ABSTRACT
Cancer is a major health problem, not only in developed countries, but also in developing countries where the number of cancer-related ailments is growing. Chemotherapy is the most commonly used treatment option but side effects associated with its use necessitates the search for alternatives. Over 80% of the population in developing countries relies on ethnomedicinal plants for primary healthcare including cancer. There are concerns about the safety and efficacy of such ethnomedicines but unfortunately, the prerequisite laboratory set up for such evaluation is usually lacking. An inexpensive, sensitive, field oriented assay would greatly facilitate and improve research into alternative anticancer plant based medicinal therapies. This study proposes to evaluate the suitability of Dugesia dorotocephala as an alternative laboratory method for antiproliferative properties of indigenous plant extracts. Brown planaria, D. dorotocephala maintained under laboratory settings were divided into three groups, each containing a minimum of three planaria. Each planaria was dissected into two using a sterile scalpel. The tail section was transferred into a 24 well plate, after measuring its length in mm. Root and bark extracts of Colophospermum mopane and Schinziophyton rautanenii were prepared at concentrations (5 and 20 µg/ml) and incubated with dissected planaria for 8 days, fresh extracts were replaced every two days and the planaria was observed for its length in addition to the development of eye spots. Planaria regeneration was observed in control wells receiving no treatment, however, a growth promoting effect was exhibited by S. rautanenii root extract in a time and concentration dependant manner at 5 µg/ml. An anti-proliferative effect was observed for S. rautanenii bark extracts and this was observed at both concentrations, with the higher extract of 20 µg/ml exhibiting more growth antiproliferative activity. The extract of C. mopane root had a cytotoxic effect at concentration 20 µg/ml, causing planaria death. The use of Planaria represents an inexpensive, quantifiable, field oriented method to evaluate the effect of indigenous plant extracts in the absence of cell culture. This method is capable of distinguishing between different treatments, extract concentrations as well as time points.
Key words: Dugesia dorotocephala, plant extracts, anti-proliferative, alternative method.
Cancer is a group of related diseases which are characterized by uncontrolled cellular division. Cancer is initiated when a normal cell is transformed into an abnormal cell as a result of injury at the molecular level, resulting in mutated cells. Mutations such as deletions in the colorectal cancer (DCC) gene causes colorectal cancer (Khan et al., 2011) while others such as increased copy numbers of genes such a KIT cause melanomas (Beadling et al., 2008). Cancer is caused by a number of factors such as bacteria (Marie and Lory, 2012), viruses (Bosch et al., 2002), carcinogenic chemicals such as aflatoxins (Wild and Montesano, 2009) while factors such as being overweight, lack of exercise, bad eating habits or excessive alcohol or tobacco consumption can accelerate the risk of cancer development. Cancer continues to be a growing health problem not only in developing countries but also in the developed world causing about 12.7 million cancer incidences and about 7.8 million deaths in 2008 alone (Jemal et al., 2011). In Namibia, (Namibian Cancer Registry, 2011) statistics continues to note an increase in various cancer incidences with a total of 6363 neoplasmas between 2000 to 2005, as compared to a total of 4949 carcinomas between 2006 to 2009 (Namibian Cancer Registry, 2009). Treatment involves radiotherapy, chemotherapy, surgery or a combination of these and the most common treatment being chemotherapy. Chemotherapy, although being the most commonly used treatment for cancer also comes with side effects, including nausea, alopecia, weight-loss, fatigue, vomiting, nausea, hot flushes among others (Dou et al., 2011; Han et al., 2013; Turk et al., 2011). In recent years, efforts have been directed towards a search for alternative less cytotoxic treatments and much of this attention has been directed towards ethnomedicinal plants (Aggarwal et al., 2003; Doughari et al., 2009; Johnson et al., 2001; Russo et al., 2010; Susanti et al., 2012).
Namibia has a wealth of indigenous plants currently being used as ethnomedicines within various traditional settings, (Cheikhyoussef et al., 2011; Chinsembu, 2009; Chinsembu and Hedimbi, 2010; Chinsembu et al., 2011). Ethnomedicinal surveys indicate that indigenous people utilize medicinal plants to treat symptoms similar to cancers. In the traditional setting, there is a need for science based evaluation of medicinal plants effectiveness and safety. These studies require cell culture, small animal model studies to access cytotoxicity and mode of action before progression to clinical trials. However, cell culture opportunities are not always readily available, while funds are required to buy laboratory machinery, chemical reagents and well as ethical clearance for studies involving animal models. There is therefore a need for a study model to access preliminary therapeutic activity of indigenous plants which is quantifiable, suitable for use under field conditions and is inexpensive.
Planaria are flatworms from the phylum of Platyhelminthes. Planaria have been observed to possess regenerative properties (Alvarado, 2012; Cebria, 2007; Iglesias et al., 2008), with different body parts demonstrating differential rates. Planaria is a simple multicellular organism and mutilation of its body using a surgical instrument or the self-inflicted fission process results in two or more separate body parts. The organisms’ ability to regenerate body parts at the site of the incision through proliferation (Reddien and Alvarado, 2004) and to remodel pre-existing tissues and proportion has claimed the interest of scientist over the past years. Planaria offers a good model for the study of antiproliferative or growth promoting effects of plant extracts since it’s a closed system as opposed to cell culture, being able to show the effect and fate of metabolic byproducts produced during extract metabolism. It offers an easier model since assay can be conducted without the need for specialized equipment. In addition, planaria culture and use in assay does not require the ethical clearance as animal models and clinical trials do.
This paper presents an alternative method to determine preliminary therapeutic properties of a plant extract in the absence of the suitable cell lines. This method herewith does not seek to replace the need for cell lines but is merely a field assay to help provide a presumptive answer regarding the potential anti-proliferative properties of plant extracts. Furthermore, this paper presents the potential anticancer properties of indigenous plants derived from the ethnomedicinal practices of various tribes in Namibia.
Preparation of plant extracts
Plant material, root and bark of Schinziophyton rautanenii and Colophospermum mopane were harvested in March 2013; voucher specimen were prepared and deposited with the National Botanical Research Institute for scientific validation. Plant material was air- dried for two weeks before being ground to powder using an industrial blender. Plant material, about 10 g, was macerated in 100 ml methanol for 24 h. This was followed by filtration and rotary evaporation, freeze drying to dryness. C. mopane and S. rautanenii root and bark crude extracts were dissolved in dimethyl sulphoxide and further diluted in water to a concentration of 20 and 5 µg/ml. A 24 well plate was labeled and 1 ml of the appropriate treatment preparation was pipetted into each well. Mineral water, dimethyl sulphoxide were used as negative and positive controls. Data was normalized by deducting change in planaria length resulting from dimethyl sulphoxide.
Experimental animals
Planaria was obtained from Carolina biological laboratories and was maintained under laboratory conditions. A total of thirty-three planaria were used in this study. Planaria were grouped in three groups as follows: Group 1 (Negative control) (3 planaria), Group 2 (Dimethyl sulphoxide) (6 planaria), Group 3 (Plant extracts treatment) (24 planaria). A group was grown in the presence of dimethyl sulphoxide or negative control (mineral water) or plant extract treatment at two concentrations (5 or 20 µg/ml of a plant extract).
Planaria regeneration assay
Prior to experiment, planaria was fed using raw liver and maintained in mineral water. Each planaria was transferred to a petri dish containing water, using a soft brush. Planaria was then disserted using a sterile scalpel, below the sensory lobes. A ruler placed below the petri dish was used to measure the tail section of each planaria in millimeters (mm). Before transferring it to a well using a soft bristle paint brush. The 24 well plate was kept in the dark by wrapping with aluminium foil. Observations on the length of each planaria, and the presence or absence of eye spots were made on every second day. On every second day, new preparations of the appropriate plant extract was replaced to ensure a constant presence of plant extracts. The mean planaria length under each treatment was used to determine the effect of plant extracts on the regenerative ability of planaria and was expressed as mean ± SE. Comparisons were done at 0.05 confidence level.
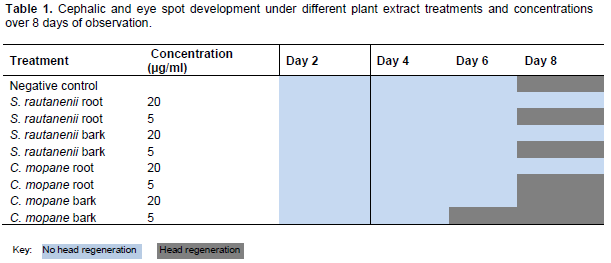
This paper discusses the change in planaria length and regeneration of the planaria’s cephalic region under different conditions as an indication of the antiproliferative properties of indigenous plants. The development of a cephalic region with distinct eye spots was observed under a dissecting microscope. Planaria were noted as either presenting visible eye spots on every second day or not. Table 1 shows that full regeneration occurred towards the end of the experiment, see key below table. C. mopane bark at 5 µg/ml, exhibited early head regeneration as compared to negative control and other plant extracts. All other plant extracts at concentrations of 20 µg/ml, with the exception of C. mopane bark, inhibited planaria head regeneration, as seen in Table 1.
Maintenance of planaria tail sections in different plant extracts of C. mopane and S. rautanenii resulted in the following observations: at plant extract concentration of 20 µg/ml, the root extract of S. rautanenii promoted planaria regeneration. However, planaria length promotion was less in comparison to the negative control (Figure 1A). A concentration effect was observed, at lower plant extract concentrations (5 µg/ml), planaria regeneration was comparable to negative control (Figure 1A). Plant extract S. rautanenii root had a growth promoting effect (Figure 1A). The planaria antiproliferative assay can be used to study plant extracts that have an immune-stimulating effect, (Prasad and Mukthiraj, 2011) or plant extracts that may have cell growth stimulating effects. These properties are important in palliative care as they may be useful as tools to remediate side effects of chemotherapy, example being the promotion of hair follicle regeneration (Kang et al., 2011; Pathan et al., 2012). There was no significant difference in differing extract concentrations on day 2, 4, 6 and 8 (p=0.45, p=0.77, p=0.3 and p=0.28) respectively.
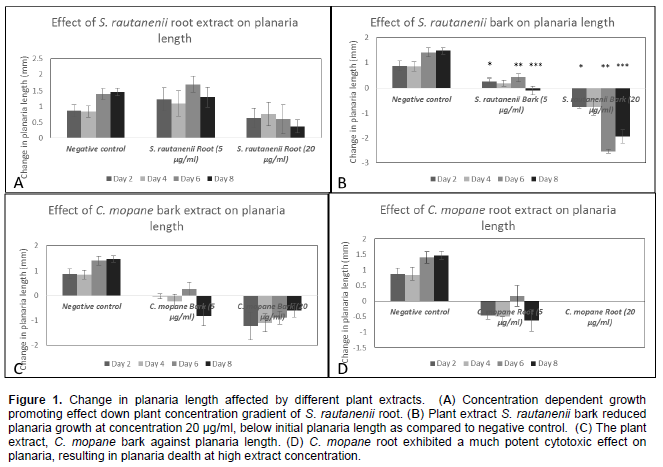
Treatment of planaria with bark extracts of S. rautanenii revealed a growth inhibiting effect at high extract concentration in comparison to negative, while a growth promoting effect at lower extract concentration was comparable to negative control over the four observations made (Figure 1B). As experiment progressed, a day response effect was observed in that the growth inhibiting effect increased over the experimental time, while a growth promoting similar response effect is observed at lower extract concentration.
In addition, a mean change in planaria length of on day 2 (-0.77 ± 0.04 mm) and that observed on day 6 (-2.53 ±0.07 mm) was significantly different indicating that the planaria assay was sensitive to detect changes to planaria caused by plant extract over a period of observation (Figure 1B). Plant extract C. mopane bark exhibited a strong initial effect on the planaria’s regenerative abilities, which is observable at both highand low extract concentrations. However, as experiment progressed, the plant extract become less potent in causing a growth inhibition (Figure 1C). Figure 1C, further displays that C. mopane bark is toxic to planaria and inhibits cellular regeneration. Figure 1D, shows the effect of C. mopane root extract on planaria regeneration at different concentrations. C. mopane root extract exhibited a cytotoxic effect at 20 µg/ml since planaria could not survive beyond 24 h plant extract, despite repeated experiments. At a lower extract concentration, C. mopane root extract exhibited antiproliferation activity with increased anti-proliferative activity as experiment progressed. Therefore, C. mopane root is a antiproliferative extract as compared to C. mopane bark and warrants additional cytotoxic assays involving cellular cancer cultures. Since planaria is a living system, requiring nutrition and energy to build regeneration blastema when spliced (Montgomery and Coward, 1974), contents, whether chemical (ions, compounds, extracts) or biological (bacteria or viruses) in its immediate environment can influence its regeneration. Planaria undergoing regeneration use up preexisting totipotent stem cells which proliferate to form the blastema (Cebria and Vispo, 1997) and some break down to produce the energy needed for survival since planarians undergoing regeneration do not feed (Montgomery and Coward, 1974). However, the contents of the medium into which planarians are kept during regeneration has been shown to either aid or retard regeneration (Inglesias et al., 2008), which implies that regenerating planarians take up nutrients from its environment, perhaps via diffusion, which may have positive or negative repercussions towards ability to regenerate. It is therefore a potential model for the study of antiproliferative or growth promoting activities of plant extracts. With many indigenous plants already in use within different traditional communities in Namibia and elsewhere around the world, a need for evaluation and science based evidence of the efficacy and safety of these plants is necessary. And while phytochemical profiles may direct scientist as to the potential pharmaceutical properties a plant extract may possess, in vitro and in vivo mammal models offer a more reliable method for pharmacological activity analysis. However, these are not readily accessible in most laboratories. In a field setting, during plant specimen collection, the planaria assay may serve as a preliminary screen to eliminate extracts that are inactive. Another source, Spjut (1985) alluded that only 10% of all collected plants in the National Cancer Institute programme dedicated towards screening of plant material for anticancer activity were potent. In a much recent study, Fouche et al. (2008) found very few hits of potently active plant extracts as compared to the total species collected.
Planaria as a biological model for the study of the biological effect of plant extracts offers a number of advantages. Firstly, the experiment is versatile and can differentiate between growth promoting and growth inhibiting effects. In addition, differential effects are easily noticed at different extract concentrations while the animal’s response towards the extract can be monitored easily. This advantages the use of planaria over cell culture since an experiment of this nature using the latter can only be maintained for not more than three days, as opposed to eight or longer with planaria. Planaria maintenance is inexpensive, not requiring additional equipment nor additional reagents or specialized personnel to perform. Planaria have the ability to regenerate from as little 0.08 mm3 of its original size when spliced, offering an inexpensive breeding method for experimental procedures (Montgomery and Coward, 1974). While cell culture may often get contaminated and the integrity of the experiment compromised by mycoplasma or other unrelated cells (Drexler and Uphoff, 2002) and animal models requiring ethical clearance, planaria culture offers a midpoint stand between the two options. But most importantly, planaria is a biological system giving a better representation of the plant’s effect within a living system as opposed to cell culture.
Planaria response to various plant extracts is a quantifiable assay that can be used to determine effect of plants on cellular regeneration to infer preliminary cytotoxic or growth promoting effects of plants. The assay is inexpensive, versatile and offers the best of both cell culture as well as small mammal animal models. Further studies are required to determine the reproducibility of the experiment, in order for it to be used as a preliminary screen for undergraduate research or other instances where cell culture or small mammal studies are not possible.
The authors of this article declare no Conflict interest.
The authors of this study wishes to acknowledge the support of the Multidisciplinary Research Center and the Biological Sciences Department at the University of Namibia. The funds were provided by the University of Namibia Research and Publications Office and the German Academic Exchange Programme (DAAD).
REFERENCES
|
Aggarwal BA, Kumar A, Bharti AC (2003). Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 23:363-398. |
|
|
Alvarado AS (2012). Q&A: What is regeneration, and why look to planarians for answers? BMC Biol. 10:88.
Crossref |
|
|
Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, Town A, Harlow A, Cruz F, Azar S, Rubin BP, Muller S, West R, Heinrich MC, Corless CL (2008). KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 14:6821-6828.
Crossref |
|
|
Bosch FX, Lorincz A, Munoz N, Meijer CJL, Shah KV (2002). The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265.
Crossref |
|
|
Cebria F, Vispo M (1997). Myocyte differentiation and body wall muscle regeneration. Dev. Genes. Evol. 207:306-316.
Crossref |
|
|
Cebria F (2007). Regenerating the central nervous system: How easy for planarians! Dev. Genes. Evol. 217:733-748.
Crossref |
|
|
Cheikhyoussef A, Shapi M, Matengu K, Mu Ashekele H (2011). Ethnomedicinal study of indigenous knowledge on medicinal plant use by traditional healers in Oshikoto region, Namibia. J. Ethnobiol. Ethnomed. 7:10.
Crossref |
|
|
|
Chinsembu KC, Hedimbi M, Mukaru WC (2011). Putative medicinal properties of plants from the Kavango region, Namibia. J. Med. Plants Res. 5(31):6787-6797. |
|
|
Chinsembu KC, Hedimbi M (2010). An ethnobatanical survey of plants used to manage HIV/AIDS opportunistic infections in katima mulilo, Caprivi region, Namibia. J. Ethnobiol. Ethnomed. 6:25.
Crossref |
|
|
Chinsembu KC (2009). Model and experiences of initiating collaboration with traditional healers in validation of ethnomedicines for HIV/AIDS in Namibia. J. Ethnobiol. Ethnomed. 5:30.
Crossref |
|
|
|
Dou D, Tao W, Li L, Jia T, Loo WTY, Cheung MNB, Chow LWC, Dou Y, Luo Z (2011). Immuno-stabilizing effect of thymosin-alpha-1 on post-modified radical mastectomy (MRM) of breast cancer patients. Afr. J. Pharma. Pharmacol. 5(22):2428-2434. |
|
|
|
Doughari JH, Human IS, Bennade S, Ndakidemi PA (2009). Phytochemicals as chemotherapeutic agents and antioxidants: Possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. J. Med. Plants. Res. 3(11):839-848. |
|
|
Drexler HG, Uphoff CC (2002). Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39:75-90.
Crossref |
|
|
Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J (2008). In vitro anticancer screening of South African plants. J. Ethnopharmacol. 119(3):455-461.
Crossref |
|
|
Han L, Wu J-J, Yang L-X (2013). Effect of chemotherapy with cisplatin and rapamycin on HeLa cells in vitro. Afr. J. Pharma. Pharmacol. 7(6):263-268.
Crossref |
|
|
Inglesias M, Gomez-Skarmeta JL, Salo E, Adell T (2008). Silencing of Smed-βcatenin1 generates radial-like hypercephalized planarians. Development 135:1215-1221.
Crossref |
|
|
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011). Global cancer statistics. CA Cancer. J. Clin. 61:69-90.
Crossref |
|
|
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA (2001). Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer. 84(10):1424-1431.
Crossref |
|
|
|
Kang BS, Yoon JS, Kim D-Y, Jeong J-H, Kim E-Y, Nam SY, Yun YW, Kim J-S, Lee BJ (2011). Effects of herbal extracts on hair growth promotion in experimental animal model. J Biomed. Mater. Res. 12(2):113-120. |
|
|
|
Khan NP, Pandith AA, Hussain MUI, Yousuf A, Khan MS, Siddiqi MA, Wani KA, Mudassar S (2011). Loss of heterozygosity (LOH) of deleted in colorectal cancer (DCC) gene and predisposition to colorectal cancer: significant association in colorectal patients in Kashmir. J. Cancer Res. Exp. Oncol. 3(8):88-94. |
|
|
|
Marie MAM, Lory S (2012). Heliobacter pylori and asthma pathogenesis, role of HP-NAP? Afr. J. Microbiol. Res. 6(3):481-485. |
|
|
Montgomery JR, Coward SJ (1974). On the minimal size of a planarian capable of regeneration. Trans. Am. Microsc. Soc. 93(3):386-391.
Crossref |
|
|
|
Namibian Cancer Registry (2009). Cancer in Namibia 2000-2005. Windhoek: Cancer Association of Namibia. |
|
|
|
Namibian cancer registry (2011). Cancer in Namibia 2006-2009. Windhoek: Cancer Association of Namibia. |
|
|
|
Pathan A, Pathan M, Garud N, Garud A (2012). Effects of some novel medicinal plants and polyherbal formulation on stress induced alopecia. Pharmacol. OnLine. 3:150-157. |
|
|
|
Prasad G, Mukthiraj S (2011). Effect of methanolic extract of Andrographic paniculata (Nees) on growth and haematology of Oreochromis mossambicus (Peters). World J. Fish Mar. Sci. 3(6):473-479. |
|
|
Reddien PW, Alvarado AS (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20:725-757.
Crossref |
|
|
Russo M, Spagnuolo C, Tedesco I, Russo GL (2010). Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins 2:517-551.
Crossref |
|
|
Spjut R (1985). Limitations of random screen: Search for new anticancer drugs in higher plants. Econ. Bot. 39(3):266-288.
Crossref |
|
|
|
Susanti S, Iwasaki H, Taira N, Itokazu Y, Kakazu N, Shimabukuro M, Oku H (2012). Studies on the enhancement of cancer-selective cytotoxicity of Kampo medicine by combination. J. Med. Plants. Res. 6(39):5299-5305. |
|
|
|
Turk M, Kaya B, Menemen Y, Ogoztuzun S (2011). Apoptotic and necrotic effects of plant extracts belonging to the genus Alchemilla L. species on HeLa cells in vitro. J. Med. Plants. Res. 5(18): 566-4571. |
|
|
Wild CP, Montesano R (2009). A model of interaction: Aflatoxins and hepatitis viruses in liver cancer etiology and prevention. Cancer Lett. 286:22-28.
Crossref |