamura EB (2001). Biology and integrated pest management for the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Integrated Pest Management Reviews 6:79-155.
Crossref |
|
|
|
Harris WV (1947). The banana borer. E. Afr. Agric. J. 13:15-18. |
|
|
|
Hinton HE (1981). Biology of Insect Eggs. Vol 1. Pergamon Press, Oxford. 473P. |
|
|
Johnson AW, Hays SB (1969). Laboratory mating behavior of the plum curculio. J. Econ. Entomol. 62(2):438-440.
Crossref |
|
|
|
Khairmode PV, Sathe TV, Dasai AS (2015). Biology, Ecology and Control of Weevils (Coleoptera: Curculionidae) on Banana from Kolhapur region, India. Biolife 3(1):16-20. |
|
|
|
Lawrence WS (1990). Effects of body size and repeated matings on female milkweed beetle (Coleoptera : Cerambycidae) reproductive success. Ann. Entomol. Soc. Am. 83(6):1096-1100
Crossref
Longoria A (1968). Diferencias sexuales en la morfologia externa de C. sordidus Germer (Coleoptera: Curculionidae). Ciencias Biol. La Habana 1:1-11.
|
|
|
Martins C, Saad E, Almeida L, Zarbin P (2013). Cuticular Compounds Recognition and Mating Behavior of the Rice Water Weevil Oryzophagus oryzae (Coleoptera: Curculionidae). J. Insect Behavior 26(6):812.
Crossref |
|
|
|
Mesquita ALM, Alves EJ, Caldas RC (1984).Resistance of bananas cultivars to Cosmopolites sordidus (Germar, 1824). Fruits 39(4):254-257. |
|
|
Ostmark HE (1974). Economic insect pests of bananas. Ann. Rev. Entomol. 19:161-176
Crossref |
|
|
|
Pavis, C. (1988). Quelques aspects comportemenaux le charancon du bananier Cosmopolites sordidus Germar (Coleoptera: Curculionidae). In: INIBAP 1988, Nematodes and the Borer weevil in Bananas: Present Status of Research and Outlook, Proceedings of a workshop held in Bujumbura, Burundi 7 – 11 December, 1987, pp. 58-61. |
|
|
Pyle, DW, Gromko, MH (1978). Repeated mating by female Drosophila melanogaster: The adaptive importance. Experientia 34(4):449-450.
Crossref |
|
|
|
Ravi G, Palaniswami, MS (2002). Evidence for a female-produced sex pheromone in banana pseudostem weevil, Odoiporus longicollis Olivier. Curr. Sci. 83(7):893-898. |
|
|
Reddy GVP, Zerlene TC, Fritz Naz, Rangaswamy M (2008). A Pheromone-based trapping system for monitoring the population of Cosmopolites sordidus (GERMAR) (Coleoptera: Curculionidae). J. Plant Prot. Res. 48(4):515-527.
Crossref |
|
|
Roth LM, Willis ER (1963). The humidity behaviour of Cosmopolites sordidus (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 56:41-52.
Crossref |
|
|
Rukazambuga ND, Gold CS, Gowen SR (1998). Yield loss in East African highland banana (Musa spp. AAA-EA group caused by the banana weevil, Cosmopolites sordidus Germar. Crop Prot. 17:551-589.
Crossref |
|
|
|
Selander J (1978). Evidence of pheromone-mediated behaviour in the large pine weevil, Hylobius abietis (Coleoptera : Curculionidae). Ann. Enomol. fenn. 44:105-112 |
|
|
|
Simmonds NW (1966). Bananas. 2nd edn. Longmans Press, London. 512P. |
|
|
|
Simmonds NW, Simmonds FJ (1953). Experiments on the banana borer Cosmopolites sordidus in Trinidad. BW1. Trop. Agr. 30:216-223 |
|
|
Tiles DA, Eidmann HH, Solbreck, B. (1988). Mating stimulant of the pine weevil, Hylobius abietis (L). J. Chem Ecol. 14(6):1495-1503.
Crossref |
|
|
|
Tinzaara W, Dicke M, van Huis A, Gold CS (2002). Use of infochemicals in pest management with special reference to the banana weevil Cosmopolites sordidus Germar (Coleopteran: Curculionidae); Ins. Sci. Applic. 22:241-261. |
|
|
Tinzaara W, Gold CS, Dicke M, van Huis A, Ragama PE (2011). Effect of age, female mating status and density on the banana weevil response to aggregation pheromone. Afr. Crop Sci. J.19(2):105-116.
Crossref |
|
|
|
Uzakah, R.P. (1995). Reproductive biology, behaviour and pheromones of the banana weevil, Cosmopolites sordidus Germar (Coleoptera : Curculionidae). PhD dissertation, University of Ibadan, Ibadan Nigeria. 177P. |
|
|
|
Viswanath BN (1981). Development of Cosmopolites sordidus (Coleoptera : Curculionidae) on banana varieties in South India. Colemania 1(1):57-58. |
|
|
|
Wolcott GN (1933). An economic entomology of the West Indies. Entomol Soc of Puerto Rico, San Juan, pp. 482-492. |