ABSTRACT
Effects of annual spraying of copper (Cu) fungicide by cocoa farmers in Nigeria needs immediate investigation to avoid copper toxicity which will affect uptake of other essential nutrients for plant growth. Laboratory and Screenhouse studies were carried out to investigate the effects of Cu application on availability of P, Zn, Fe and growth of maize. In the laboratory, copper was applied as CuSO4 at 0, 10, 20, 30, 40 and 50 mg Cu kg-1 to 100 g soil and left for 5 weeks for equilibration while in the Screenhouse, the same rate of Cu was thoroughly mixed with 1 kg soil in a plastic container. Maize was used as the test crop and growth parameters were monitored. Soil and tissue samples were analysed for Cu, P, Zn and Fe at the end of Laboratory and Screenhouse experiments. The experiment was arranged in a completely randomized design and replicated three times. Data obtained were subjected to analysis of variance; differences in means were tested using the Duncan Multiple Range Test. The results from Laboratory and Screenhouse experiments showed significant decrease in soil available P, Zn and Fe as rates of Cu increase over control experiment. The effect was more pronounced at application rate above 20 mg Cu kg-1. Gradual decrease in maize plant height, stem girth, leaf areas index, P, Zn and Fe uptake were observed as rate of Cu application increased. For instant, at application rate of 10 and 20 mgkg-1, available P uptake was 5.49 mg/pot and 3.08 mg/pot respectively. The negative impact of Cu accumulation on available P was consistent in all the experiments. The result of the experiment clearly revealed strong negative impact of excess Cu on availabilities and uptake of P, Zn and Fe in soil.
Key words: Copper, fungicides, application rate, Screenhouse, nutrients.
Copper is an essential element for various metabolic processes in soils (Scheiber et al., 2013). It is required only in trace amounts and becomes toxic at high concentrations (Delas, 1963; Alva and Chen, 1995). The critical copper deficiency level in vegetative plant parts is generally 3 to 5 mg kg-1 dry weight (Robson and Reuther, 1981). Contrastingly, high Cu levels may inhibit root elongation and damage of root cell membranes of non-tolerant plants (Wainwright and Woolhouse, 1977). Particularly, cocoa farmers in Nigeria apply Cu fungicides annually to curb the menace of black pod disease, which is caused by Phythophthora spp. However, high application rates of Cu reduced soil nutrients which affects plant productivity (Lindsay, 1974). When copper gets into the soil, it binds strongly to organic matter, clay minerals and hydrated oxides of iron (Fe), aluminium (Al) and manganese (Mn) (Schnitzer, 1969), and either reduces the concentration of these nutrients (Fe, Mn and Al) in the soil or makes them unavailable for plant uptake. For example, Savithri et al. (2003) found that as the copper content in the soils of grape farms increased with continuous application of copper fungicides in form of Bodeaux mixture (CuSO4 + Ca(OH)2), the amount of micronutrient such as zinc, manganese and iron decreased. Similarly, some macronutrients are also affected, for example, available phosphorus contents of the soils decreased with application of Cu fungicide at both surface and subsurface layers (Spencer, 1966). Akinnifesi et al. (2006) reported that increasing copper content of soils in cocoa plantations reduced the amount of plant available phosphorus that caused nutrient imbalance, which may affect nutrient uptake by plants. In fact, increasing amounts of Cu in soil may cause translocation of Cu to various vegetative parts. Grain crops such as maize requires relatively high amount of Cu to improve quality and yield but at some certain level, it becomes toxic to the plants (Rogerio et al., 2013). Thus, this study was carried out to determine both the interactions between increasing application rates of Cu and soil available P, Zn and Fe as well as to determine the effect of increasing Cu application rates on nutrient uptake (P, Zn and Fe) and plant growth of maize plant.
Soil samples (0 to 15 cm) were collected from Teaching and Research Farm (T&RF), Obafemi Awolowo University, Ile-Ife in Southwestern Nigeria. The T&RF is situated within the rain forest zone and located between latitudes 7° 32’ N and 7° 33’ N and longitudes 4° 32’ E and 4° 40’ E. The altitude is 244 m above the sea level. The local classification of the soil is Iwo series while the USDA equivalent is Ultisol. The soil samples were air dried and subjected to physical and chemical analysis. These were particle size analysis, soil pH, organic matter, exchangeable cations, Total N, available P and micronutrients. Experiments were carried out for five weeks at the Department of Soil Science and Land Resources Management, Obafemi Awolowo University, Ile-Ife, Nigeria, to determine the effect of Cu on soil P, Zn and Fe, growth parameters and nutrient uptake of maize plant. The particle size analysis was carried out using the modified hydrometer method by Bouyoucous (1965). Soil pH in 0.01 M CaCl2 was determined using the soil-solution ratio 1:2 (Peech et al. 1953). The soil organic matter content of the soil was determined using the Chromic Acid Digestion Method by Walkley-Black (1934) as reviewed by Allison (1965). Exchangeable basic cations (Ca, Mg, K and Na) were extracted with 1 N NH4OAc at pH 7 (Thomas, 1982) while available P was determined by Bray 1 method (Bray and Kurtz, 1945). Available Cu, Zn and extractable Fe were extracted with 0.1 N HCl (Wears and Sommer, 1948). The micronutrients (Cu, Zn and Fe), Ca and Mg were determined using an Atomic Absorption Spectrophotometer (AAS) (PG 990 model) while Na, K were determined using Flame photometer. Laboratory experiment was carried out for 5 weeks in order to assess the effect of addition of copper to soil on other soil chemical properties. Copper was applied as CuSO4 at 0, 10, 20, 30, 40 and 50 mg Cu kg-1 to 100 g soil and left for 5 weeks to equilibrate. The moisture content was determined by filling 1000-ml flask with soil and cotton wool was spread on the soil. 100 ml water was poured into the flask and covered with polythene bags with 6 holes on it for good aeration. The experiment was left for 48 h. Soil sample was taken from the middle of the flask and oven-dried until constant weight was achieved. The soil field moisture content was then determined.
In the screenhouse, the same rate of Cu was applied to 1 kg soil in a plastic container and moistened to 70% field moisture capacity to determine the effects of increasing rate of Cu application on nutrient uptake and plant growth of maize. The experiment was replicated three times and arranged in completely randomized design. 17 ml of water per 100 g of soil was calculated for field moisture content while 70% of that gave 12 ml. 120 mls of deionized water was then added to 1 kg soil. The watering was done every 3 days throughout the experimental period in the Screenhouse to maintain moisture content. Maize (Zea mays L.) was used as the test crop and growth parameters (plant height, stem girth and leaf areas) were monitored forthnightly. Soil and tissue samples were analysed for Cu, P, Zn and Fe. All the experiments were carried out in triplicates and arranged in completely randomized design. Data obtained were subjected to analysis of variance; differences in means were tested using the Duncan Multiple Range Test.
Physical and chemical properties of the soil used for laboratory and screenhouse studies
The results of physical and chemical properties of the soil used for the experiment are presented in Table 1. The soil was slightly acidic. The soil organic matter (OM) was low while total nitrogen was moderate. The exchangeable bases were moderately high. The available P was equally high while that of available Cu was moderately high. The extractable Fe and available Zn were generally high. In general, the soil may be considered as moderately fertile.
Effects of added Cu on available P, Zn and Fe in the incubation experiment
The available Cu increased significantly (p<0.05) over the Control as rates of Cu application increased (Table 2). Soil P, Zn and Fe decreased as Cu rates increased. Available P showed significant (p<0.05) decrease with increasing rates of Cu application over the Control experiment. For instant, at 0 mg Cu kg-1 and 10 mg Cu kg-1, available P were 27.66 and 26.35 mgkg-1 respectively. The result showed antagonistic interaction between available Cu and P. Similarly, application of Cu resulted in significant (p<0.05) decrease in available Zn values when compared with the Control. This might be due to the fact that Cu and Zn have similar charge and ionic size. Robson and Pitman (1983) reported that such interactions are more common between nutrients of similar size, charge and geometry of coordination and electronic configuration. Similarly, available Fe decreased significantly (p<0.05) following the application of different rates of Cu when compared with the Control (Table 2).
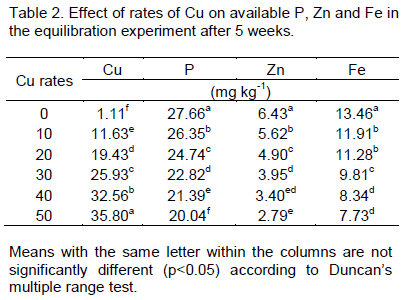
Effect of Cu additions on available P, Zn and Fe in the screenhouse experiment
Soil available P, Zn and Fe
The experiment was carried out in the Screenhouse for five weeks after which the soil was analyzed to determine the interactions among Cu, Zn, Fe and P following the application of different rates of Cu (Table 3). The Cu content in the soil after harvesting increased significantly (p<0.05) as the rate of application increased when compared with the Control. Significant (p<0.05) decrease in available P after harvesting over the Control was also observed. It was observed that significant difference (p<0.05) was recorded among Cu treated pots. Application rate above 20 mg Cu kg-1 significantly reduced available P. For instance, available P at 20 mg Cu kg-1 was 18.09 mg P kg-1 while 12.76 mg kg-1 was recorded at application rate of 30 mg Cu kg-1. Similarly, significant decrease in available Zn (p<0.05) was observed following the application of different rates of Cu to the soil over the Control. As copper and Zn have similar ionic size, the presence of Cu in the soil may, therefore, have some negative effects on Zn as both elements compete for the same adsorption sites. There was no significant (p>0.05) difference in extractable Fe content following the application of 10 mg/kg of Cu salt when compared with the Control but significant decrease (p<0.05) was recorded as application rate increased beyond 10 mg Cu kg-1. Significant difference was recorded in extractable Fe among Cu treated pots, for example, 8.17 mgkg-1 and 7.04 mgkg-1 at 30 and 40 mg Cu kg-1 were recorded. Presence of excess Cu in the soil has negative effect on extractable Fe content as earlier observed in the equilibration study (Table 3). Cu applications changed availability of nutrients, which affected plant nutrition (that is, total contents in biomass). This points to the fact that an excessive use of Cu fungicides may reduce plant productivity.
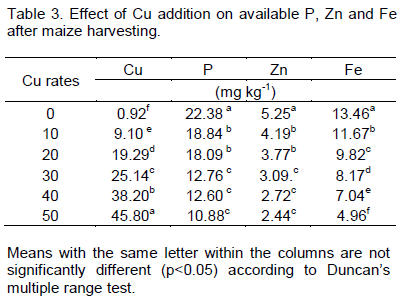
Growth of maize plant
The effects of different rates of Cu on growth parameters, viz, plant height, stem girth and leaf areas and nutrient uptake by maize were also monitored in the screenhouse. The plant height showed no significant difference (p>0.05) in all the Cu treated pots except at the application rate of 50 mg Cu kg-1 when compared with the Control (Table 4). This confirms earlier work reported by Karataglis and Babalonas (1985), that plant height, shoot and root biomass, flower and fruit production decreased with increasing Cu concentration. This suggests that excess Cu in soil affects uptake of other nutrients for plant height development and invariably results into low productivity. No significant effect (p>0.05) of added Cu was found on stem girth when compared with the Control. The results showed gradual reduction in stem girth following different rates of Cu application to soil. Leaf area index showed significant decrease (p<0.05) at 30, 40 and 50 mgkg-1 Cu treated pot when compared with control. However, there was no significant effect (p>0.05) of added Cu on leaf area index at 10 and 20 mg/kg Cu treated pot when compared with the Control. This implies that the presence of Cu in the soil had negative impact on the ability of maize plant to absorb nutrients necessary for leaf area development. This is in line with a report of Zheng et al. (2004) that excessive Cu reduced plant root length, root dry weight, total dry weight, and root to shoot ratio, leaf area and specific leaf area in three ornamental crops grown in solution culture.
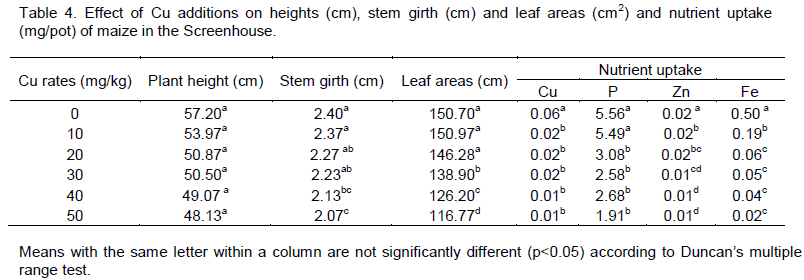
Nutrients uptake
There was no significant effect (p>0.05) of added Cu in Cu uptake by maize as Cu rates increased in all the Cu treated pots (Table 4). However, significant decrease (p>0.05) was observed when compared with the Control. This may be attributed to immobilization of Cu by humic substances in the soil. This result showed that increase in Cu content of the soil may lead to low absorption of Cu by plant. No significant effect of added Cu was observed in P uptake at application rate of 10 mg Cu kg-1 when compared with the Control. Significant decrease was observed among Cu treated pots, for instance, P uptake at 10 and 20 mg Cu kg-1 were 5.49 and 3.08 mgkg-1. There was gradual reduction in P uptake as rate of Cu increased. This result also supports the observation made in the equilibration study and analysis of soil after harvesting of maize plant in the Screenhouse. Significant decrease in Zn uptake was recorded in all the Cu treated pots over the Control. Gradual decrease was observed in Zn uptake as the rate of Cu application increases. This result follows the same pattern as observed in the equilibration study. Other studies have shown that Cu and Zn interact with each other due to antagonistic relationship (Dangarwala, 2001). The application of Cu significantly reduced the Fe content in the plant tissue in all the Cu treated pots over the Control, a finding similar to the observation in the equilibration study. The results were in agreement with those of Brar and Sekhon (1978), who observed that excess Cu antagonistically affected the translocation of Fe from stem to the leaves. Excess Cu in the soil may cause Fe chlorosis in plants and thereby affecting the productivity and biodiversity (Alva and Graham, 1991). It was reported that response of excess Cu has frequently been attributed to an interference with Fe metabolism (Yau et al., 1991; Ouzounidou et al., 1995). Kim et al. (1978) and Gonçalves et al. (2009) reported that the interference of heavy metals in excess amounts with normal Fe metabolism was known to induce physiological Fe deficiency.
The study investigated the effects of Cu fungicides on available P, Zn and Fe and on growth and nutrient uptake by maize (Zea mays L.). The results of the study showed that available Cu increased significantly over the Control as rate of Cu application increased. Significant decrease in available P was observed as rate of Cu increased. The result showed antagonistic relationship between available Cu and P as the rate of Cu application increased. It could be that accumulation of Cu hindered the uptake of P by the plant or reduced its availability in soil. Significant decrease was observed in Zn contents as the rate of Cu application increased. This relationship might be due to the fact that Cu and Zn have similar charges and ionic sizes. Significant decrease was also observed in extractable Fe content over the Control as the rate of Cu application increased. Gradual decrease in plant height, stem girth and leaf area index were recorded as the rate of Cu application increased. This pointed to the fact that presence of excess Cu in the soil prevented the absorption of essential nutrients needed for adequate development of plant height, stem girth and leaf area. There should be proper monitoring of Fe, P and Zn levels in soils after Cu applications to ensure nutrient balance because excess Cu may reduce plant productivity and thus reduce economical income by farmers.
The authors have not declared any conflict of interests.
REFERENCES
Akinnifesi TA, Asubiojo OI, Amusan AA (2006). Effects of fungicide residues on the physico-chemical characteristics of soils of a major cocoa-producing area of Nigeria. Sci. Total Environ. 366:876-879.
Crossref |
|
|
|
Allison LE (1965). Organic Carbon. In: Black et al. (ed.) Methods of Soil Analysis. Part 2 Monograph No 9. America Soc. Agro. Madison pp. 1367-1378. |
|
|
Alva AK, Chen Q (1995). Effects of external copper concentrations on uptake of trace elements by citrus seedlings. Soil Sci.159:59-64.
Crossref |
|
|
|
Alva A, Graham JH (1991). The role of copper in citriculture. Adv. Agron. 1:145-170. |
|
|
|
Bouyoucos GJ (1965). Hydrometer methods improved for making particle size analysis of soils. Soil Sci. Soc. Am. Proc. 26:917- 925. |
|
|
|
Brar MS, Sekhon GS (1978). Effect of zinc and copper application on the yield and micronutrient content of wheat. J. Ind. Soc. Soil Sci. 26:84-86. |
|
|
|
Bray RH, Kurtz LT (1945). Determination of total, organic and available form of Phosphorus in soils. J. Soil Sci. 59:45-59. |
|
|
|
Dangarwala RT (2001). Need for sustaining balanced supply of micronutrients in soil rather than their correction. J. Ind. Soc. Soil Sci. 49:647-652. |
|
|
|
Delas J (1963). The toxicity of copper accumulated in soils. Agrochemica 7:258-288. |
|
|
|
Gonçalves JF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, de Moraes Flores EM and Nicoloso FT (2009). Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Phy. Biochem. 47:814-821. |
|
|
|
Karataglis S, Babalonas D (1985). The toxic effects of copper on the growth of Solanum lycopersicum L. collected from Zn and Pb-soil. Angewandte Botanik 59:45-52. |
|
|
|
Kim BY, Kim KS, Kim BJ, Han KM (1978). Uptake and yield of heavy metal Cu, Ni, Cr, Co and Mn. Rep. Off. Rural Development pp. 1-10. |
|
|
|
Lindsay WL (1974). "Role of Chelation in Micronutrient Availability." In: The Plant Root and Its Environment, edited by EE Carson 507-524. Charlottesville: University Press of Virginia. Ornamental Crops in Solution Culture. Hortsci. 39:1116-1120. |
|
|
Ouzounidou G, Ciamporova M, Moustakas M, Karataglis S (1995). Responses of maize (Zea mays L.) plants to copper stress--I. Growth, mineral content and ultrastructure of roots. Environ. Exper. Bot. 35(2):167-176.
Crossref |
|
|
Peech M, Olsen RA, Bolt GH (1953). The significant of potentiometric measurements involving liquid junction in clay and soil suspension. Soil Sci. Soc. Am. Proc. 17:214-220.
Crossref |
|
|
|
Robson AD, Pitman MG (1983). Interactions between Nutrients in higher plants. Encyclopedia of plant physiology 15:147-180. |
|
|
|
Robson AD, Reuther DJ (1981). Diagnosis of copper deficiency and toxicity. In: Loneragan JF, Robson AD, Graham RD (Ed.). Copper in soils and plants. Orlando: Academic Press. pp. 287-312. |
|
|
|
Rogério HB, Luciane AT, Fábio RM, Márcio P, Samir OK, Daísa B (2013).Foliar copper uptake by maize plants: effects on growth and yield. Cienc. Rural vol.43 no.9 Santa Maria. |
|
Crossref |
|
|
|
|
Savithri P, Joseph BIJU, Poongothai S (2003). Effect of copper fungicide sprays on the status of micronutrient in soils of hot semi-arid region of India. Tamil Nadu Agricultural University, Coimbatore 641 003. |
|
|
Scheiber Ivo D, Ralf M, Julian FB (2013). "Chapter 11. Copper: Effects of Deficiency and Overload". In Astrid Sigel, Helmut Sigel and Roland K. O. Sigel. Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences 13:359-387.
Crossref |
|
|
Schnitzer M (1969). Reactions between fulvic acid, a soil humic compound and inorganic soil constituents. Soil Sci. Soc. Am. Proc. 33:75-81.
Crossref |
|
|
Spencer WF (1966). Effect of copper on yield and uptake of phosphorus and iron by citrus seedlings grown at various phosphorus levels. Soil Sci.102:296-299.
Crossref |
|
|
|
Thomas GW (1982). Exchangeable cations In.; AL Page, R. H. Miller, D. Keeney (Eds.): Methods of Soil Analysis. 22nd Edition. Medison: America Soci. Agron. 57-164. |
|
|
Wainwright SJ, Woolhouse HW (1977). Some physiological aspects of copper and zinc tolerance in Agrostis tenuis Sibth: Cell elongation and membrane damage. J. Exper. Bot. 28:1029-1036.
Crossref |
|
|
Walkley A, Black IA (1934). An examination of the method for determining soil organic matter and proposed modification of the chromic acid titration method. Soil Sci.37:29-38.
Crossref |
|
|
Wears JI, Sommer AL (1948). Acid extractable zinc of soils in relation to occurrence of zinc deficiency symptoms of corn: A method of analysis. Soil Sci. Soc. Am. Proc. 12:143-144.
Crossref |
|
|
|
Yau PY, Loh CF, Azmil IAR (1991). Copper toxicity of clove (Syzygium aromaticum (L.) Merr. and Perry) seedlings. Mardisson, J. Res. 19:49-53. |
|
|
|
Zheng YB, Wang LP and Dixon MA (2004). Response to copper toxicity for three ornamental crops in solution culture. Hortscience 39:1116-1120. |