ABSTRACT
The corn crop is one of the most cultivated in the world and one of the most need studies that seek alternatives on the use of Azospirillum brasilense. This bacterium produces growth hormones that may benefit the corn crop. The work objective was to verify the agronomical performance of corn crop, in function of foliar Azospirillum brasilense inoculation, associated with nitrogen doses. The research was performed in Selvíria, Mato Grosso do Sul State, Brazil, which is in the Brazilian Cerrado. The corn crop was cultivated during season (Spring/Summer) and late season (Summer/Autumn), under conventional tillage system. Experimental design of randomized blocks was used, with factorial scheme 4 × 2. The treatments were made in four nitrogen doses (0, 30, 60 and 90 Kg ha-1) with and without the foliar applying (in stage V6) of the inoculant containing A. brasilense. The inoculant used had the strains AbV5 and AbV6 of A. brasilense (2×108 viable cells mL-1) and, the dose 200 mL ha-1 was used. The nitrogen fertilization was made with ammonium sulfate, also on phenological stage V6. The following evaluations were made: Final plant population, foliar nitrogen content, foliar chlorophyll index, ear insertion height, plants height, stalk diameter, ears length, ears diameter, thousand grain weight and grain productivity. The foliar inoculation with the A. brasilense bacterium proved to be advantageous for the corn crop, and therefore, an option for the farmer. Because of the large volume of products used in seed (fungicide, insecticide, plant growth regulators) and the lack of information on the degree of interference of these products on the bacteria, it becomes pertinent the study of other inoculation forms on crops. Other studies that verify the influence of A. brasilense bacteria in foliar application on the physiology of the shoot and root of crops should be encouraged.
Key words: Zea mays L., inoculation, nitrogen, diazotrophic bacteria.
Abbreviation:
CEC, Cation Exchange Capacity; BS, Base Saturation
The corn crop (Zea mays L.) has indisputable role in the world and Brazilian economy, due to its outstanding position among the agricultural species explored (Môro and Fritsche, 2015). Besides human feeding, corn is the main component in animal food, highlighting birds, cattle and pigs. Therefore, much research should be conducted in order to increase productivity (Purwanto et al., 2015).
Plant growth-promoting bacteria, called associative, as they do not make symbiosis with the host plant and belong to the genus Azospirillum, can help through several mechanisms in the crops nitrogen nutrition (Bashan and Bashan, 2005). Among the mechanisms, the process of nitrogen biological fixation and the plant growth promotion stand out for the production of several vegetable hormones, which result in higher growth, water and nutrients absorption (Moreira et al., 2010) which may increase the productive potential of crops. According Bashan et al. (2004), the most common explanation for the effect of the bacteria in plants is the production of hormones that alter the metabolism and the morphology of the plant, improving the nutrient and water uptake.
Costa et al. (2015) studied inoculation with Azospirillum brasilense in seed and nitrogen doses in corn crop in the Cerrado region and reported that the use of bacteria in corn promoted greater plant height, stalk diameter, chlorophyll index, dry mass of stalk and root, ear length, thousand grain weight and yield. Similarly, Souza (2014) also in Cerrado region, noted that the use of bacteria in seed promoted greater plant height and ear insertion height, hundred grain weight and yield. Morais et al. (2016) researched several doses of inoculant containing A. brasilense applied in sowing furrow of corn and verified that the dose of 200 mL ha-1 promoted increased in grain yield. One of the most pronounced effects of inoculation with Azospirillum on root morphology is represented by the proliferation of root hairs (Saikia et al., 2012) and, according Fulchieri et al. (1993) the promotion of root growth can lead to better exploration of the soil and enhance the growth and development of plants by the greater water and nutrients uptake.
Studies that promote knowledge about bacteria of genera Azospirillum must be stimulated (Moreira et al., 2010; Lana et al., 2012) aiming not only low cost and low environmental impact agriculture, but also the biotechnological potential that these bacteria present (Moreira et al., 2010). Identifying the managing conditions which may contribute to the maximization of processes made by them is a challenge for the present research (Moreira et al., 2010), as an example, the study about foliar applying of the inoculant containing the bacterium A. brasilense can be mentioned. According to Fukami et al. (2016) little is known about the effects of pesticides used to treat seeds on Azospirillum. The authors report that alternative methods of inoculation in crops are needed and highlight the foliar application in the vegetative stage.
This way, the objective of the work was to evaluate the corn crop performance (season and late season) when submitted to foliar inoculation of A. brasilense and nitrogen doses applied in topdressing, in low altitude Cerrado region.
The experiment was developed during the season in 2011/12 (Spring/Summer) and the late season in 2012/13 (Summer/Autumn) at the Teaching, Research and Extension Farm of Engineering College of Ilha Solteira - UNESP, located in Selvíria city, Mato Grosso do Sul State, Brazil, within the geographical coordinates of 22° 23’ south latitude and 51° 27’ west longitude and 335 m of altitude. Soil is classified according to Santos et al. (2013), in RED OXISOL typical dystrophic clayed. Before of experiment installation was collected soil in the layer from 0.0 to 0.2 m for the chemical characterization of all experimental area, with the same values for all experimental plots (Table 1).
The soil preparation was made with scarifier and leveling harrow. The conventional seeding system was used. The corn seeding on season period was made under soybean residues and on late season period under corn residues. The experimental area was cropped with soybeans on summer of 2010/2011 and remained fallow until the sowing of corn on season period (2011/12). After the cropped of corn on season period (2011/2012), the area remained fallow until the sowing of corn on late season period (2013). It was opted to sow the corn in the beginning of period that characterizes the late season (January) to match the period of much of the crop development with a part of the rainy period, providing better water condition. Agricultural activity in the Cerrado is concentrated in the rainy period, usually between the months of October to March, when it occurs between 80 and 90% of the annual precipitation (Assad et al., 1993), so there is a preference for sow crops in the period that provides better conditions for development.
The annual average rainfall in the region is 1,313 mm, maximum annual temperature is 31°C, minimum annual temperature is 19°C and average annual temperature is 25°C (Portugal et al., 2015) with climate type Aw, according to Köppen’s classification. The daily rainfall values, maximum temperature and minimum temperature on the experiment leading period are on Figure 1.
The experimental design was of randomized blocks with 8 treatments, disposed in factorial scheme 4x2 with four replications. The treatments were composed by 4 nitrogen doses in ammonium sulfate form (0, 30, 60 and 90 Kg ha-1) with and without the foliar application of the inoculant containing A. brasilense. The plots were composed by 5 lines with 5 m long, being considered as useful areas, the 2 central ones.
For the season corn the simple hybrid AG 8088 YG was used, of early cycle and hard orange seed; while for the late season corn, the simple hybrid DKB 390 VT PRO was used, of early cycle and semi hard yellow-orange seed (Cruz et al., 2013). The foliar inoculation was performed with Masterfix Gramineas® inoculant, with the strains AbV5 and AbV6 of A. brasilense (2×108 viable cells mL-1) with dose of 200 mL ha-1 diluted with water only. The sowing was made on 11/9th/2011 (season corn) and 01/2nd/2013 (late season corn) with row spacing of 0.90 m and the initial population was established in 50,000 plants ha-1 for the corn in both cultivation seasons. According to Fornasieri-Filho (2007) the ideal population for the corn crop varies from 30,000 to 90,000 plants ha-1. The mineral fertilization in the sowing furrow was defined based on the chemical soil analysis and expected productivity of 4,000 to 6,000 kg ha-1, according to the recommendations of Raij and Cantarella (1997) using 250 Kg ha-1 of 08-28-16 (season) and 400 kg ha-1 of formulated 04-30-10 (late season).
The foliar inoculation with the bacterium, as well as the topdressing fertilization, were made when the plants were on developing stage V6 (six expanded leaves), according to the phenological scale proposed by Ritchie et al. (2003), at that phase the crop was with 17 DAE (days after the emergency) and 22 DAE on season and late season, respectively. The topdressing fertilization and the inoculant applying were made in the late afternoon, aiming mild temperature conditions, especially to favor the inoculation with the bacteria. For the inoculant applying, costal spray with cone jet tip and 180L ha-1 output was used.
Aiming to keep the crop free from competition with weeds, atrazine and tembotrione were used in doses of 1,000 and 100 g ha-1 of a.i., respectively, in tank mixture form. The adjuvant soy methylated ester was added to the application mixture (500 g ha-1 of a.i.). The application was made through the use of tractor powered spray with flat jet tips and adjusted to apply 200 L ha-1 of the mixture.
During the research, the following evaluations were performed: (a) final plant population, made through counting the plants in the useful area at the harvest moment and later extrapolated for hectare. This evaluation has an important role on the yield of corn crop, since small variations have great influence on the final yield (Cruz et al., 2015); (b) foliar nitrogen content, gotten through collect of the mid third of five opposite leaves and under the main corn ear on stage R1(flowering – at 52 DAE and 48 DAE of season and late season corn, respectively), according to the phenological scale proposed by Ritchie et al. (2003), after that the drying of leaves was made and the nitrogen content was determined in laboratory. The analysis of plant tissue as a dignostic criterion is based on the premise there is a relationship between growth and crops production and the nutrients content in their tissues (Coelho, 2008) among them, the nitrogen; (c) foliar chlorophyll index, made at the same moment and with the same leaf collect to obtain the foliar nitrogen content, using portable chlorophyll meter (model CFL 1030), which through sensors, analyzes three light frequency lines and through absorption relation of different frequencies, provides measurement of chlorophyll a, b, and total (a+b) levels, expressed in dimensioned units called FCI (Foliar Chlorophyll Index). The foliar chlorophyll index is a good parameter to indicate the nitrogen level in cereals (Argenta et al., 2001), because 50 to 70% of total nitrogen of the leaves is integral of enzymes that are associated to the chloroplasts (Lima et al., 2009); (d) plants height, gotten by measurement of five random plants per plot, using a graduated ruler, from the ground to the flag leaf, on R6 stage (physiological maturity), according to the phenological scale proposed by Ritchie et al. (2003); (e) ear insertion height, measured in the plants and at the same moment of plant height, however, the measurement was made from the ground to the ear insertion on the stalk. Higher plant height and ear insertion height on the stalk can contribute to the increase of crop lodging (Brachtvogel et al., 2012); (f) stalk diameter, measured at the second internode from the plants base, with digital caliper rule CD-6 CSX-B (Mitutoyo Sul Americana®), five random plants were considered. The stalk diameter is one of the characteristics that has been more related to the percentage of lodging and breakage of plants in corn crop (Kappes et al., 2013); (g) ear length, made after harvest, considering ten random ears without straw in each plot, which were measured from the base to the apex with graduated ruler; (h) ear diameter, was obtained by measuring the central third of the same ears used to measure the length, with the digital caliper ruler. The length and diameter of corn ears directly influence the yield of crop grain (Kappes et al., 2009); (i) thousand grain weight, obtained by counting a subsample of 250 grains per plot, which was submitted to weighting and humidity determination, making it possible to estimate the grains weight corrected to 13% of humidity (wet basis - w.b.). The grain mass depends entirely of the factors that control the supply of assimilates for grain filling (Fageria, 1989) and correlates positively with crop yield (Duarte et al., 2007); (j) grain productivity, it was obtained from the threshing and weighting of grains from the ears collected from the two central lines in each plot, the values were extrapolated to kg ha-1 and corrected to 13% humidity (w.b.). This is the most important variable to check if the crop was responsive to the different treatments in which it was subjected.
The corn ears harvest on the area was performed on 03/15/2012, at 120 DAE of corn on season and on 16/05/2013, at 127 DAE on late season.
The results were submitted to the F test of analysis of variance, comparing the treatments average with A. brasilense by the Tukey test at 5% de probability. The N doses average in topdressing were submitted to the regression analysis, adjusting meaningful equation models through the F test.
During the corn crop cycle cropped in the season period (Figure 1A), the accumulated rainfall registered was 682 mm and on corn cycle cropped in late season (Figure 1B), the accumulated rainfall was 603 mm. In both cultivation seasons the precipitation was adequate to the corn crop because, according to Fancelli (2015) this crop requires 400 to 600 mm of rainfall during the cycle. It is noticed that in the period of late season occurred less rainfall and irregular distribution at the end of the reproductive phase.
The F test was significant at 5% to the inoculation in the final population variable only in the season period (Table 2). The foliar inoculation with A. brasilense provided higher final plant population. After 11 days of the inoculant applying, there was a 14-day dry period, what may have not favored the non-inoculated plants (Figure 1). At the initial development phase, the corn plants have fast absorption of nutrients and water; therefore, long periods without water may impair the development. It can be explained by the hormones production by the bacteria, which promoted the growth of the root system and, consequently the soil volume to be explored in search of water and/or nutrients (Okon and Labandera-Gonzalez, 1994), providing higher survival rate, while the non-inoculated plants did not have this competitive advantage. According Fornasieri-Filho (2007) in corn crops with excess of plants regarding the water supply capacity occur death of plants. Therefore, the dry periods are the main cause of mortality of plants in Cerrado region. What did not occur with the late season corn, because there was proper rainfall distribution in the vegetative and reproductive phases. For both corn crop periods, the F test showed that there was no influence of nitrogen doses (Table 2).
As to the foliar nitrogen content, it is noticed that on season corn, the F test was significant at 1% for inoculation and nitrogen doses, and the late season corn was significant at 5% only for nitrogen doses (Table 2). The inoculation had positive influence, resulting in higher foliar N content. However, both treatments obtained values within the suitable range, which, according to Cantarella and Furlani (1997) is between 27 and 35 g Kg-1. In relation to the N doses, it’s observed that in both crops, the foliar N contents had a linear positive response, the same was reported by Farinelli and Lemos (2012), Mota et al. (2015), Lange et al. (2006), Moda et al. (2014) and Valderrama et al. (2014).

For the foliar chlorophyll index the F test was significant at 5% for nitrogen doses in both crops (Table 2). On season, it is noticed that the foliar chlorophyll index adjusted to the quadratic equation, which had higher values with the approximate dose 58 kg ha-1 of N. Maestro et al. (2014), working with N doses in corn at the same region of the present work, it was observed a higher foliar chlorophyll index with doses of 85 and 96 kg ha-1, in the years 2009 and 2010, respectively. Torres et al. (2015) observed higher values for the chlorophyll index with the dose of 142 kg ha-1, the authors report that above these doses, there was chlorophyll content reduction due to the fact that they stopped responding to the increase of N offer.
The foliar chlorophyll index is used to predict the nitrogen content in the leaves, however, making comparison it’s observed that the chlorophyll index did not reflect on the foliar N content on season. According to Blackmer and Schepers (1995) the luxury consumption of nitrogen by the plant, in nitrate form is not detected by the chlorophyll meter. It happens because the N does not associate to the chlorophyll molecule (Sangoi et al., 2015). On late season, the foliar chlorophyll index adjusted to the positive linear equation with the nitrogen dose increase, reliably reflecting the foliar nitrogen level, the same was reported by Costa et al. (2012) and Mota
et al. (2015).
In relation to plant height, the F test was significant at 1% for inoculation only on late season corn (Table 3). The plants height was benefited by the foliar inoculation with inoculant containing A. brasilense, representing increase a little above of 7 cm under inoculation treat-ment. The growth hormones production characteristic, given by the bacteria, may be the reason for such superiority in plants height. According to Bashan and Bashan (2010) and Vacheron et al. (2013) these hormones change the plants metabolism and morphology, leading to better mineral and water absorption, consequently higher plants. Kappes et al. (2013), researching inoculation, applying nitrogen foliar and in topdressing in Cerrado region, obtained increase of 12.6 cm in corn plant height. Puente et al. (2009), working with seeds inoculation with A. brasilense (without inoculation, 12 mL kg-1 of seeds and 0.41 mL kg-1 of seeds in three study locations) in Argentina, observed that at the second location, the plant height with treatment 0.41 mL kg-1 of seeds was superior to the ones without inoculation.
It is observed that for the ear insertion height the F test was significant at 5% for the interaction between inoculation and N doses on season corn (Table 3). According to the Table 4 values, it is noticed that when the nitrogen in topdressing was not applied (0 kg ha-1), the presence of inoculation with A. brasilense provided superior ear insertion height. The same thing did not happen when N in topdressing was made. This way, it is possible to infer that the inoculation with the bacteria A. brasilense supplied, sufficiently, the N demanded by the corn plants. According to Vacheron et al. (2013) plant growth promoting bacteria can fix and provide nitrogen to the plant, promoting growth. From 30 kg ha-1 of N may be available in the soil amount of nitrogen in the ammonium form sufficient to promote reduction of the bacteria activity, mainly, may have coincided with the V9 stage of development (elongation stage of stalk). According to Rudnick et al. (1997) and Hartmann (1988) the addition of nitrogen to the soil, especially in ammoniacal form decreases the activity of A. brasilense bacterium. Therefore, the presence of ammoniacal nitrogen in the soil at this stage of the plant, could have influenced the bacteria and their activity, interfering in the ear insertion height. According to Ritchie et al. (2003) in the V9 stage the plant stalk is in rapid elongation and one female inflorescence will develop from each of the nodes above the soil surface, except for the last six to eight nodes below tassel. The authors explain that the growth of female inflorescences of lower insertions in the stalk, occasionally stays slower and only one or two female inflorescences in upper position in the plant will develop into productive ears.

Still on Table 4, it is noticed that in inoculation absence, the insertion height adjusted to a quadratic equation with N doses increase in topdressing, which presented maximum point with estimated dose of 76 kg ha-1 of N.
On late season, the F test was significant at 5% for the ear insertion height (Table 3). This variable was positively influenced by the inoculation with A. brasilense, in a similar way to what happened to plant height, also on late season period. Therefore, we have the same explanation.
It is noteworthy that higher plant height and ear insertion height can bring some implications. According to Sousa and Ferreira (2015), Carvalho et al. (2015) and Cabral et al. (2016) corn plants and ear insertion height higher have a greater tendency to tip over and stalk breakage, especially in regions of strong winds. However, in this study it was not observed such implication.
The corn stalk diameter did not respond to any of the treatments (Table 3). The same way, Fernandes et al. (2005), Meira et al. (2009), Goes et al. (2014) and Gazola et al. (2014) did not observe stalk diameter response with the increase in N doses. Dotto et al. (2010), Kappes et al. (2013), Souza (2014) and Marini et al. (2015) working with diazotrophic bacteria inoculation, did not observe influence on the corn stalk diameter.
According to the significance of 1% of the F test, it is verified that there was interaction between inoculation and N doses for ear length evaluation on season corn (Table 5).

According to the deployment shown on Table 6, it is noticed that only in doses of 30 and 60 kg ha-1 of N the inoculation presence provided superior ear length. Probably, until the dose of 60 kg ha-1 of N there was significant reduction of ammoniacal nitrogen in the soil in the course of time, allowing the bacteria resume their activity on the main moment in which the plant determines the ear length (V12). According to Rudnick et al. (1997) and Hartmann (1988) the addition of nitrogen to the soil, especially in the ammoniacal form decreases the activity of A. brasilense bacteria. In the course of time, after nitrogen fertilization in topdressing (V6), the ammoniacal nitrogen passes to the nitrate form by the action of Nitrosomonas and Nitrobacter bacteria. Cantarella (2007) states that in soils with aerobic conditions and high temperatures, the ammoniacal nitrogen are oxidized to nitrate form in approximately 15 to 30 days. This process decreases the amount of ammoniacal nitrogen in the soil that would be a limiting factor for the bacterium. Possibly, at this stage there was a reduction of the amount of ammoniacal nitrogen and allowed the Azospirillum bacteria resume their activity, resulting in increased ear length.
At the inoculation presence, the ear length adjusted to a quadratic model with N doses increase, showing longer length with the dose of 70 kg ha-1, but at the inoculation absence, there was the opposite, in other words, the ear length adjusted to a quadratic model, however, showing minimum point with the dose of 33 kg ha-1. Dotto et al. (2010) report that a higher contribution of inoculation associated to nitrogen fertilization is generally noticed. Nevertheless, there are many papers performed in Brazil in regions with similar climate to the present study (Kappes et al., 2013, Cunha et al., 2014) and regions with different climate (Cavallet et al., 2000; Repke et al., 2013; Marini et al., 2015) showing absence of significant results for the interaction between seed inoculation with A. brasilense and N doses for the ear length. According to Basi (2013) it is essential that field experiments be performed to evaluate the effects of inoculation with A. brasilense in order to obtain more results of this technology in corn crop in different conditions of year, climate and soil. This information reinforces the importance of the study of other forms of inoculation, since researches with foliar inoculation in different crops are scarce. Morais et al. (2016) reported that the use seed inoculation becomes impractical in the field, because the seeds generally marketed have already been treated with phytosanitary products, and the need to treat seeds again with bacteria is not attractive to farmers.
On late season, the F test showed significance of 1% for the inoculation on the ear length (Table 5). The inoculation presence provided decrease of 6.5% on ear length, in relation to the inoculation absent treatment. Differently, Cavallet et al. (2000) and Kappes et al. (2013), obtained increase of 6 and 3.7%, respectively, on the ear length.
The ear diameter was not influenced by the inoculation and the N doses, as seen by the F test that was not significant (Table 5). Similarly, Cunha et al. (2014) and Marini et al. (2015), using inoculation and Kappes et al. (2013), using inoculation and N doses applying in topdressing, did not find differences from the treatments about the ear diameter of corn.
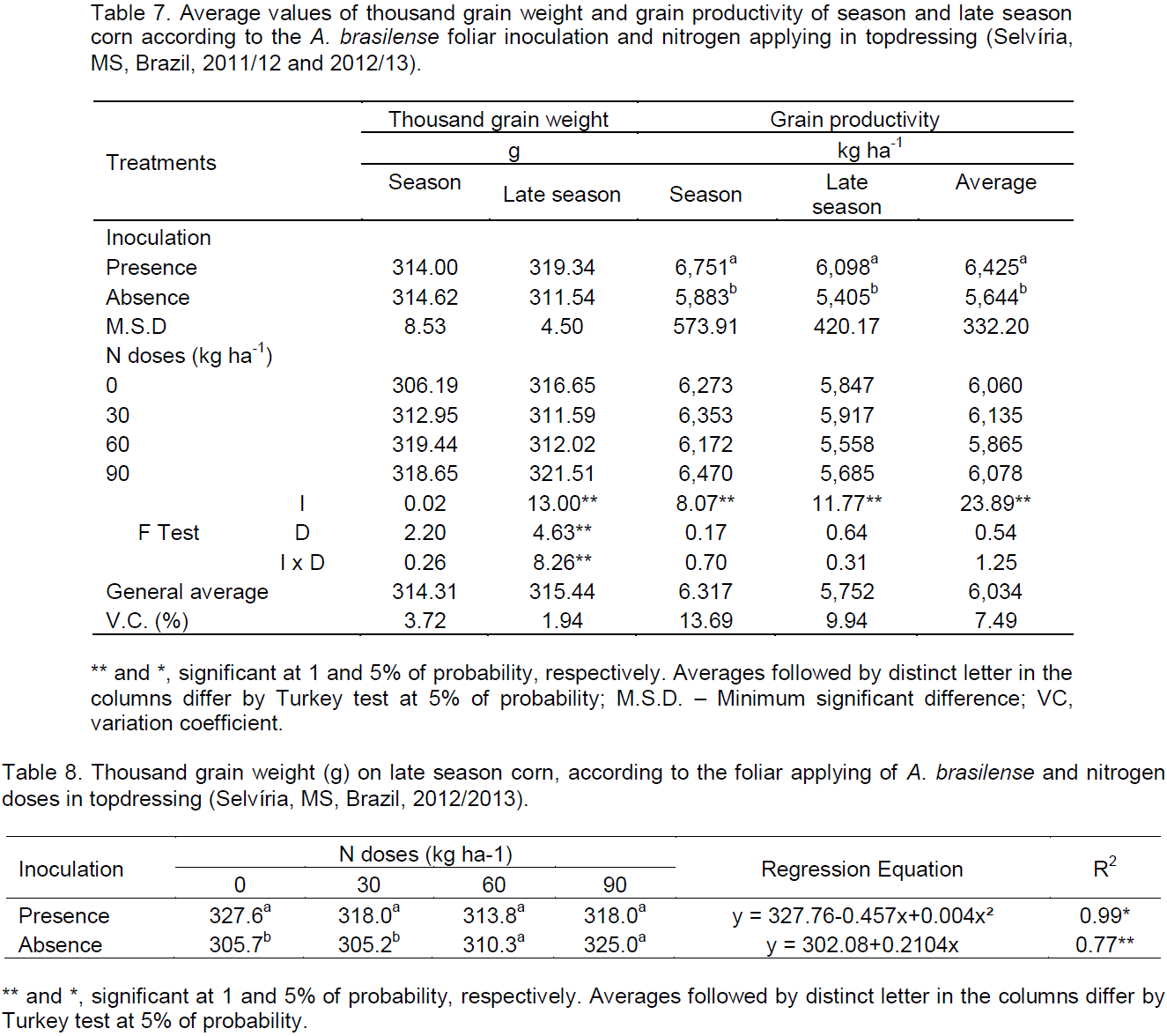
The thousand grain weight on season was not influenced by the treatments, as verified by non-significant F test. But on late season period, the F test was significant at 1% for the interaction between inoculation and N doses (Table 7). According to the deployment (Table 8), it is noticed that on the doses of 0 and 30 kg ha-1 of N the inoculation presence provided increase of 7% and 4% on the thousand grain weight, respectively. Biari et al. (2008) verified increase of 30% on the hundred grain weight, from the inoculation treatment with A. brasilense associated to the applying of 50 kg ha-1 N in topdressing in comparison to the control treatment.
When the inoculation was performed (Table 8), the thousand grain weight adjusted to a quadratic model, presenting minimum point with the dose of 57 kg ha-1 of N. At inoculation absence, the thousand grain weight adjusted to a positive linear model with increase of N doses.
Costa et al. (2015) verified that the thousand grain weight responded in a positive linear way with N doses increase, at the inoculant presence on the seed, foliar and also on the inoculant absence. Rodrigues et al. (2014) observed that the hundred grain weight of corn responded in a negative linear way, with the N doses increase, when the seeds were inoculated with the strain AbV5 of Azospirillum.
In relation to the corn productivity, the F test was significant at 1% for inoculation in both crops and in the average (Table 7). The presence of foliar inoculation with A. brasilense provided higher values, whereas the average of the two periods of cultivation, there was increase of 14% in productivity. Several authors reported increase in corn grain productivity with the use of inoculation in seeds with A. brasilense. Cavallet et al. (2000) reported increase of 17% in productivity, Hungria et al. (2010) 27%, Bartchechen et al. (2010) 15%, Lana et al. (2012) 11%, Braccini et al. (2012) 26%, Kappes et al. (2013) 9%, Souza (2014) 6% and Mazzuchelli et al. (2014) reported increase of 22% in productivity. Works that evaluate foliar inoculation with A. brasilense in corn crops are scarce. Costa et al. (2015), working with inoculation in the seed, foliar (stage V4) and control, associated to foliar applying of N doses (0, 25, 50, 75 and 100%, with the dose of 100% corresponding to 50 kg ha-1 of N), verified that there was increase of 36% in corn grains productivity when the seed inoculation was made in relation to the control. The authors also verified increase of 22% in productivity with foliar applying without foliar N applying. According to Okon and Labandera-Gonzalez (1994) the productivity increase in response to the inoculation is usually about 5 to 30%.
In general, it is noticed that the crops were little responsive to the doses of N. This can be attributed to the fact that corn was sown under soybean residues, which showed average yield of 3,600 kg ha-1. According to Duarte et al. (2013) the nitrogen present in soybean residues can be used by the late season corn. The authors estimate that for the corn grown in succession, are availed about 15 kg of N for each ton of soybean, or 54 kg ha-1 of N when produces 3.6 ton ha-1 of soybean.
When observing the previous assessments, it is noticed that for corn grown on season, what probably influenced the higher productivity in the presence of inoculation was the final plant population, expressing the higher potential to overcome the productivity of the treatment without inoculation. According to Von Pinho et al. (2008), there is linear relation between grain productivity and plants density. The authors found that for each increase of 1,000 plants ha-1 in plant population, there was increase in grains productivity.
For the late season corn, the increase on plant heightand the thousand weight grains with the treatment in inoculation presence, influenced to culminate in higher productivity. Higher plants tend to be more productive because suffer less stress during the development and accumulate greater amounts of reservation in the stalk (Silva et al., 2006). According to Braccini et al. (2012) the corn inoculation with A. brasilense promotes increase in plant height and in corn grains productivity, when compared to the control group.
The foliar inoculation with the A. brasilense bacterium proved to be advantageous for the corn crop, and therefore, an option for the farmer. Because of the large volume of products used in seed (fungicide, insecticide, plant growth regulators) and the lack of information on the degree of interference of these products on the bacteria, it becomes pertinent the study of other inoculation forms on crops. Other studies that verify the influence of A. brasilense bacteria in foliar application on the physiology of the shoot and root of crops should be encouraged.
The authors have not declared any conflict of interests.
REFERENCES
|
Argenta G, Silva PRF, Bortolini CG (2001). Teor de clorofila na folha como indicador do nível de N em cereais. Ciênc. Rural 31(3):715-722.
Crossref
|
|
|
|
Assad ED, Sano EE, Matsumoto R, Castro LHR, Silva FAM (1993). Veranicos da região dos cerrados brasileiros frequência e probabilidade de ocorrência. Pesqui. Agropecu. Bras. 28(9):993-1003.
|
|
|
|
|
Bartchechen A, Fiori CCL, Watanabe SH, Guarido RC (2010). Efeito da inoculação de Azospirillum brasiliense na produtividade da cultura do milho (Zea mays L). Campo Digital 5(1):56-59.
|
|
|
|
|
Bashan Y, de Bashan LE (2005). Plant growth-promoting. Encycl. soils Environ. 1:103-115.
|
|
|
|
|
Bashan Y, Bashan LE (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth – a critical assessment, Adv. Agron. 108:77-136.
Crossref
|
|
|
|
|
Bashan Y, Holguin G, Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microb. 50:521-577.
Crossref
|
|
|
|
|
Basi S (2013). Associação de Azospirillum brasilense e de nitrogênio em cobertura na cultura do milho. 50f. Dissertação (Mestrado em Agronomia), Universidade Federal do Centro-Oeste, Guarapuava
View
|
|
|
|
|
Biari A, Gholami A, Rahmani HA (2008). Growth Promotion and Enhanced Nutrient Uptake of Maize (Zea mays L.) by Application of Plant Growth Promoting Rhizobacteria in Arid Region of Iran. J. Biol. Sci. 8(6):1015-1020.
Crossref
|
|
|
|
|
Blackmer TM, Schepers JS (1995). Use of a Chlorophyll Meter to Monitor Nitrogen Status and Schedule Fertigation for Corn. J. Prod. Agric. 8(1):56-60.
Crossref
|
|
|
|
|
Braccini AL, Dan LGM, Piccinin GG, Albrecht LP, Barbosa MC, Ortiz AHT (2012). Seed inoculation with Azospirillum brasilense, associated with the use of bio-regulators in maize. Rev. Caatinga 25(2):58-64.
|
|
|
|
|
Brachtvogel EL, Pereira FRS, Cruz SCS, Abreu ML, Bicudo SJ (2012). População, arranjo de plantas uniforme e a competição intraespecífica em milho. Rev. Trop. Cienc. Agra. Biol. 6(1):75-83.
|
|
|
|
|
Cabral PDS, Amaral Júnior AT, Freitas ILJ, Ribeiro RM, Silva TRC (2016). Relação causa e efeito de caracteres quantitativos sobre a capacidade de expansão do grão em milho-pipoca. Rev. Cienc. Agron. 47(1):108-117.
|
|
|
|
|
Cantarella H (2007). Nitrogênio. In: Novais RF, Alvarez VVH, Barros NF, Fontes RLF, Cantarutti RB, Neves JCL (Eds.). Fertilidade do solo. Viçosa: SBCS: pp. 375-470.
|
|
|
|
|
Cantarella H, Furlani PR (1997). Cereais. In: Raij B van, Cantarella H, Quaggio JA, Furlani AMC (Eds.). Recomendações de calagem e adubação para o Estado de São Paulo. 2.ed. Campinas: IAC, 285 p. (Boletim técnico, 100).
|
|
|
|
|
Carvalho IDE, Ferreira PV, Silva JP, Costa KDS, Oliveira FS (2015). Comportamento produtivo de genótipos de milho (Zea mays L.) em diferentes espaçamentos sob adubação orgânica. Agropecu. Cient. Semiárido 11(1):97-107.
|
|
|
|
|
Cavallet LE, Pessoa ACS, Helmich J, Helmich PR, Ost CF (2000). Produtividade do milho em resposta à aplicação de nitrogênio e inoculação das sementes com Azospirillum spp. Rev. Bras. Eng. Agric. Ambient. 4(1):129-132.
Crossref
|
|
|
|
|
Coelho AM (2008). Cultivo do Sorgo: nutrição e adubação. Sete Lagoas: Embrapa Milho Sorgo. Available at:
View
|
|
|
|
|
Costa NM, Andreotti M, Gameiro RA, Pariz CM, Buzetti S, Lopes SM (2012). Adubação nitrogenada no consórcio de milho com duas espécies de braquiária em sistema plantio direto. Pesqui. Agropecu. Bras. 47(8):1038-1047.
Crossref
|
|
|
|
|
Costa RRGF, Quirino GSF, Naves DCF, Santos CB, Rocha AFS (2015). Efficiency of inoculant with Azospirillum brasilense on the growth and yield of second-harvest maize. Pesqui. Agropecu. Trop. 45(3):304-311.
Crossref
|
|
|
|
|
Cruz JC, Pereira FIA, Queiroz LR (2013). Milho: cultivares para 2013/2014. Sete Lagoas: Embrapa Milho e Sorgo. Available at:
View
|
|
|
|
|
Cruz JC, Pereira Filho IA, Alvarenga RC (2015). Preparo do solo e plantio. In: Borém A, Galvão JCC, Pimentel MA (Eds.). Milho: do plantio à colheita. 1.ed. Viçosa: UFV: 77-107.
|
|
|
|
|
Cunha FN, Silva NF, Bastos FJC, Carvalho JJ, Moura LMF, Teixeira MB, Rocha AC, Souchie EL (2014). Efeito de Azospirillum brasilense na produtividade de milho no sudoeste goiano. Rev. Bras. Milho Sorgo 13(3):261-272.
Crossref
|
|
|
|
|
Dotto AP, Lana MC, Steiner F, Francoloso JF (2010). Produtividade do milho em resposta à inoculação com Herbaspirillum seropedicae sob diferentes níveis de nitrogênio. Rev. Bras. Cienc. Agrar. 5(3):376-382.
Crossref
|
|
|
|
|
Duarte AP, Henriques DR, Corrêa PC, Paterniani MEAGZ (2007). Produtividade, aparência, densidade e suscetibilidade à quebra dos grãos em híbridos de milho, na safrinha. Rev. Bras. Milho Sorgo 6(2):174-185.
Crossref
|
|
|
|
|
Duarte AP, Kurihara CH, Cantarella H (2013). Adubação do Milho Safrinha em Consórcio com Braquiária. In: Ceccon G (Org.). Consórcio Milho-Braquiária. Brasília: Embrapa, cap. 6:113-142.
|
|
|
|
|
Fageria NK (1989). Solos tropicais e aspectos fisiológicos das culturas. Brasília: EMBRAPA-CNPAF: 425 p
|
|
|
|
|
Fancelli AL (2015). Ecofisiologia, fenologia e implicações básicas de manejo. In: Borém A, Galvão JCC, Pimentel MA (Eds.). Milho: do plantio à colheita. 1.ed. Viçosa: UFV: 50-76.
|
|
|
|
|
Farinelli R, Lemos LB (2012). Nitrogênio em cobertura na cultura do milho em preparo convencional e plantio direto consolidados. Pesqui. Agrocu. Trop. 42(1):63-70.
Crossref
|
|
|
|
|
Fernandes FCS, Buzetti S, Arf O, Andrade JAC (2005). Doses, eficiência e uso de nitrogênio por seis cultivares de milho. Rev. Bras. Milho Sorgo 4(2):195-204.
Crossref
|
|
|
|
|
Fornasieri-Filho D (2007). Manual da cultura do milho. 1. ed. Jaboticabal: Funep: 273 p.
|
|
|
|
|
Fukami J, Nogueira MA, Araújo RS, Hungria M (2016). Accessing inoculation methods of maize and wheat Azospirillum brasilense. AMB Express 6(3):1-13.
Crossref
|
|
|
|
|
Fulchieri M, Lucangeli C, Bottini R (1993). Inoculation with Azospirillum lipoferum affects growth and gibberellin status of corn seedling roots. Plant. Cell. Physiol. 34:1305-1309.
|
|
|
|
|
Gazola D, Zucareli C, Silva RR, Fonseca ICB (2014). Aplicação foliar de aminoácidos e adubação nitrogenada de cobertura na cultura do milho safrinha. Rev. Bras. Eng. Agric. Ambient. 18(7):700-707.
Crossref
|
|
|
|
|
Goes RJ, Rodrigues RAF, Takasu AT, Arf O (2014). Fontes e doses de nitrogênio em cobertura para a cultura do milho em espaçamento reduzido. Agrarian 7(24):257-263.
|
|
|
|
|
Hartmann A (1988). Ecophysiological aspects of growth and nitrogen fixation in Azospirillum sp. Plant Soil 110(2):225-238.
Crossref
|
|
|
|
|
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010). Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331:413-425.
Crossref
|
|
|
|
|
Kappes C, Carvalho MAC, Yamashita OM, Silva JAN (2009). Influência do nitrogênio no desempenho produtivo do milho cultivado na segunda safra em sucessão à soja. Pesqui. Agropecu. Trop. 39(3):251-259.
|
|
|
|
|
Kappes C, Orf O, Arf MV, Ferreira JP, Dal Bem EA, Portugal JR, Vilela RG (2013). Inoculação de sementes com bactéria diazotrófica e aplicação de nitrogênio em cobertura e foliar em milho. Semina: Ciênc. Agrárias 34(2):527-538.
|
|
|
|
|
Lana MC, Dartora J, Marini D, Hann JE (2012). Inoculation with Azospirillum, associated with nitrogen fertilization in maize. Rev. Ceres 59(3):399-405.
Crossref
|
|
|
|
|
Lange A, Carvalho JLN, Damin V, Cruz JC, Guilherme LRG, Marques JJ (2006). Doses de nitrogênio e de palha em sistema plantio direto de milho no cerrado. Rev. Ceres 53(306):171-178.
|
|
|
|
|
Lima AGS, Mendes CR, Nascimento R, Lopes NF, Carvalho MAP (2009). Avaliação bioquímica de plantas de milho pulverizadas com ureia isolada e em associação com aminoácidos. Rev. Ceres 56(3):358-363.
|
|
|
|
|
Maestro PR, Buzetti S, Teixeira Filho MCM, Garcia CM, Rodrigues MAC, Lino ACM, Andreotti M (2014). Aplicação de ureia revestida em cobertura no milho irrigado sob sistema de semeadura direta. Rev. Bras. Ciênc. Agrar. 9(2):192-199.
Crossref
|
|
|
|
|
Marini D, Guimarães VF, Dartora J, Lana MC, Pinto JAS (2015). Growth and yield of corn hybrids in response to association with Azospirillum brasilense and nitrogen fertilization. Rev. Ceres 62(1):117-123.
Crossref
|
|
|
|
|
Mazzuchelli RCL, Sossai BF, Araujo FF (2014). Inoculação de Bacillus subtilis e Azospirillum brasilense na cultura do milho. In: Colloquium Agrariae 10(2):40-47.
Crossref
|
|
|
|
|
Meira FA, Buzetti S, Andreotti M, Arf O, Sá ME, Andrade JAC (2009). Fontes e épocas de aplicação do nitrogênio na cultura do milho irrigado. Semina: Cienc. Agrar. 30(2):275-284.
Crossref
|
|
|
|
|
Moda LR, Santos CLR, Flores RA, Borges BMMN, Andrioli I, Prado RM (2014). Resposta do milho cultivado em sistema de plantio direto à aplicação de doses de nitrogênio e cultivo de plantas de Cobertura em pré-safra. Biosci. J. 30(1):178-187.
|
|
|
|
|
Morais TP, Brito CH, Brandão AM, Rezende WS (2016). Inoculation of maize with Azospirillum brasilense in the seed furrow. Rev. Cienc. Agron. 47(2):290-298.
|
|
|
|
|
Moreira FMS, Silva K, Nóbrega RSA, Carvalho F (2010). Bactérias diazotróficas associativas: diversidade, ecologia e potencial de aplicações. Comunicata Sci. 1(2):74-99.
|
|
|
|
|
Môro GV, Fritsche NR (2015). Importância e usos do milho no Brasil. In: Borém A, Galvão JCC, Pimentel MA (Eds.). Milho: do plantio à colheita. 1.ed. Viçosa: UFV: 09-25.
|
|
|
|
|
Mota MR, Sangoi L, Sshenatto E, Giordani W, Boniatti CM, Dall'lgna (2015). Fontes estabilizadas de nitrogênio como alternativa para aumentar o rendimento de grãos e a eficiência de uso do nitrogênio pelo milho. Rev. Bras. Cienc. Solo 39:512-522.
Crossref
|
|
|
|
|
Okon Y, Labandera-Gonzalez CA (1994). Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 26(12):1591-1601.
Crossref
|
|
|
|
|
Portugal JR, Peres AR, Rodrigues RAF (2015). Aspectos climáticos no feijoeiro. In: Arf O, Lemos LB, Soratto RP, Ferrari S (Eds.) Aspectos gerais da cultura do feijão Phaseolus vulgaris L. Botucatu: FEPAF, cap. 4:65-75.
|
|
|
|
|
Puente ML, Garcia JE, Alejandro P (2009). Effect of the bacterial concentration of Azospirillum brasilense in the inoculum and its plant growth regulator compounds on crop yield of corn (Zea mays L.) in the field. World J. Agric. Sci. 5(5):604-608.
|
|
|
|
|
Purwanto, Minardi S, Supriyadi (2015). Optimization of Nitrogen Fertilization Input on Zea mays L. Cultivation through the Biological Inhibition of Nitrification. Agri. Sci. 6:201-207.
|
|
|
|
|
Raij B, Cantarella H (1997). Cereais. In: Raij B van, Cantarella H, Quaggio JA, Furlani AMC (Eds.). Recomendações de calagem e adubação para o Estado de São Paulo. 2 ed. Campinas: IAC, 285 p.
|
|
|
|
|
Repke RA, Cruz SJS, Silva CJ, Figueiredo PG, Bicudo SJ (2013). Eficiência de Azospirillum brasilense combinada com doses de nitrogênio no desenvolvimento de plantas de milho. Rev. Bras. Milho Sorgo 12(3):214-226.
Crossref
|
|
|
|
|
Ritchie SW, Hanway JJ, Benson GO (2003). Como a planta de milho se desenvolve. Inf. Agron. 103:1-19.
|
|
|
|
|
Rodrigues LFOS, Guimarães VF, Silva MB, Pinto Junior AS, Klein, J, Costa, ACPR (2014). Características agronômicas do trigo em função de Azospirillum brasilense, ácidos húmicos e nitrogênio em casa de vegetação. Rev. Bras. Eng. Agric. Ambient. 18(1):31-37.
Crossref
|
|
|
|
|
Rudnick P, Meletzus D, Green A, He L, Kennedy C (1997). Regulation of nitrogen fixation by ammonium in diazotrophic species of proteobacteria. Soil. Biol. Biochem. 29(5/6):831-841.
Crossref
|
|
|
|
|
Saikia SP, Bora D, Goswami A, Mudoi KD, Gogoi A (2012). A review on the role of Azospirillum in the yield improvement of non-leguminous crops. Afr. J. Microbiol. Res. 6(6):1085-1102.
|
|
|
|
|
Sangoi L, Silva LMM, Mota MR, Panison F, Schmitt A, Souza NM, Giordani W, Schenatto DE (2015). Desempenho Agronômico do Milho em Razão do Tratamento de Sementes com Azospirillum sp. e da Aplicação de Doses de Nitrogênio Mineral. Rev. Bras. Cienc. Solo 39(4):1141-1150.
Crossref
|
|
|
|
|
Santos HG, Jacomine PKT, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Cunha TJF, Oliveira JB (2013). Sistema brasileiro de classificação de solos. 3. ed. Brasília: Embrapa, 353 p.
|
|
|
|
|
Silva DA, Vitorino ACT, Souza LCF, Gonçalves MC, Roscoe R (2006). Culturas antecessoras e adubação nitrogenada na cultura do milho, em sistema plantio direto. Rev. Bras. Milho Sorgo 5(1):75-88.
Crossref
|
|
|
|
|
Sousa ALB, Ferreira LM (2015). Competição de cultivares de milho em sistema de plantio direto (SPD) na região do Alto Rio Negro, Amazonas. Rev. Educ. Cienc. Tecnol. IFAM 9(1):59-69.
|
|
|
|
|
Souza WCRD (2014). Manejo da adubação nitrogenada na cultura do milho pelo uso da inoculação com azospirillum brasilense em consórcio com capim xaraés. Available at:
View
|
|
|
|
|
Torres JLR, Faria MV, Lana RMQ, Prudente T, Vasconcelos AC (2015). Corn agronomic evaluation under different doses of nitrogen and seed inoculation in savanna. Afr. J. Agric. Res. 10(26):2568-2575.
Crossref
|
|
|
|
|
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moenne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dye F, Prigent-Combaret C (2013). Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4(356):1-19.
Crossref
|
|
|
|
|
Valderrama M, Buzetti S, Teixeira Filho MCM, Bebett CGS, Andreotti M (2014). Adubação nitrogenada na cultura do milho com ureia revestida por diferentes fontes de polímeros. Semina: Cienc. Agrar. 35(2):659-670.
Crossref
|
|
|
|
|
Von Pinho RG, Gross MR, Steola AG, Mendes MC (2008). Adubação nitrogenada, densidade e espaçamento de híbridos de milho em sistema plantio direto na região sudeste do Tocantins. Bragantia 67(3):733-739.
Crossref
|
|