ABSTRACT
Corynespora cassiicola which cause the target spot in soybeans can lead to significant reductions in grain yield. Chemical control mechanisms recommended for disease control was performed with low efficacy in the field due to loss of the pathogen sensitivity to fungicides. This study evaluated the effect of fungicides in inhibiting C. cassiicola using in vitro test. Four isolates from different regions of Rio Verde - GO were used. The experimental design was completely randomized with nine treatments and five doses (0.0, 0.1, 1.0, 10 and 100 mg). The fungicides, in the various concentrations, were added in PDA medium and poured into Petri dishes, 80 mm in diameter. Then 5 mm discs, containing fungy mycelia, were transferred to the center of the plate and incubated in growth chamber at 25°C with photoperiod of 12 h. The mycelial growth in colony diameter was measured every 24 h. The inhibition percentage of each fungicide on various isolates of fungi was determined, by observing area under the curve of mycelial progress (AUCMP) and by determining the mycelial growth speed rate (MGSR) was determined. All treatments showed a decrease in SRMG with increased applied dose, the fungicide fluazinam had the best performance, with 100% mycelial growth inhibition at all dose tested and in both areas in which the isolate was obtained. The choice of product and dose to be applied directly will be helpful in the chemical control programs ensuring higher yields at the end of the crop cycle.
Key words: Target spot, Corynespora cassiicola, in vitro, test.
The fungus, Corynespora cassiicola (Berk. & MA Curtis) CT Wei, causal agent of the target spot, is associated with wide range of host species (Silva et al., 1995). In Brazil, the target spot has existed in soybeans since 1976 (Almeida et al., 1976), and as a result of higher susceptible seeding, its incidence has increased in recent seasons, being found in almost all soybean production regions in Brazil (Godoy et al., 2012). In soybean, the losses in yield is up to 20 - 50% (Silva et al., 2008). Control strategies recommended for the disease is the use of resistant cultivars, seed treatment, the rotation/succession of culture with corn and grass species and chemical control (Almeida et al., 2005; Silva et al., 2008).
Fungicides described for complex late season diseases (CLSD) are the same recommended for target spot control in the shoot of soybean culture, being: azoxystrobin, azoxystrobin + cyproconazole, carbendazim, difenoconazole, flutriafol, pyraclostrobin + epoxiconazole, tebuconazole, methyl thiophanate, methyl thiophanate + flutriafol, trifloxystrobin + cyproconazole, trifloxystrobin + propiconazole (Embrapa, 2007). However, there are concerns about chemical control options, as fungicides from benzimidazole, triazole and strobilurin groups recommended for the control of this disease have presented low efficacy in the field (Godoy et al., 2012).
After these reports on the difficulty in the chemical control of the disease in recent harvests in the Midwest region of Brasil, some studies have shown a variability between populations of C. cassiicola and consequently the reduction or loss of the pathogen sensitivity to fungicides (Avozani et al., 2014; Teramoto et al., 2012; Soares et al., 2012). This response has occurred when successive applications of the same product are done in association with improper application conditions (eradicant applications, subdoses and inadequate technology) (Reis et al., 2010).
However, studies that characterize isolates from different regions are scarce and little is known about the variability of the same, making it an obstacle for genetic improvement programs and also to evaluate the efficacy of chemical control due to possible variability of these pathogens.
Considering the difficulties in the control strategy of fungi that cause the target spot and the need for studies on sensitive populations to fungicides, this study aimed at evaluating the sensitivity of C. cassiicola isolated from experimental areas of the city of Rio Verde.
Assay
The experiment was carried out in Phytopathology Laboratory at the University of Rio Verde – UniRV – 2014/2015. The experimental design was a completely randomization with six replicates, using nine fungicides in four doses of active ingredient (AI) [100 ppm (20 mg), 10 ppm (2 mg), 1 ppm (0.2 mg) and 0.1 ppm (0.02 mg)] obtained from the stock solution (Table 1). Four isolates of C. cassiicola from different locations in the city of Rio Verde, where field trials (efficacy test to products) had already been done were used in sensitivity tests (Table 2). For each treatment, a control was added without fungicides application.
Isolation and in vitro test
For isolation, the trefoils of three plants were selected in the plots with disease symptoms. The material was taken to the Phytopathology Laboratory and the fragments plant tissues were disinfected in a solution of sodium hypochlorite 1% by three minutes. Later, the fragments were washed with distilled water to remove excess. Then, the peace of fragments were distributed into acrylic boxes gerbox (11 x 11 x 3.5 cm) containing a nylon foam and two overlapping sheets of filter paper, moistened with sterile distilled water and kept in chamber growth at 25°C ± 2:12 photoperiod. After fungal growth, the colonies visually recognised were transferred to another dish containing medium of potato dextrose agar (PDA), later kept in chamber growth at 25ºC ± 2:12 photoperiod.
For the in vitro tests, the different doses of fungicides were prepared by dissolving the commercial fungicide formulation in sterile deionized water (SDW) until use. They were then further diluted to obtain the desired concentration and poured into plastic Petri dishes (80 mm diameter) and added at the time of PDA culture medium preparation and after been poured into Petri dishes of 80 mm.
The day after culture medium preparation, 6 mm-diameter mycelial plugs of each isolate, taken from seven-day-old colonies, were placed on the center of each dish. The plates were sealed with PVC plastic film and incubated in a growth chamber at 25 ± 2°C and 12 h photoperiod provided by three fluorescent 40 W lamps placed at 50 cm above the plates. When the colony in the control treatment reached the edge of the plates, the diameter of all colonies was measured with a digital calliper as described by Avozani et al. (2014).
Evaluations
The first evaluation took place after 48 h of the experiment. The diameter of each colony was measured in two directions(represented the total growth percentage), at 48 h intervals from the time of inoculation up to the end of the experiment. After measurements, the percentages of inhibition in fungal growth were determined in each treatment, calculating the mycelial growth speed rate (MGSR), used to calculate the inhibition of mycelial growth. This performed MGSR was calculated based on equation MGSR= Σ[(D-Da)/N] (Dias et al., 2005). Where: D = current average diameter of the colony; Da = the average diameter of the colony in the previous day; N = number of hours or days after inoculation.
A completely randomized experimental design using four replicates was adopted. A Petri dish was used as an experimental unit. Data on fungal colony diameter were transformed into growth percentage. The inhibitory concentration (IC50) able to inhibit 50% of mycelial growth for evaluated fungicides and each isolate was calculated from the generated equation.
Classification of isolates based on fungicides sensitivity used was performed according to the criteria proposed by Edginton et al. (1971), in which chemical compounds with IC50 less than 1 mg/L was considered highly fungitoxic, with IC50 between 1 and 50 mg/L are moderately fungitoxic and IC50 higher than 50 mg/L are not fungitoxic. A useful tool to quantify the shift in sensitivity to a fungicide in a fungus is the sensitivity reduction factor (SRF) (Kunz et al., 1998), which is calculated by dividing the IC50 of the fungal strain suspected of having reduced/lost its sensitivity by the IC50 of the sensitive strain. SRF value of 1 means no change in sensitivity, while values > 1 indicate the shift for sensitivity reduction (Reis et al., 2010; Russel, 2004).
Data analysis
All the assays were repeated twice using a completely randomised experimental design with four replicates per treatment. Data were subjected to Shapiro-Wilkand Bartlett tests (signiï¬cance level, P>0.05) for normality and homoscedasticity, respectively. Distribution of isolates (% inhibition colonisation) was subjected to one-way ANOVA, and means were compared using Scott-Knott tests (P< 0.05) (Scott and Knott, 1974). The regression model was ï¬t to the quantitative variables as log transformation using the Sigma Plot 11.0 program.
Evaluation of mycelial growth speed rate (MGSR) of C. cassicola isolates by different fungicides doses
After the calculation of the MGSR and in accordance with
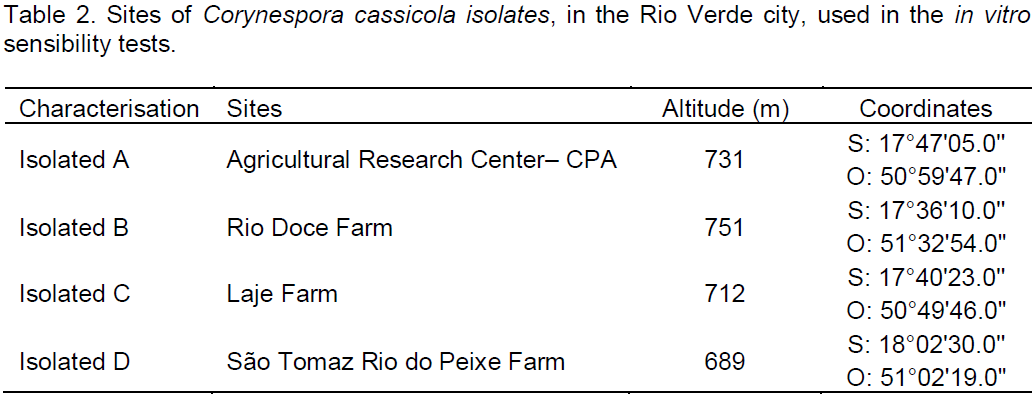
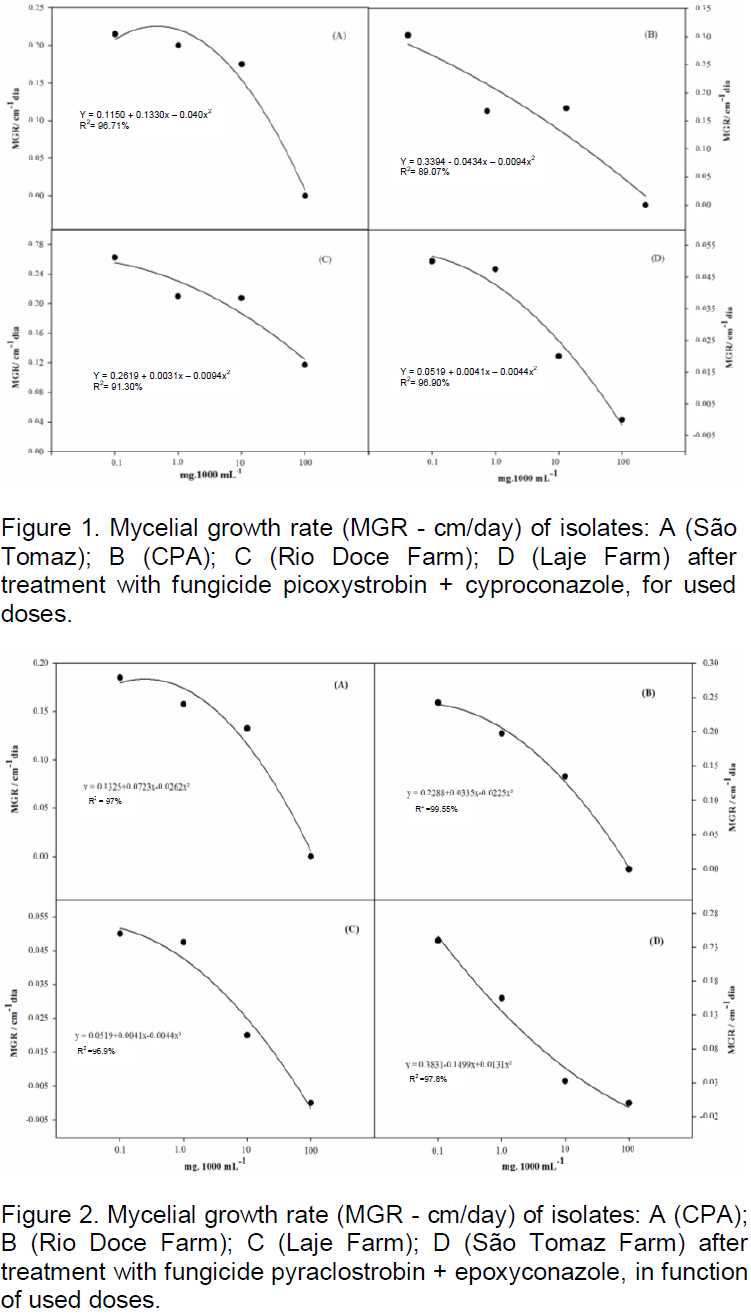
the regression analysis for each variable, it was observed that generally all fungicides produced decrease in growth of fungal mycelia with increasing dose. However, the picoxystrobin + cyproconazole treatments (Figure 1), pyraclostrobin + epoxyconazole (Figure 2), azoxystrobin + cyproconazole (Figure 3), pyraclostrobin + epoxiconazole + fluxapyroxad (Figure 4) and procymidone (Figure 5) showed significant reduction (p < 0.05) in mycelial growth with increased rates of fungicides for all isolates.
For the treatment containing the fungicide trifloxystrobin + prothioconazole (Figure 6) according to regression analysis to MGSR, the isolates from CPA, Rio Doce Farm and São Tomaz Rio do Peixe Farm showed a significant reduction in mycelial growth of C. cassicola. However, for the isolate from Laje farm, there was no dose effect in reducing growth in the studied treatment.
Treatment containing the fungicide carbendazim (Figure 7) and methyl thiophanate (Figure 8) showed a significant reduction in mycelial growth of C. cassicola in isolates from CPA, Laje Farm and São Tomaz Rio do Peixe Farm observed by regression analysis of MGSR. However, isolates from Rio Doce Farm showed no dose effect in reducing the growth.
Inhibition percentage of C. cassicola isolate by different doses of fungicides
In the inhibition evaluations, a partial or total inhibition of C. cassicola was observed. The control (0.0 mg) had mycelial growth of 100% in all evaluated replications. For isolates from CPA, there was 100% of inhibition at doses of 100 mg in all the treatments, except for treatment with methyl thiophanate where inhibition was 68.54% (Table 3). The treatment containing trifloxystrobin + prothioconazole inhibited 100% of the growth of C. cassicola at doses of 10 and 100 mg from São Tomaz Rio do Peixe Farm. On the same property, it was noted that the pyraclostrobin + epoxiconazole + fluxapyroxad, trifloxystrobin + prothioconazole, procymidone, fluazinam and carbendazim treatments, inhibited 100% of mycelial growth of C. cassicola in doses of 10 and 100 mg, with a significant difference when compared with the doses of 1.0 and 0.1 mg. For azoxystrobin + cyproconazole treatments and methyl thiophanate in the dose of 100 mg, 79.22 and 55.52% of inhibition respectively were observed, which were lower percentages than other treatments that reached 100% of inhibition when 100 mg of active ingredient was used (Table 3).
In assessing the isolates from Rio Doce Farm, treatments that stood out with 100% of mycelial growth inhibition of C. cassicola were fluazinam and methyl thiophanate in four doses: 0.1, 1.0, 10 and 100 mg. On the other hand, picoxystrobin + cyproconazole and procymidone treatments achieved the maximum inhibition of 66.72 and 80.97%, respectively (Table 3). The treatment containing fluazinam had the best result in the mycelial growth inhibition, similar in the four doses (0.1, 1.0, 10 and 100 mg), inhibiting 100% of the growth in all isolates of the study areas (Table 3).
Evaluation of the inhibitory concentration of the C. cassicola isolates
Low concentrations of the fungicide picoxystrobin + cyproconazole reduced growth of isolates from CPA and Sao Tomaz farm at IC50 fungal growth, showing significant difference considering the other isolates. The IC50 for picoxystrobin + cyproconazole fungicide for isolate from Rio Doce and Laje Farms was not significant.
Treatment with pyraclostrobin + epoxiconazole in both study areas with their isolates was significant at the level of 0.01%, so the IC50 had significant effect on this active ingredient, being classified as highly fungitoxic (Table 4). In most cases, azoxystrobin + cyproconazole when compared with the other treatments applied in the Laje Farm, showed no significant effect. In the other areas of study, with the exception of Laje Farm, the IC50 demonstrated that azoxystrobin + cyproconazole has fungicidal action. Pyraclostrobin + epoxiconazole + fluxapyroxad had similar effect on other treatments for its areas, where the IC50 indicated high fungitoxic action of the active ingredient used.
The trifloxystrobin + prothioconazole treatment showed significant effect on the four study areas, where the IC50 showed high fungicidal activity of its active ingredient. Procymidone (Table 4) also showed IC50 with high fungicidal action in all the studied areas. Treatment with fluazinam at IC50 showed no significant effects. The carbendazim treatment showed no significant difference for the isolates from Rio Doce and Laje Farms.
Methyl thiophanate demonstrated significance level of 0.01% for isolates from CPA and Laje Farm, as for isolates from Rio Doce and São Tomaz farms, there was no significant difference.
The fungicide pyraclostrobin + epoxiconazole was highly toxic for isolates from CPA and Laje Farm, however, to isolates from São Tomaz and Rio Doce farms it was moderately toxic. The fungicide azoxystrobin + cyproconazole was highly toxic to isolates from CPA and moderately toxic for isolated from São Tomaz and Rio Doce farms. However, to isolate from Laje Farm, the cyproconazole + azoxystrobin fungicide was not toxic. The pyraclostrobin + epoxiconazole + fluxapyroxad fungicide was highly toxic for all isolates tested. The trifloxystrobin + prothioconazole fungicide was moderately toxic for isolates from São Tomaz Farm and, the other isolates were highly fungitoxic. Procymidone was moderately toxic to isolate from São Tomaz Farmand to the others, it was highly toxic.
Fluazinam was highly toxic to all isolates used. Carbendazim was also highly toxic to isolates from CPA and São Tomaz Farm. For the isolate from Laje Farm, the fungicide carbendazim was moderately toxic and showed no antifungal effect on isolate from Rio Doce Farm. For the fungicide, methyl thiophanate was slightly toxic to isolates from CPA and Laje Farm, were slightly toxic and showed no fungitoxicity for all other isolates.
The differences in the behavior of the isolates from different areas indicate the possible change of genetic variability of these isolates causing low sensitivity to fungicides. It is known that the abuse of systemic molecules to control pathogens causes reduction in the sensitivity to products (Reis et al., 2010). In some studies, the fungicide carbendazim had low efficiency in controlling the target spot, which could have been as a result of the resistance to this chemical group on the pathogen (Teramoto et al., 2013; Avozani et al., 2014). However, in this work, carbendazim fungicide did not appear to be inefficient in its toxicity. On the other hand, there was a highlight for methyl thiophanate considering its percentage inhibition of mycelial growth, which was less efficient in almost all locations.
The fungicides belonging to the chemical group of benzimidazoles act on fungi by inhibiting α and β tubulin specific proteins (Coutinho et al., 2006). The affinity of benzimidazole with tubulin is the main factor that determines the fungicidal activity in organisms. The higher the affinity, the more sensitive the organism to the fungicide.
Probably due to various selection factors, a mutation occurred to β-tubulin protein gene leading to formation of β-tubulin protein that has reduced binding affinity with benzimidazole leading to a new generation of resistant population (Brent, 1995; Hewitt, 1998). Therefore, the high selection pressure caused by intensive use of fungicides such as benzimidazoles, may result in the selection of resistant fungus at a short period of time (Parreira et al., 2009), explaining the difference of inhibition displayed by methyl thiophanate.
According to Deising et al. (2008), the resistance acquired by the pathogen population to the product is directly proportional to applied doses, frequency of application, degree of coverage, persistence in culture or in soil and the size of the treated area. This justifies the lower results observed for azoxystrobin + cyproconazole treatment at the highest dose when compared with the other treatments (Table 3). In the evaluations performed in this research, the product Fluazinam showed 100% efficient in the percentage inhibition of mycelial growth in all doses and in both areas in which they were obtained.
In previous work carried out by Töfoli et al. (2003), the fungicide fluazinam was also responsible for higher levels of mycelial growth inhibition in isolates of Alternaria solani and noted that the action of this fungicide showed complete inhibition of spore germination of A. solanis from doses of 1 μg.mL-1. In other studies using the same product, fluazinam, Guimarães et al. (2008) found efficiency in the control of Monosporascus cannonballus at different doses.
The inhibitory concentration (IC50) studies for different fungicides and specific to C. cassiicola in soybean are scarce, yet it is very useful in carrying out research and sensitivity monitoring, especially in areas where the control of this disease is not being efficient (Avozani et al., 2014). The isolates from the Laje Farm treated with azoxystrobin + cyproconazole showed no significant effect on the IC50 values but had high IC50 value.
This effect may be due to high-pressure selectivity for this area specifically, or the inappropriate use of the product in the past situations, which may, according to the obtained data, have selected individuals resistant to the products. The high value of IC50 (Table 4) clearly shows that this area of study (Laje Farm), the respective active component has low fungicide action. Since treatment with fluazinam at the IC50 showed no significant results, however, was highly fungitoxic for all used isolates. In this study, thecarbendazim treatment showed no significant difference for the isolate from Rio Doce and Laje farms, and for the Laje Farm, according to the IC50, this fungicide can be classified as moderately fungitoxic, according to the criteria proposed by Edgington et al. (1971). Furthermore, Avozani et al. (2014) found that the isolates of C. cassiicola showed less sensitivity to the carbendazim active ingredient and the cyproconazole active ingredient presented best valore of IC50. In studying the sensitivity of isolates submitted to the treatments, it was noted that the increased resistance of the isolates from the Laje Farm for some treatments, should necessitate the investigation of the previous management methods in this region that could have contribute to the multiplication of resistant populations.
Generally, fungicides used showed good control levels distinguishing between places where the experiments were carried out. All treatments caused an increase in productivity as compared to the control treatment. The fluazinam fungicide was better among the other fungicides with 100% of mycelial growth inhibition in all doses and in all areas in which the isolate was obtained so it is considered highly fungitoxic. The low sensitivity of these pathogens to some molecules can guide the development of management strategies reducing the loss in yield and quality of crops around the world.
The authors have not declared any conflict of interests.
REFERENCES
|
Almeida AMR, Ferreira LP, Yorinori JT, Silva JFV, Henning AA, Godoy CV, Costamilan LM, Meyer MC (2005). Doenças da Soja. In: Kimati H, Amorim L, Rezende JAM, Bergamin FA, Camargo LEA (Eds.). Manual de Fitopatologia 2: Doenças das plantas cultivadas. São Paulo, 4 ed. Agron. Ceres pp. 569-588.
|
|
|
|
Almeida AMR, Machado CC, Ferreira LP, Lehman OS, Antonio H (1976). Ocorrência de Corynespora cassiicola no Estado de São Paulo. Fitopatol. Bras. 1:111-112.
|
|
|
|
|
Avozani A, Reis EM, Tonin RB (2014). Sensitivity loss by Corynespora cassiicola, isolated from soybean, to the fungicide carbendazim. Summa Phytopathol. 40:273-276.
Crossref
|
|
|
|
|
Brent KJ (1995). Fungicide resistance in crop pathogens: how can it be managed? Brussels: FRAC Monograph 1., 1995.
|
|
|
|
|
Coutinho CBF, Galli A, Mazo LH, Machado SAS (2006). Carbendazim e o meio ambiente: degradação e toxidez. Pesticidas. Rev. Ecotoxicol. Meio Ambiente 16:63-70.
|
|
|
|
|
Deising HB, Reimann S, Pascholati SF (2008). Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 39:286-295.
Crossref
|
|
|
|
|
Dias MD, Pozza EA, Abreu MS, Miranda EO (2005). Efeito da temperatura no crescimento micelial, produção e germinação de conídios de Colletotrichum spp. isolados de Coffea arabica L. Ciênc. Agrotécnologia 29:545-552.
|
|
|
|
|
Edgington LV, Khew KL, Barron GL (1971). Fungitoxie spectrum of benzimidazole compounds. Phytopathology 61:42-44.
Crossref
|
|
|
|
|
Embrapa (2007). Sistema de produção: Tecnologia de Produção de Soja da Região Central do Brasil. Londrina PR: Embrapa. P 225.
|
|
|
|
|
Godoy CV, Utiamada CM, Meyer MC, Campos HD, Pimenta CB, Borges EP, Siqueri FV, Nunes Junior J, Silva LHCP, Sato LN, Madalosso M (2012). Eficiência de fungicidas para o controle da mancha-alvo, Corynespora cassiicola, na safra 2011/12: resultados sumarizados dos ensaios cooperativos. Londrina PR. Embrapa Soja.(Circular Técnica 94).
|
|
|
|
|
Guimarães IM, Junior SR, Silva PJK, Michereff SJ, Nogueira DRS (2008). Efeito do Fluazinam no controle de Monosporascus cannonballus, agente causal do declínio de ramas em meloeiro. Rev. Caatinga 21:147-153.
|
|
|
|
|
Hewitt HG (1998). Fungicides in crop protection. Oxon, UK: CAB International, 1998. 221p.
|
|
|
|
|
Kunz S, Lutz B, Deising H, Mendgen K (1998). Assessment of sensitivity to anilopyrimidine-and strobilurin-fungicides in populations of the apple scab fungus Venturia inaequalis. J. Phytopathol. 146:231-238.
Crossref
|
|
|
|
|
Parreira DF, Neves WS, Zambolim L (2009). Resistência de fungos a fungicidas inibidores de quinona. Rev. Tróp. 3(2):24.
|
|
|
|
|
Reis EM, Reis AC, Carmona MA (2010). Manual de fungicidas: guia para o Controle Químico de Doenças de plantas. 6 ed. Passo Fundo: Editora UPF. 226 p.
|
|
|
|
|
Scott AJ, Knott M (1974). A Cluster Analysis Method for Grouping Means in the Analysis of Variance. Biometrics 30:507-512.
Crossref
|
|
|
|
|
Silva L, Campos H, Silva J (2008). Fortalecida e agressiva. Revista Cultivar–Grandes Culturas. Ano X (114):20-22.
|
|
|
|
|
Silva WPK, Multani DS, Deverall BJ, Lyon BR (1995). RFLP and RAPD analyses in the identification and differentiation of isolates of the leaf spot fungus Corynespora cassiicola. Aust. J. Bot. 43:609-618.
Crossref
|
|
|
|
|
Soares RM, Almeida Filho KM, Meyer MC, Teramoto A, Godoy CV (2012). Comparação da virulência de isolados de Corynespora cassiicola obtidos em soja. In: VI CONGRESSO BRASILEIRO DE SOJA 2012, Cuiabá, MT. P 118. Available at:
View
|
|
|
|
|
Teramoto A, Machado TA, Nascimento LM, Meyer MC, Cunha MG (2012). Sensibilidade a fungicidas de isolados de Corynespora cassiicola provenientes do estado de Goiás. In: VI CONGRESSO BRASILEIRO DE SOJA. 2012, Cuiabá, MT. P 117.
|
|
|
|
|
Teramoto A, Parisi MCM, Cunha MG (2013). Caracterização fisiológica de isolados de Corynespora cassiicola. Trop. Plant Pathol. 38:313-322.
Crossref
|
|
|
|
|
Töfoli JG, Domingues RJ, Kurozawa C (2003). Ação "in vitro" de fungicidas no crescimento micelial e germinação de conídios de Alternaria solani, agente causal da pinta preta do tomateiro. Arq. Inst. Biol. São Paulo 70:337-345.
|
|