The cashew tree (Anacardium occidentale L.) is indigenous to Brazil and is an evergreen nut-bearing tropical plant that grows in latitude 15° north and south of the equator. It is a multipurpose tree crop with great economic importance to third world countries including Benin Republic, Brazil,Cote d’Ivore, Guinea Bissau, Ghana, India, Mozambique, Nigeria, Philippines, Sri Lanka, Tanzania and Vietnam. Morphologically, the architecture of cashew tree makes it a foremost tree crop for reclaiming land area to enhanced productivity, through the prevention of desertification and soil erosion. The drought resistant, evergreen cashew tree is economically grown for its nut, apple and wood. Products derived from the nuts include the world’s highly delighted roasted kernel snacks, kernel oil, cashew nut shell liquid, and from the apple: juice, jam and alcohol among others.
Cashew wood is also used for furniture and fishing boats. Of all, cashew nut is the most economic part of the cashew tree providing foreign exchange earnings for producer countries. In Nigeria cashew nuts exports represent 7 to 8% non-oil export earnings. The estimated export value varies from US$ 25 to 35 million annually (Nugawela and Oroch, 2005). Cultivation and processing activities in cashew provides employment and income generation for women and smallholder farmers in Nigeria (Akinwale, 2000; Topper et al., 2001). It supplements the income of about 50,000 farmers and an additional 55,000 people employed down its’ value chain (Nugawela and Oroch, 2005) as harvesters, transporters, processors, marketers, exporters etc. Women are particularly involved in the cashew sub-sector more than in any other cash crop of the nation.
Although cashew was introduced into Nigeria more than 400 years ago, extensive cultivation started only in the early 1950’s. From 1965 to 1990 cashew production was relatively static at 25,000 tonnes with estimated land area of 50,000 ha in 1990. Currently, cashew cultivation has spread to about 27 states of the country and in the past 12 years, production has increased almost thirty-fold from 30,000 MT in 1990 to 836,500 MT in 2012 from estimated land area of 366,000 ha (FAOSTAT, 2013).
The many importance of cashew makes it a topmost tree crop for intensive research. Breeding activities on Cashew in Nigeria started with germplasm introductions, followed by evaluations, selections, and release of identified superior varieties to farmers (Akinwale and Esan, 1989). Other research areas included assessments of morphological and molecular characteristics, ploidy status, reproductive biology, development of improved technology for large-scale production of value added cashew products, formulation of comprehensive farm management practices, soil and mineral requirements assessments and effective strategies for pest and disease control (Aliyu, 2012, 2004; Oduwole et al., 2001; Ibiremo et al., 2012; Asogwa et al., 2008). Attempts have also been made to develop rapid methods of propagation, including budding, grafting, marcotting and tissue culture protocols for mass multiplication (Aliyu, 2000, 2001, 2005). Efforts in these directions have broadened knowledge on cashew as a crop and contributed to enhancing its productivity. Presently however, increase in cashew production in Nigeria is attributed to increase in cultivated area, rather than to increase in yield per hectare. Moreover, plantings materials are usually unimproved open pollinated seed nuts that do not breed true to type. There is therefore the need to step up research to produce cashew varieties with improved yields and quality as well as standardise effective propagation techniques to clone them.
It is noteworthy to remark here that the Nigerian cashew nuts sell at a discount in the world market in the region of 20 to 30% (Nugawela and Oroch, 2005; Oroch, 2005; Topper, 2008). Among the limiting factor for good pricing of Nigerian cashew includes: Low quality, small nut and kernel size, and more importantly poor kernel peelability (that is, the difficulty in the removal of the testa from the kernel) which adds more to the cost of processing. Poor peelability may possibly be resulting from the single or complex effect of poor harvest, poor post harvest handling, abiotic factors or inherent genetic composition of the Nigerian cashew. Understanding the cause(s) of this problem would be a relevant research pursuit.
Biologically, Analeptes trifaciata (cashew stem girdler) and shoot wilt disease have been identified to cause significant yield reduction of cashew in Nigeria. Identification of effective control measure for this pest and disease is a requisite for enhanced cashew productivity in Nigeria.
Considering the significance of cashew in the livelihood and economy of the nation, a concerted research effort on improving nut quality and increasing production per land area is key. While this may change the place of Nigeria productivity status globally, it will also impact on local processing and consumption. The current status of Nigeria cashew production, research efforts, achievements, constraints and areas of possible improvement are hereby discussed.
Cashew (
Anacardium occidentale L.) belongs to the order Sapindales, family Anacardiaceae and genus
Anacardium. The Anacardiaceae family consists of about 75 genera and 700 species (Nakasone and Paull, 1998). Botanically, the Anacardiaceae includes primarily trees and shrubs with resin canals, resinous bark and clear to milky exudates. The trees or shrubs have alternate, often trifoliate or pinnate leaves. Flowers are generally not highly conspicuous and can either be unisexual or bisexual. Only one carpel matures, forming a drupe (a fleshy fruit with a stoney seed). In some cases, the drupy fruits produce an irritant called urushiol. Cashew is related to Mango (
Magnifera indica L), Pistachio (
Pistacia vera L), Poison ivy (
Toxicodendron rydbergii) and Poison oak (
Toxicodendron diversilobum) which are also in the Anacardiaceae family. In the genus
Anacardium, nine species are identified under numerical taxonomy (Mitchell and Mori, 1987). These include
Anacardium corymbosum Barb.Rodr.p, Anacardium excelsum L.,
Anacardium giganteum (Bertero & Balb. ex Kunth) Skeels,
Anacardium humile Hance ex Engl.,
Anacardium microcarpum A.St.-Hil.,,ppp,
Anacardium nanum A.St.-Hil.,
Anacardium negrense Pires & Froes,
Anacardium occidentale L. and
Anacardium spruceanum Benth. ex Engl. Of all, only cashew (
A. occidentale) is of economic
importance because of its edible apple and nutritious kernel.
CASHEW CENTRE OF ORIGIN AND SPREAD
Cashew originated in Latin America, specifically North-eastern Brazil (Ohler, 1979). Portuguese explorers introduced it to the tropics of Asia and Africa from where it spread into other parts of the world. At present, cashew is produced in 32 countries of the world with sufficient warm and humid climate. The main producers however are Brazil, Benin Republic, Cote d’Ivore, Ghana, Guinea Bissau, India, Mozambique, Nigeria, Philippines, Srilanka, Tanzania and Vietnam.
CASHEW IN NIGERIA
History
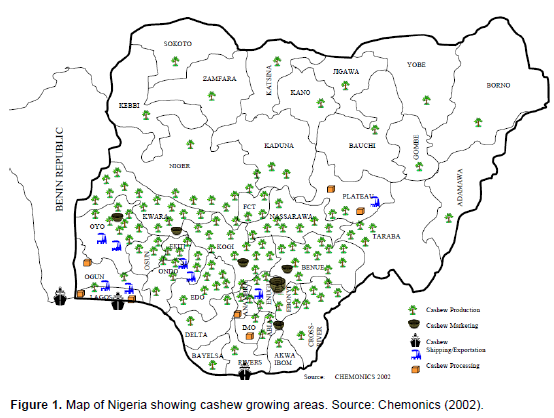
Cashew was introduced into Nigeria by the Portuguese traders around the 16th century (Woodroof 1967; Ohler, 1979). It was first planted in Agege, Lagos State, from it spread to a few other parts of the country through transfer of nuts by man. For over 400 years after introduction, cashew trees were exploited mainly for apple; no commercial value was attached to the nuts (Aliyu, 2012). Many of the trees flourished in the wild while being utilised for aforestation and erosion control scheme particularly in the escarpment areas of Udi in Anambra state. The first commercial cashew planting in Nigeria was in the mid 1950 at Ogbe, Oji, Udi and Mbala by the defunct Eastern Nigeria Development Corporation (ENDC) and Iwo, Eruwa and Upper Ogun by the defunct Western Nigeria Development Corporation (WNDC) (Akinwale and Esan, 1989; Asogwa et al., 2009). These plantations were established with introduced Indian cashew varieties. Progress in the cashew industry then was low due to general neglect and poor management of the plantations. With the involvement of private entrepreneurs, Federal and State Governments, and affluent farmers more nuts were obtained in 1978, 1980 and 1982 from India, Tanzania, Mozambique and Brazil to broaden the cashew genetic base of the country. Today, cashew cultivation has spread to almost all the states of Nigeria with increased processing, shipping and exporting activities. The major Cashew growing areas in the different parts of Nigeria in the order of the level of productivity with respect to the different regions of the country are: Enugu, Abia, Imo, Anambra, Ebonyi and Cross River States in the east and southern part, Oyo, Osun, Ondo, Ekiti and Ogun States in the western part, Kwara, Kogi, Nassarawa, Benue, Taraba, Niger, Federal Capital Territory (Abuja), Kaduna and Plateau in the Middle Belt and Sokoto and Kebbi States in the North-western of the country (Ezeagu, 2002; Chemonic 2002) (Figure 1). It is noteworthy that the majority of export quality nuts come from the Western and Eastern parts of the country.
Research efforts
Germplasm (different genetic composition) collection or assemblage is the first step in crop improvement programme. Desai (2008) reported that the initial germplasm collection and evaluation for cashew breeding programme in India, Brazil, Tanzania, Australia, and Mozambique started in 1970 and onwards. Similarly, research into the breeding, cultivation and use of cashew in Nigeria started in 1972 by the Cocoa Research Institute of Nigeria (CRIN). Many breeding trials were established from locally collected materials, and half-sib accessions from India, Tanzania, Mozambique, and Brazil (Sanwo et al., 1972; Adebola and Esan, 2002). Some of the trials conducted included grading, planting and evaluation of cashew according to nut weight and size. There were experiments to evaluate the performance of the Brazilian Jumbo nut type at different spacing of 9 × 9 m, 8 × 8 m and 6 × 6 m. The trials facilitated better understanding of the crops morphology and agronomy. Hybridisation trials showed both self and cross compatibility in cashew genotypes, but low percentage fruit set was recorded for hand pollinated flowers (Akinwale and Esan, 1989; Aliyu 2007, 2008). With respect to entomology and pathological research, suitable fungicide and insecticide mixtures were developed and successfully used to control the incidence of ravaging inflorescence blight disease of cashew. The screening and breeding of cashew genotypes for resistance to the disease and others including the cashew stem girdler (Analeptes trifaciata) insect pest is a feat to be achieved. Although cashew flourishes in soils where most other crops do not (Ohler, 1979) it does perform better with nutritional assistance. Cashew responded well to fertilizer application, especially during the vegetative growing period (Hammed et al., 2011). The use of organic fertilizer amended with phosphate fertilizer and arbuscular mycorrhizal fungi (AMF) inoculation were found to have positive influence on the growth of cashew and the chemical properties of the soil. Nigerian Sokoto rock phosphate was discovered a viable option to single super phosphate for cashew production (Ibiremo, 2010; Ibiremo et al., 2012). Recent work by Adewale et al. (2013) also revealed that the trend of growth and development of cashew genotypes differed in response to varied combination of soil nutrients.
On-farm evaluation of cashew accessions introduced from India, Tanzania and Mozambique led to the initial selections and subsequent release to farmers of half-sib cashew genotypes called the “G-series” in the 1980s with potential for high yield of 1000 kg nuts/ha (Akinwale and Esan, 1989; Aliyu, 2012). Although, Nigeria was first to release cashew seeds that have been evaluated to a certain degree to farmers compared with other African countries like Guinea, Guinea Bissau, Cote d’lvoire and Ghana (Topper, 2002); there is still much to be done to improve seeds varieties and production.
Cashew nut production
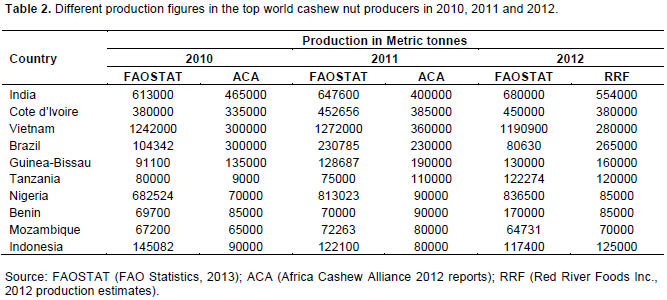
In recent times, there has been a steady increase in Nigeria’s annual cashew nut production from 466,000 MT in the year 2000 to 836,500 MT in year 2012. The production figure of 2012 for Nigeria worth 45% of cashew nuts produced in African (FAOSTAT, 2013; Table 1). Africa contributed over 45% of an estimate of 4,152,315 MT of the global cashew production in 2012. Other major cashew producing countries in Africa are Cote d’lvoire, Tanzania, Mozambique and Guinea Bissau.
There are conflicting records of the position of Nigeria in cashew nut production in Africa and in the World. There are reports that Nigeria has led Africa in cashew nut production in the past decade with about half the African production (Table 1). Nigeria ranked second in the world in 2010, 2011 and 2012 with estimated nut production of 650,000, 813,023 and 835,500 MT respectively (Ogunsina and Lucas, 2008; Aliyu, 2011; FAOSTAT, 2013). African Cashew Alliance (ACA) 2011 report however gave cashew nut production estimates in Nigerian as 70,000 and 90,000MT for 2010 and 2011 respectively. This record placed Nigeria fourth position behind Cote d’lvoire (9,385,000MT), Guinea-Bissau (190,000MT) and Tanzania (110,000MT) and seventh in the world (ACA 2012; Tables 1 and 2). These records of production may be based on unimproved planting materials in aged plantations and wild grooves. A committed research attention leading to release of better performing genetic materials may revolutionize cashew production in Nigeria. Potentials of Nigeria in cashew production seem promising.

The discrepancies in the above reports on cashew nut production in Nigeria could be largely due to poor commercialization and lack of proper documentations (Chemonics, 2002). Estimated production values cited for Nigeria are reported to be often times obtained from import records of buyers and informal sources (Chemonics, 2002). Since there is a lot of unknown border crossing of raw cashew nuts and unregistered small scale local processors; proper accounting is often inhibited. Production statistics are reported to be difficult to accumulate and only the Food and Agricultural Organisation (FAO) make an attempt at estimating and collating country data (Jaeger, 1999). More concerted efforts is required through a national cashew production survey for assessment and documentation of true production estimates in all producing states and regions of the country.
Cashew nut yield
Cashew tree yield of 1.5 to 4 kg of nuts/tree have been reported for Africa (ACA, 2011), and 7 to 11 kg of nut/tree for South Asia (ACA, 2011). In Nigeria, mature cashew tree nut yields of <1 kg to over 20 kg of nuts is obtained (Martin and Kasuga, 1995; Aliyu, 2004). Nut yield in the range of 7.82 to 14.04 kg/tree were also observed in some Nigerian cashew germplasm collections (Aliyu and Awopetu, 2007a). Desai (2008) obtained nut yields of 0.25, 2.41, 8.65, 10.02 and 30.50 kg/tree for some Tanzanian varieties. In India, cashew varieties with tree yield capacity of 10 to 13 kg of nuts/tree have been distributed to farmers (The Hindu, 2000). In Nigeria variability in tree yield is observed not only in different fields but also within particular farms and plantations. The wide margin in nut yield per tree is dependent on the genetic source of the materials (Martins and Kasuga, 1998; Aliyu, 2004; Aliyu, 2007). The contribution of environment, especially soil fertility and plant population may be very significant. Desai (2008) summarily attributed differences in nut yield to agro climatic conditions, age, inherent genetic makeup of the genotype or cultivar and the interaction of both with the environment.
Studies on cashew yield in Nigeria and Tanzania showed that about 30% of the tree population in a hectare produced 80% of the nut yield, while 20% of the yield comes from the remaining 70% of the trees (Martin and Kasuga 1995; Aliyu, 2004). This is a reflection of the productive capacity of most Cashew plantations in Nigeria.
Productivity by cashew genotypes in plantations is hinged on the procedure of planting material selection. Cashew cultivation in Nigeria is almost solely by sexual means. The planting materials are the readily available open pollinated and unselected nuts obtained in farmers’ field or that of his neighbour (Chemonics, 2002). Continuity in the practise of the use of this type of planting materials may strongly impede the trend of both production and improvement of the crop.
Old age of tree is also implicated as another factor that contributes to low and variable yield. Available reports revealed that about 60% of Nigeria cashew plantations host very old cashew trees which have outlived their productive years with age above 30 years (Chemonics, 2002; Oluyinka, 2012). Research focused on generation and distribution of genotypes with high productive capacity to farmers may enhance cashew productivity in Nigeria. Rejuvenation of old plantations and establishment of new ones with improved varieties is a necessary immediate measure to increase the average cashew nut yield in Nigeria. Planting with clonal seedling as opposed to open pollinated seed and seedling is advised. The use of clonal seedlings has advantage of producing uniformly yielding trees that reaches fruit bearing in about two years after establishment as against the long gestated sexual seedlings from open pollinated nuts (IRD, 2011). The incorporation of bee farming within a cashew plantation has also been reported to enhance pollination for increased fruit set (IRD, 2011), adoption of this technology in Nigeria cashew plantation could improve nut productivity.
Mode of cashew propagation
Cashew is propagated mainly from the seed in Nigeria. Since seed nut incorporate a wide range of genetic diversity, the genetic integrity of a particular clone or genotype can only be preserved through vegetative propagation. Several methods of vegetative propagation have been attempted in cashew viz., air layering, inarching, budding, marcotting or grafting which may be epicotyls, soft wood, or flush side grafting. The degree of success of each varied in different countries with attending limitations. Of all the methods, grafting wasreported to be the best for large-scale clonal seedling production of cashew. Tip or bud grafting is used in East Africa, India, Brazil, and Ghana and up to 100% success rate have been obtained with 10-week old seedlings. Some factors identified to affect the success of grafting includes period of the year or season of grafting. For example, period with high maximum temperature and minimum humidity were marked with higher rate of grafting success. Moreover, the type of propagation structure (mist house, green house, open air and under shade) and the length of the scion have equally been identified as success determining factors (Sagar, 2007).
In Nigeria, standardization of vegetative propagation techniques has been one of the important thrust areas in research. Budding and grafting method were carried out with low level of successes. Earlier trials revealed the effect of age of root stock and defoliation or non defoliation of stock and scion on the success of budding and grafting. In an experiment by Aliyu (2001), grafting on 2, 3 and 4 months old root stock gave better result than the ones on 4, 5, 6 and 7 months old rootstock. In addition, budding success was between 10 to 44% while sprouting percentage was 0 to 14%. In the cleft grafting method, success was 4 to 40%, while sprouting was ranged between 4 to 8%. However in both cases, the sprouts did not reach maturity (Aliyu, 2000, 2001). The significant low success turn out seem to inform the need to standardize the vegetative propagation methods in Cashew for higher success rate to meet commercial demands.
Efforts have been made in the application of tissue culture technique for mass propagation of cashew (Aliyu, 2005; Aliyu and Awopetu, 2005). In vitro culture has proved successful for many horticultural fruit species (Ammirato et. al., 1984). The recalcitrant nature of cashew, abnormal development of calli from explants, and browning of explants were some of the limiting factors to the tissue culture success observed in cashew in Nigeria (Aliyu, 2005). Browning of explants has been reduced through frequent transfers, addition of activated charcoal and dark treatments. Furthermore, use of explants from in vitro germinated seedlings, or fungicidal treated young flush in MS (Murashige and Skog) basal salt medium supplemented with cytokinins was found to improve the in vitro success rate of cashew significantly (Aliyu, 2005). However culture to full plant and successful transfer to field has not been achieved.
Cashew varieties
Genetic variability exists in cashew germplasm evaluated in Nigeria. Some of the most important morphological distinguishing characteristics of cashew are: Nut size, form of tree, apple colour (yellow, orange or red), disease resistance, fruit bearing capacity, etc. (Aliyu, 2007). Based on nut size, there are six different size classes capable of meaningful description of cashew characterization (Plate 1). The nut weight significantly correlates with the size, hence the six different sizes of Jumbo (>16 g), extra large (12-15 g), large (8-11 g), medium (6-7 g), small (2-5 g) and madras (≤2 g). Earlier report by Hammed et al. (2008) documented that compensatory nut yield exists among cultivars in thedifferent size classes: cashew trees with heavier nuts (Jumbo) yield less (8-10 kg/tree) while trees with lighter nuts (e.g. medium and madras) of equal maturity age yields more (30-250 kg/tree). The varieties released in 1980 by the Cocoa Research Institute of Nigeria (Akinwale and Esan, 1989) were characterised with minimum-sized nuts and a yield of 1000kg/ha. Further efforts at evaluating and documenting the existing germplasm in the country lead to the identification of three varieties with superior characters (Table 3). The Indian accessions were found to be highly productive (2500 kg/ha) but produced small sized nuts with kernel quality in the range of W320 to 450. It is noteworthy that the benchmark grade for high quality cashew kernel is W320; this refers to a standard where 320 white cashew kernel amount to a pound by weight. From Table 3, the lesser yielding Jumbo varieties however produce highest exportable cashew kernel grade of W180 and higher volume of apple juice (Aliyu, 2004; 2007, 2011). Presently, majority (about 80%) of Nigerian cashew trees produce small to medium nut size, supposedly obtained from the Asian genetic sources, remaining 20% of trees are grown from large sized (Brazilian) nuts (Aliyu, 2011). Cultivation is recommended for cultivars that have a high kernel grade of W180, 210 and 240 which attract higher prices in the world market. Combining higher nut yield with high kernel grade in one genotype is an important breeding focus to meet up with the global quality challenge and good pricing.

Genetic diversity
For any meaningful genetic improvement programs, a vast understanding of the genetic diversity of existing germplasm is essentially important. DNA based markers in genetic analysis of cashew have been attempted in Brazil (Neto et al., 1995), India (Karihaloo and Archak, 2000), and Tanzania (Mneney et al., 2001). Both morphogenetic marker study and RAPD analysis were used to elucidate the genetic relationship of Indian cashew germplasm (Dhanaraj et al., 2002 Samal et al., 2003). Similarly, phenotypic and molecular markers have been used to quantify the extent of variation among the cashew germplasm collections in Nigeria (Aliyu and Awopetu, 2007a). Application of molecular methods of classification using Simple Sequence Repeat (SSR) markers and the application of Poly Acrylamide gel electrophoresis (PAGE) analysis to some Nigeria cashew accessions (Aliyu and Awopetu, 2007b) revealed results which corroborated those obtained from morphogenetic marker. The analysis also revealed genetic redundancy and existence of narrow genetic base within the Nigeria cashew germplasm (Akinwale and Esan, 1989; Aliyu and Awopetu, 2007a, b). Similar diversity studies undertaken in Tanzania and India revealed a narrow genetic base within geographic cashew variety groups (Mneney et al., 2001; Archak et al., 2003). Further exploration of the Brazilian cashew biotype will broaden its genetic base in Nigeria (Hammed et al., 2008).
Breeding for improved genotypes
Good planting material is a very important input in crop production because it determines the upper limits on yield and the ultimate productivity of other inputs. Objectives of crop improvement in cashew generally include development of new high yielding commercial varieties with such characters as desired tree size (dwarf / semi dwarf canopy), bold nut size (>8 g) with higher shelling percentage (>28%) and higher kernel grade (180 to 210W), bigger and juicy apple, resistance / tolerance to biotic stress (pests and diseases) and abiotic stress (Bhaskara Rao, 1998; Bhaskara Rao et al., 1998; Harries et al., 1998). Besides the above (with respect to Nigerian cashew quality) breeding for increased testa peelability is very novel so as to improve Nigerian competitiveness in the cashew global market. Screening of the wide germplasm for these and other traits may lead to the identification of some specific genotypes which may be selected, multiplied and released to farmers. While such procedure may lead to short term variety release, wide crossing of parents in a breeding programme would be necessary for introgression of some economic traits with composite features in hybrid varieties. Currently, Nigeria Cashew germplasm consists mainly of exotic varieties (Adebola and Esan, 2002); there is no record of an improved hybrid cashew as is obtained in some other producing countries.
There are reports of improved hybrids in Australia (Blaikie et al., 2002) and developed clones in Tanzania (Masawe et al., 1998). Diversity within the Tanzanian cashew gene pool was improved through the development of over 100 hybrids (NARI, 2008). High-yielding dwarf genotype was reported (Ashante et al., 2002) to have been developed in Brazil; the variety gave good yields, precocious fruiting and bold nuts. Lack of hybrid production in Nigeria may be due to the low rate of fertilization and lack of progression to maturity obtained in previous hybridization trials (Aliyu, 2008). Poor fertilization may be due to incompatibility (Aliyu and Awopetu, 2005), leading to reduced fruit set. From records, fruit-set in cashew can be as low as between 0.7 and 4.1% (Rao, 1956; Aliyu, 2008). In earlier reports, poor fruit set, excessive premature fruit drop have been reported in the crop (Nawale et al., 1984; Patnaik et al., 1985). Another reason for difficulty of hybrid generation in cashew may be due to differences in ploidy level of parents. Polymorphic chromosome number of 2n = 24, 30, 40, 42 have been observed in some cashew populations (Deckers et al., 2001; cited in Aliyu and Awopetu, 2007b). Cytological examination of some Nigeria cashew populations revealed a diploid and haploid chromosomes of 2n = 42 and n = 21 respectively (Aliyu and Awopetu, 2007b), further screening for ploidy levels and particularly of parent before hybridisation may be useful in promoting proper match of chromosome for higher fruit set. Identification of good parental lines is important in enhancing the effectiveness of hybridization programme. Furthermore, adoption of polyploid breeding in cashew might open opportunity for development of improved varieties and widening of the genetic base of the crop.
Cashew quality
Generally, the mode of production, collection and storage practises affect the quality of cashew nuts. Smallholding farmers may harvest apple to meet urgent cash needs, without minding the maturity status of nuts. This practice contributes to about 40% post-harvest losses of cashew nuts. Immature nuts have high moisture content and are unfit for export. Inadequate drying and improper storage, for example, the use of polythene bags instead of jute bags to store harvested cashew nuts enhances the deterioration of stored kernels. Training farmers on good cashew production practises right from the field to storage might help to alleviate defects in nut quality due to these factors. In addition, government support for smallholder farmers to improve their livelihood would reduce the menace of harvesting immature nuts.
Due to poor peelability of cashew testa from the kernel, about 64% of the total labour for processing 180 metric tonnes of raw nuts by a small-scale processing plant per month is expended on peeling testa alone (Chemonics, 2002). This has brought significant losses to processors and indirectly Nigerian Cashew farmers. While this problem explain for loses and poor pricing of the Nigerian Cashew, it equally answers for its poor acceptability in the global market. The possible cause either genetic or environmental needs to be investigated. Solution through research would be most welcome as this would enhance the acceptability and worthwhile pricing of Nigerian Cashew. It would also encourage small-scale cashew processors who cannot afford high cost peeling machines. Probable research activities to solving this problem may include: Exploration and collection of cashew genetic resources, evaluation for peelability and trait-specific selection for onward breeding programme.
Cashew from Benin Republic has the highest kernel peeling ability in Africa (OLAM, per. Comm.).
Pests and diseases
On the field, production of cashew is mostly impaired by insect pest complexes (Hammed et al., 2008). The Entomological unit of Cocoa Research Institute of Nigeria has archived the collection and identification of insect pests of cashew since 1971. Moreover, information on their symptoms was harnessed and protocols to reduce their menace were formulated (Asogwa et al., 2008). The inexhaustible list of the major economic insect pests of cashew in Nigeria is presented in Table 4.

The insect species have been implicated with economic losses estimated between 52 and 75% of the production level (Ojelade, 1998). Analeptes trifasciata was reported to produce a significant damage to cashew in Nigeria (Topper, 2002; Chemonics, 2002; Asogwa et al., 2011) while low level of Helopeltis incidences is recorded (Topper, 2002); although, they are the main insect pests of cashew in East Africa and India (Boma et al., 1998, Topper et al., 1998; Topper, 2002). A survey in Nigeria showed a wide spread of Analeptes trifaciata infestation in almost all cashew producing states; making it an economic pest (Igboekwe, 1984; 1985; Asogwa et al., 2011). In the past, little or no importance was accorded to the insect (Asogwa et al., 2011), but over time, there seemed to be a progress in the pest spread as cashew continued to expand and increase. The life cycle of the insect starts with the brightly coloured adult male and female “longicorn” beetles (black with 3 orange bands on wings). These feed by scrapping the back of cashew stem causing a V- shaped groove which leads to eventual girdling and falling off of the affected stem/branch and loss of all the fruit such branch may be carrying. Cashew yield loss due to A. trifaciata infestation could be up to 54.8% (ERLS, 1988). The dead wood tissue so formed provides a breeding site for the eggs laid by the adult female after mating. The eggs mature to larvae which burrow the dead wood and develop through the pupal stage into adults.
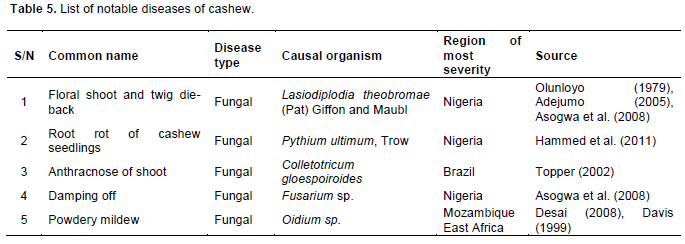
Similarly, cashew production is seriously affected by three major diseases (Table 5). Of the disease pathogens, Lasiodiplodia theobromae is implicated with up to 70% reduction in nut yield, and more than 50% death of vegetative shoot (Hammed et al., 2008).
Among control measures identified for keeping these insect pests and diseases below economic threshold includes good farm sanitation, picking and burning of infected twigs, and chemical spray. Frequent application of insecticides was recommended (Topper, 2002) for the control of Analeptes trifasciata. The body chemical components or the hard cuticle of the insect may however prevent prompt response to chemical treatment. Analeptes trifasciata has been found most responsive to chemical control at the time of the year when the body fat content is low. Removal of alternative hosts (Adansonia digitata and Ficus mucosa) in addition to burning of infected twigs may be necessary to effectively check the spread and damage of A. trifasciata on cashew trees.
Use of botanicals has also been found effective in the control of some pests. Field application of Pipper guineense as a spray at 5 and 10% and combination of garlic (Allium sativum), Pipper guineense, Ocimum gratissimum and Chromonaena odorata at 5, 7.5 and 10% have been found to reduce incidence of inflorescence die-back disease (Lasiodiplodia theobromae) (Adejumo and Otunoye, 2002; Adejumo, 2005). Furthermore, the use of resistant varieties of Cashew seems a promising option for the control measure programmes. Screening of some genotypes of cashew led to the identification of ten genotypes with relative tolerance to cashew inflorescence blight disease in Nigeria (Olunloyo, 1994; Adejumo, 2005).
Organic cashew
Cashew is grown without the use of agro-chemicals in Nigeria, the organic nature of Nigeria cashew is currently a pride (Hammed et al., 2008). The use of chemicals is mostly on experimental fields. Efforts must be made to uphold the status by breeding Cashew for resistance to major pest and diseases. This will in turn prevent chemical pesticides contamination. Promotion of certified organic cashews will improve the export value of cashew products and open new markets to Nigerian exporters. Organic cashew snack products are in strong demand, with sales increasing at over 80% per year in the US market (Chemonics, 2002).
End use production
Research into end use potentials of cashew promoted the development of various innovative products from its apple and nuts. Value addition through processing of agricultural produce has potential to improve it shelve life and increase income of producers (Lawal and Jaiyeola, 2007). Cashew kernels are roasted, fried, spiced, or honey coated and sold in different packages and sizes as snacks. Oil has been mechanically extracted from the cashew kernel which has higher stability at 80 degrees centigrade compared with other commercial oils like palm oil, groundnut oil, corn oil, or cocoa butter. The cashew kernel oil is promising for food and industrial uses. There has been development of improved technique for processing cashew apples into wine, jam, and non-alcoholic beverage of a high nutritional value with vitamin C content of 170-180 mg/100 ml juice.
Local juice extractor/processor that produces cashew apple juice adaptable for use on a cottage industry scale has been fabricated and found economically viable (Akinwale et al., 2001; Oduwole et al., 2001). Developed cashew meal from the kernel including bread, candy, cake, biscuits coated with chocolate is found to have good and acceptable organoleptic properties. Cashew nut shells have been incorporated in fertilizer composition, ruminant feeds and hydraulic paints. In view of the food, industrial and medicinal uses to which cashew tree and its products can be put (Table 6), it appears to be one of the most intensively utilised plant in the world. It offers continual opportunity for investment, as well as great potential for economic development (Olife et al., 2013).
Toward improved cashew nut production in Nigeria; a lesson from Vietnam
At present, Vietnam is the leading producer of cashew nuts in the world. Cashew was introduced to Vietnam in the 18th century, much later than in Nigeria; where the trees gained significance as the most important plant of the countries’ National Poverty Reduction Programme. Cashew was made a greening factor for reproducing bare hills and vacant lands, which lead to rapid expansion of total area of cashew trees. Moreover, high yielding varieties cover an area of 305,791 ha, recording higher yields per hectare compared to all other producer countries (Table 7). Survey of cashew production across Nigeria in 2001 revealed that less than 20% of the available crop able lands are under cultivation in most of the Cashew producing states in Nigeria (Topper et al., 2001). Daramola et al. (2005) also reported that only 34.2 million hectares (about 48%) of the cultivable land area (71.2 million hectares) are actually being cultivated out of the total Nigerian land area of 98.3 million hectares.

This implies that there are prospect for future expansion of Cashew production in Nigeria. Nigeria has the potential to become the World’s number one raw cashew nuts producer if more land is put into the cultivation of improved high yielding and good quality cashew genotypes.