ABSTRACT
Cowpea (Vigna unguiculata L. Walp) is a leguminous plant which is an important source of protein, grown in arid and semi-arid areas of Africa subject to climate changes including soil salinization. The current study aimed to test the hypothesis that screening for salt-tolerant Bradyrhizobim-cowpea combination would promise a salt tolerant symbiotic system. Four selected Bradyrhizobium were characterized for their abilities to grow freely in salt medium and for their symbiotic efficiency in improving cowpea growth in Leonard jars at 3 levels of salinity (0, 40 and 80 mM). The effectiveness of inoculated bacteria was assessed by measuring shoot and root dry matters, salt tolerance index, leaf chlorophyll content and leaf area. This study did not show a correlation between strains tolerance to high salt concentrations in free-living form and symbiotic efficiency on cowpea. Furthermore, results showed that all the strain improved root/shoot ratio (RSR) and yield of cowpea plants up to 40 mM. However at 80 mM, only LCM4767 and ORS3257 strains were efficient. Selected strains improved and/or maintained plant growth and production under salt stress. The results suggest that cowpea production can be improved by introduction of efficient legume-Bradyrhizobium symbiotic combinations in saline areas.
Key words: Salt stress-tolerance, Bradyrhizobium, cowpea, symbiosis, Senegal.
It is predicted that more than 50% of the arable land would be salinized by the year 2050 (Shrivastava and Kumar, 2015). To avoid or minimize arable land loss, saline soils have to be rehabilitated with crops tolerant to salinity (Yan et al., 2015). In arid and semi-arid regions, progressive salinization and drought are the major environmental factors limiting crop yield and arable land area. Salt stress is more chronic than drought stress (Tilakarathna and Raizada, 2017). Their negative impact is attributed to ion toxicity and associated osmotic stress.This excess ions affects plant systems, decreases yield and crop quality (Mahajan and Tuteja, 2005), especially legumes. Leguminous plants are classified as sensitive or moderately tolerant to salinity (Läuchli, 1984) and their ability to withstand salt stress differs from species to species (Lu et al., 2009). Among the legumes, cowpea (
Vigna unguiculata L. Walp) is a valuable component of farming systems in many areas because of its ability to restore soil fertility for succeeding cereal crops grown in rotation (Carsky et al., 2002; Tarawali et al., 2002; Sanginga et al., 2002). Cowpea is a major source of dietary protein that nutritionally complements staple low-protein cereal and tuber crops; it is a valuable and dependable commodity that produces income for farmers and traders (Singh et al., 2002; Langyintuo et al., 2003; Timko et al., 2007). Cowpea is widely grown in Senegal in Groundnut Basin, Senegal River Valley and Casamance region which are the most saline areas in Senegal (
Sadio, 1991).
According to Soil Science Office in Senegal, 1.7 million ha of arable land are affected by salinization (Anonyme, 1998) by this area increasing by the day, and it may get worse because of global climate change. Senegalese Agricultural Research Institute – ISRA is presently selecting cowpea cultivars resistant to water stress (Cissé and Hall, 2003). However, no program has focused on selection of higher yielding varieties in this salinization context (Cissé, personal communication). Cowpea is a moderately sensitive crop to salinity and exhibits greater salt tolerance during later stage of plant growth (Murillo-Amador et al., 2001). Cowpea is naturally associated with symbiosis with soil bacteria, rhizobia. The symbiotic microorganisms can help to overcome salinity stress on cowpea if their properties such as tolerance to saline conditions and genetic diversity are exploited. The eco-geographic study of associated rhizobia, already carried out by Krasova-Wade et al. (2014), has shown greater genetic diversity in the River Valley. However, cowpea-rhizobia symbiotic combinations able to survive in saline conditions are worthy studied (Thilakarathna and Raizada, 2017). Few studies have shown that many salt tolerant strains of rhizobia and bradyrhizobia increase uptake of low-molecular-weight organic solutes (glycine betaine and trehalose) at high NaCl concentrations (Wankhade et al., 1996; Diouf et al., 2015). These osmolytes allow N-fixing bacteria to square up to the changes in osmotic potential in the soil and mitigate detrimental impacts of salt in plant growth and productivity. Furthermore, in other studies, it was shown that N-fixation in saline environments depends on crop variety and rhizobia strain interactions (Argaw, 2014; Diouf et al., 2015). Thus, there is a need to develop highly salt-tolerant symbiotic associations. The objectives of this preliminary study was to evaluate the effect of inoculation of cowpea plants cultivated under 3 salt levels conditions with 4 bradyrhizobia in order to test the hypothesis that screening (from a wide collection) for salt-tolerance of cowpea-bradyrhizobia combination would promise a salt tolerant symbiotic system.
Biological material
Cowpea Melakh cultivar selected by Senegalese Agricultural Research Institute – ISRA for its fast growing cycle (45 days) and drought resistance was used in jar assays. Four native Bradyrhizobium spp. Strains: ORS3257, LCM3682, LCM3703 and LCM4767 from Laboratoire Commun de Microbiologie IRD-ISRA-UCAD collection, were used. The ability of the strains to infect cowpea was tested in Gibson tubes (Gibson, 1987). They were selected on the basis of different environment origins: ORS3257 from Groundnut Basin zone receiving 400 to 800 mm of rainfall per year (Krasova-Wade et al., 2003), LCM3682 from Senegal river valley zone receiving 100 to 300 mm of rainfall per year and containing slightly alkaline soils (Krasova-Wade et al., 2014), LCM3703 from soil with acidic pH (Soumare et al., 2013) and LCM4767 from soil characterized by high level of organic nitrogen (this study).
Salt tolerance test of Bradyrhizobium isolates
Bradyrhizobium isolates were screened for their tolerance to increased concentrations of NaCl on agar plates according to the method described by Krasova-Wade et al. (2014). Each strain was grown on yeast extracts-mannitol (YM) liquid media (Vincent, 1970) to logarithmic phase and the optical density at 600 nm (OD 600) was adjusted to 0.8. A serial dilution of 10-1 to10-6 was prepared, then 10 µl of 10-4 and 10-5 dilutions were spotted 9 times onto YM agar plates containing different concentrations of NaCl (0, 20, 40, 60, 80 and 100 mM corresponding to 0, 3.2, 6.4, 7.2, 8.5 and 12 dSm-1). The colonies growing were observed seven days after incubation in the dark at 37°C.
Bradyrhizobium viable count
The upper concentration at which bacterial growth was observed was selected for most probable number (MPN) counting. As mentioned earlier, all yeast mannitol (YM) cultured
Bradyrhizobium were reduced to the same lower optical density. Then, a serial dilution of 10
-1 to 10
-7 was prepared and 100 µl of diluted broths were spread over the yeast mannitol agar (YMA) + NaCl plates. Three replicates were used for each strain. Plates were incubated in the dark at 37°C for 7 days. Calculation of the number of viable rhizobia based on colony-forming units (CFU) per ml was done using the following formula:
No. of cells/ml = no. of colonies × dilution factor.
Leonard jar experiment
The experiment was carried out in Leonard jars in a greenhouse from July to August, 2015. The experimental design was completely randomized at 4 repetitions with 15 treatments, 4 Bradyrhizobium spp. strains and un-inoculated control and 3 salt growing conditions. The un-inoculated control received during the first 15 days after pre-germinated seeds transplantation of the complete + nitrogen (N) nutrient solution (pH 6.8) contained 3 mM of KNO3, 2.5 mM Ca(NO3)2 0.5 mM Ca(H2PO4)2, 1 mM MgSO4, 12 µM Fe (as Fe EDTA), 4 µM MnCl2, 22 µM H3BO3, 0.4 µM ZnSO4, 0.05 µM NaMoO4 and 1.6 µM CuSO4. Fifteen days after this, +N nutrient solution were replaced with -N solution with the same composition except for KNO3 which was replaced by KCl, to better evaluate the N supply of the inoculated strains.
Vermiculite preparation
Field capacity for 55 g of vermiculite corresponding to jar volume was determined. NaCl was added before pre-germinated seeds transplantation to the jars for a better appreciation of stress effect on cowpea at early growth seedling stage (Murillo-Amador et al., 2001). The salt was added to the nutrient-N solution with regards to the studied concentrations (0, 40 and 80 mM) before plant transplantation. The vermiculite-saline solution mixture was dried at room temperature before being distributed in the jars. Leonard jars with nutrient solution were covered with aluminum paper and autoclaved at 120°C for 20 min. The salinity gradient was verified with a salinometer (REFRACSEL 100) before pre-germinated seeds transplantation for each salt treatment.
Cowpea seeds were surface-sterilised as described by Krasova-Wade et al. (2003). The seeds were germinated in the dark in sterile Petri dishes containing 1% (wv-1) water agar at 28°C for 48 h. Pre-germinated seeds were transplanted to autoclaved glass jars at the rate of 1 seed per jar. Periodically, according to the plant uptake rate, the same volume of nutrient solution was added at the bottom of the Leonard jar, +N in the control and -N to jars that received 2 ml of bacteria broth inoculums containing approximately 109 cells ml-1 of each strain. Four strains: LCM4767, LCM3703, LCM3682 and ORS3257 were tested. To assess the supply of N obtained biologically in inoculated treatments, fifteen days after transplantation, the seedlings of the control were deprived of N source in the nutrient solution. A thin-layer of sterile polystyrene beads was placed on the surface of each jar to avoid evaporation and possible contaminations. Plants were cultivated in controlled environmental chambers with a 16 to 8 h light-dark photoperiod. The effectiveness of the inoculated Bradyrhizobium was assessed by measuring the following variables:
1. Chlorophyll content and leaf area which were measured three times every fifteen days. Chlorophyll levels per unit area were estimated using a SPAD 502 (Soil-Plant Analysis Development) on the 3 youngest fully expanded leaves and their leaf area was estimated by the method of Paul and Nair (2008);
2. Shoot dry matter (SDM), roots dry matter (RDM), nodule dry matter (NDM), nodule number (NN), number of pods (NP), pods dry yield (PDY), root/shoot ratio (RSR: RDM/SDM) and pod/number ratio (PNR) which were measured at the end of the experiment (45 DAS).
Salt tolerance index
Salt tolerance index (STI) was adapted from Garg and Singla (2004) method and calculated as mean total plant (shoot + root) dry mass of inoculated plants at different salt concentrations compared to the mean total plant dry mass of inoculated plants obtained without salt according to the formula: STI = (TDW at Sx/TDW at Si) × 100, where: TDW = total dry weight; Si = control treatment; Sx = x treatment.
Statistical analyses
Statistical analyses were performed by using XLSTAT software 2010. Results were analyzed by two-way analysis of variance (ANOVA). Significance was determined according to Student-Newman-Keuls test. The tolerance indices and standard deviations were also used to appreciate statistical significance results.
Screening of the Bradyrhizobium for salt tolerance and ability to sustain growth
The screening of strains for tolerance to salt stress shows 3 groups according to the upper limits of tolerance (Table 1). The LCM4767 strain started its growing at 20 mM of salt before the control without salt and stopped to grow at 40 mM. The strains ORS3257 and LCM3682 tolerated up to 60 mM, while LCM3703 strain showed its ability to grow in up to 100 mM NaCl. The number of CFU decreased with increase in salt concentration for all the strains. In the upper limit of salt tolerance corresponding to each strain, the number of cells remained relatively high (×107). There was no major difference of cells number between ORS3257 and LCM3682 strains.
Chlorophyll and leaf area index
In the first measurement, fifteen days after pre-germinated seeds transplantation (DAS), control treatment had SPAD values significantly higher than the inoculated treatments (Figure 1). The results confirm a fast availability of N from nutrient solution in the control and its rapid use in growth metabolism. On the other hand, the N resulting from biologic fixation will be accessible later, immediately after the establishment of the nodulation and the enzymatic activation. Thus, at the 30th and 45th DAS (second and third measurement, respectively), all inoculated plants presented the SPAD values and leaf area was significantly higher compared to control treatment, except for the treatment LCM3703*80 mM (Figure 1). The chlorophyll contents for inoculated plants were not statistically different among Bradyrhizobium strains treatments no matter the salinity level for all cases (0, 40 and 80 mM) (Figure 1). Leaf area showed significant differences among strains at 40 mM and especially at 80 Mm (Figure 2). Plants inoculated with LCM4767 strain maintained significantly higher leaf area followed by plants inoculated with the strain ORS3257 and finally the strain LCM3682. Treatment inoculated with strain LCM3703 at 80 mM presented the lowest leaf area when compared with the control (Figure 2).


Plant biomass and pod yield
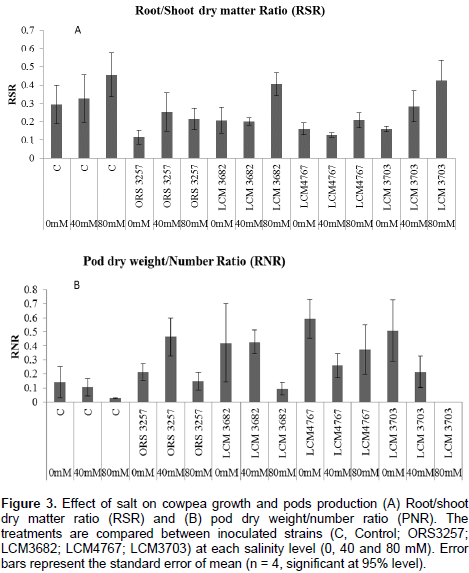
The results in Tables 2a and 3a show that the studied treatments significantly affected the growth and productivity parameters of the symbiotic cowpea system. In fact, the root/shoot ratio (RSR) of symbiotic combination: cowpea*ORS3257 and cowpea*LCM3703 in free-salt vermiculite was significantly lower than those of the control (Figure 3A and Table 2b). RSR increased with increase in the amount of salt and was generally higher in the control compared to inoculated plants, except for LCM3682*80 mM, LCM3703*40 mM and LCM3703*80 mM treatments which were significantly identical with the control treatment (Figure 3A). Results also show that the pod production pod/number ration (PNR) was significantly lower in the control compared to the inoculated plants in free-NaCl culture, except for ORS3257 and LCM3682 treatments (Figure 3B and Table 3b). Moreover, while the pods weight of the control and LCM3703 treatment decreased with increase in the amount of salt, yield of plants inoculated with ORS3257, LCM3682 and LCM4767 were significantly highest at 40 and 80 mM. The LCM4767 and ORS3257 strains lead to good productivity at 80 mM, followed by LCM3682 (Figure 3B and Table 3b). In contrast, no production was obtained with LCM3703 strain at 80 mM (Figure 3B and Table 3b).


Salt tolerance index of plants
Melakh cultivar tolerated up to 40 mM salt stress in the controlled conditions. Bradyrhizobium increased this level of tolerance when plants were inoculated with LCM4767 strain compared to the control (Table 4). The level of tolerance decreased for all of the treatments at 80 mM NaCl, but LCM4767 maintained good salt tolerance of inoculated plants.
Among the dietary legumes of the world, cowpea stands 6th in
consumption (Predeepa and Ravindran, 2010). In this study, the cultivar selected was Melakh, recommended for cropping in areas with low rainfall because of its short vegetative cycle (45 days) that supports rainfall instability and lengthening of rainfall breaks, which is a major factor in climatic deregulation (Cissé, 1997). This variety is widely used throughout Senegal. Also, recent studies have yielded data on these symbiotic capacities with inoculated strains in the field (Do Rego et al., 2015). The results of this study also showed its ability to withstand salt stress of up to 80 mM (8.5 dSm
-1) in controlled conditions, by developing as a resistance strategy, its root system, to cope with stress without lowering the vegetative production. Roots expansion improves the depth exploration of soil to reach wetter and less saline zones. The increases in RSR were more significant in the less efficient symbioses formed by strains LCM3682 and LCM3703 at 80 mM, and in non-inoculated plants. This is in accordance with Tejera et al. (2005) and could be interpreted as an imbalance of growth resulting in an expansion of the root system at the expense of shoot growth, thus leading to low pods production. However, RSR is almost stable at all level of salt for plants inoculated with LCM4767 and ORS3257 strains. The inoculations allowed an increase or maintained chlorophyll content per unit and leaf area, which is certainly linked to good N fixation. The highest values of chlorophyll content are often well correlated with highest rate of N use as already shown by Namvar et al. (2013).
In fact, N is a key component of the chlorophyll molecule, playing an important role in photosynthesis. Consequently, these results in a N status returns in higher biomass with better growth and better pods yield for the inoculated plants compared to non-inoculated plants. It was shown that cowpea exhibited no yield loss until salinity exceeded 4.9 dSm-1 (about 25 mM). But for each increase of 1 dSm-1 above this threshold, yield was reduced by 12% (Murillo-Amador et al., 2001). The results suggest that inoculum of Bradyrhizobium can establish effective N2-fixing symbioses at 80 mM (about 7.9 dSm-1) salinity and alleviate its depressive effect by improving 7 to 14 times the PNR values of yield when compared with non-inoculated cowpea. In addition, these results are confirmed by the salinity tolerance index which decreased in control plants with increase in the salt concentration of up to 80 mM, while the symbiotic combination inoculated with the strain LCM4767 tolerated the stress (STI=92).
Previous studies have shown that Bradyrhizobium could develop a wide range of phenotypic traits to salt resistance, in free-living culture. Nkot et al. (2015) found that all rhizobia isolates from Vigna subterranea were significantly affected by salt concentration with increasing salinity of up to 1% (160 mM), but some of them showed a weak growth capacity at 4% NaCl. Slow growing local isolates of soybean in Egyptian soils were the most sensitive and could not tolerate 0.5% NaCl (Youseif et al., 2014). All rhizobia isolates from cowpea in Libya were able to grow also in 0.5% NaCl (80 mM), but differently responded to levels above that (Abdelnaby et al., 2015).
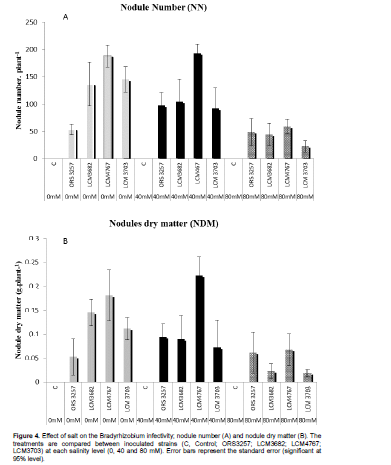
In the current study, strain behaviors in free-living form and their symbiotic capacities were compared through inoculations, highlighting four scenarios. The salt limit for LCM4767 was 40 mM in YEM Agar. However, it remained infective and efficient in symbiosis with cowpea for up to 80 mM of salt. The LCM 3703 strain has opposite phenotype. Indeed, this strain grows up to 100 mM in free-living form. However, in symbiosis, its infectivity (according to nodule number) and effectiveness (according to growth development) are greatly reduced in 80 mM NaCl resulting in significant decrease of nodulation (Figure 4). Both strains, ORS3257 and LCM3682, grow up to 60 mM salt on the YMA medium. In symbiosis, they allow the plant to maintain significant growth at 40 mM of salt. The results suggest that mechanisms used by these strains to tolerate salt stress in the free living form are different from mechanisms used in symbiosis.
The contrasting behavior of the inoculated strains shows that salt affects the infection and/or efficiency of symbiosis. There was no correlation between strains tolerance to high salt concentrations in free culture and symbiotic efficiency in cowpea. Florentino et al. (2010) reported similar results. The STI index of symbiotic system cowpea*LCM4767 was correlated with RSR report at 40 and 80 mM and higher than the one in the control and other treatments. Furthermore, some strains (LCM3703 for example in this study) maintained their infectivity in high salinity levels without any effect on plant growth. In this case, salinity leads to formation of non-functional nodules with abnormal structure and degradation of peribacteroid membrane (Bolaños et al., 2003). High variability among the studied strains suggests the need to select symbiotic combinations instead of tolerant strains or tolerant crop. In fact, the best results for symbiotic N2 fixation under salt stress are obtained if both symbiotic partners at different steps of their interaction (nodule formation, activity, etc.) resist such stress (Zahran, 1999). This is because legume-Rhizobium symbiosis and nodule formation on the legumes is more sensitive to salt or osmotic stress than the Rhizobium or the plant. The obtained results also show that the plant can increase the tolerance of the bacteria by providing a protective niche, the nodule.
The obtained results show that the selected cultivar, Melakh, can tolerate salinity of up to 80 mM at the seedling stage if inoculated with efficient Bradyrhizobium. The selected strains in this study improved plant growth in the absence of stress and/or can help to keep the growth in salty environment as compared to unstressed plants. However, the effect depends on the symbiotic combined performance. Therefore, cowpea cultivar selection for salt stress tolerance should systematically integrate the symbiotic aspect, namely its association with Bradyrhizobium partner. These results suggest that symbiotic combination should be used to improve crop production in saline areas. Further investigations are needed to identify efficient Bradyrhizobium for each cowpea cultivar in the field. This would provide scope for selection towards enhanced salinity tolerance of cowpea- Bradyrhizobium symbiotic system.
The authors have not declared any conflict of interests.
This work was supported by FIRST Grant of Ministry of Higher Education and Scientific Research, Senegal (Editions 2012 and 2013) and by National Agricultural and Agro-food Research Fund (FNRAA), Programme WAAPP Phase 2, Grant n° 16/2RA-RD/WAAPP2/FNRAA, Senegal.
REFERENCES
Abdelnaby M, Elnesairy NNB, Mohamed SH, Alkhayali YAA (2015). Symbiotic and phenotypic characteristics of rhizobia nodulaing cowpea (Vigna unguiculata L. Walp) grown in arid region of Libya (Fezzan). J. Environ. Sci. Eng. B 4 4:227-239.
https://doi.org/10.17265/2162-5263/2015.05.001 |
|
|
Argaw A (2014). Symbiotic effectiveness of inoculation with Bradyrhizobium isolates on soybean [Glycine max (L.) Merrill] genotypes with different maturities. Springer Plus 3:753.
https://doi.org/10.1186/2193-1801-3-753 |
|
|
|
Anonyme (1998). Programme d'action national de lutte contre la désertification. Ministère de l'environnement et de la protection de la nature. République du Sénégal 152p. |
|
|
Bola-os L, El-Hamdaoui A, Bonilla I (2003). Recovery of development and functionality of nodules and plant growth in salt stressed Pisum sativum – Rhizobium leguminosarum symbiosis by boron and calcium. J. Plant Physiol. 160:1493-1497.
https://doi.org/10.1078/0176-1617-01003 |
|
|
|
Carsky RJ, Vanlauwe B, Lyasse O (2002). Cowpea rotation as a resourse management technology for cereal-based systems in the savannas of West Africa. In: Challenges and Opportunities for Enhancing Sustainable Cowpea Production, ed. Fatokun, C.A., S.A. Tarawali BB, Singh PM, Kormawa, and M. Tamo M., Ibadan Nigeria. Proceedings of the World Cowpea Conference III held at the International Institute of Tropical Agriculture (IITA). pp. 252-266. |
|
|
Cissé N, Ndiaye M, Thiaw S, Hall AE (1997). Registration of Melakh cowpea. Crop Sci. P 37.
https://doi.org/10.2135/cropsci1997.0011183X003700060054x |
|
|
|
Cissé N, Hall AE (2003). Traditional Cowpea in Senegal, a Case Study 27p.http://www.fao.org/ag/AGP/AGPC/doc/publicat/cowpeaCisse/cowpeacissee.htm. |
|
|
Diouf D, Samba-Mbaye R, Lesueur D, Ba AT, Dreyfus B, de Lajudie P, Neyra M (2007). Genetic diversity of Acacia seyal Del. rhizobial populations indigenous to Senegalese soils in relation to salinity and pH of the sampling sites. Microbiol. Ecol. 54(3):553-566.
https://doi.org/10.1007/s00248-007-9243-0 |
|
|
Diouf F, Diouf D, Klonowska A, Le Queré A, Bakhoum N, Fall D, Neyra M, Parrinello H, Diouf M, Ndoye I, Moulin L (2015). Genetic and genomic diversity studies of Acacia symbionts in Senegal reveal new species of Mesorhizobium with a putative geographical pattern. Plos One, DOI:10.1371/journal.pone.0117667.
https://doi.org/10.1371/journal.pone.0117667 |
|
|
Do Rego F, Diop I, Sadio O, Da Sylva MC, Agbangba CO, Touré O, Kana K, Neyra M, Ndoye I, Krasova Wade T (2015). Response of Cowpea to Symbiotic Microorganisms Inoculation (Arbuscular Mycorrhizal Fungi and Rhizobium) in cultivated soils in Senegal. UJPS 3(2):32-42.
https://doi.org/10.13189/ujps.2015.030204 |
|
|
Florentino LA, de Sousa PM, Savana Silva J, Barroso Silva K, de Souza Moreira FM. (2010). Diversity and efficiency of Bradyrhizobium strains isolated from soil samples collected from around Sesbania virgata roots using cowpea as trap species. R. Bras. Cienc. Solo Rev. 34:1113-1123.
https://doi.org/10.1590/S0100-06832010000400011 |
|
|
Garg N, Singla R (2004). Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz. J. Plant Physiol. 16(3):137-146.
https://doi.org/10.1590/S1677-04202004000300003 |
|
|
|
Gibson AH (1987). Evaluation of nitrogen fixation by legumes in the greenhouse and growth chamber. In Symbiotic Nitrogen Fixation Technology, ed. A. H. Gibson New York, Marcel Dekker pp. 321-363. |
|
|
Krasova-Wade T, Ndoye I, Braconnier S, Sarr B, Lajudie P, Neyra M (2003). Diversity of indigenous bradyrhizobia associated with three cowpea cultivars (Vigna unguiculata (L). Walp.) grown under limited and favorable water conditions in Senegal (West Africa). Afr. J. Biotechnol. 2(1):13-22.
https://doi.org/10.5897/AJB2003.000-1003 |
|
|
Krasova-Wade T, Le Quéré A, Laguerre G, N'Zoué A, Ndione JA, do Rego F, Sadio O, Ndoye I, Neyra M (2014). Eco-geographical diversity of cowpea bradyrhizobia in Senegal is marked by dominance of two genetic types. Appl. Microbiol. Syst. 37:129-139.
https://doi.org/10.1016/j.syapm.2013.10.002 |
|
|
Langyintuo AS, Lowenberg-DeBoer J, Faye M, Lambert D, Ibro G, Moussa B, Kergna A, Kushwaha S, Musa S, Ntoukam G (2003). Cowpea supply and demand in West Africa. Field Crops Res. 82:215-231.
https://doi.org/10.1016/S0378-4290(03)00039-X |
|
|
|
Läuchli A (1984). Salt exclusion: An adaptation of legumes for crops and pastures under saline conditions. In Salinity Tolerance in Plants. Strategies for Crop Improvement. R.C. Staples and G.H. Toenniessen ed. John Wiley & Sons. New York pp. 171-187. |
|
|
Lu S, Su W, Li H, Guo Z. (2009). Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2- and NO-induced antioxidant enzyme activities. Plant Physiol. Biochem. Plant 47:132-138.
https://doi.org/10.1016/j.plaphy.2008.10.006 |
|
|
Mahajan S, Tuteja N (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Bioph. 444:139-158.
https://doi.org/10.1016/j.abb.2005.10.018 |
|
|
Murillo-Amador B, Troyo-Dieguez E, Lopez-Corted A, Jones HG, Ayala-Chairez F, Tinoco-Ojanguren CL (2001). Salt tolerance of cowpea genotypes in the emergence stage. Aust. J. Exp. Agric. 41:81-88.
https://doi.org/10.1071/EA00055 |
|
|
Namvar A, Sharifi RS, Khandan T, Moghadam MJ (2013). Seed inoculation and inorganic nitrogen fertilization effects on some physiological and agronomical traits of Chickpea (Cicer arietinum L.) in irrigated condition. JCEA 14(3):28-40.
https://doi.org/10.5513/JCEA01/14.3.1281 |
|
|
|
Nkot NL, Ngo BM, Fankem H, Adamou S, Kamguia K, Ngakou A, Nwaga D, Etoa FX (2015). Isolation and screening of indigenous Bambara groundnut (Vigna subterranea) nodulating bacteria for their tolerance to some environmental stresses. Am. J. Microbiol. Res. 3(2):65-75. |
|
|
Paul D, Nair S (2008). Stress adaptations in a plant growth promoting Rhizobacterium [PGPR] with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 48(5):378-384.
https://doi.org/10.1002/jobm.200700365 |
|
|
Predeepa RJ, Ravindran DA (2010). Nodule formation, distribution and symbiotic efficacy of Vigna unguiculata L. under different soil salinity regimes. regimes. Emir. J. Food Agric. 22(4):275-284.
https://doi.org/10.9755/ejfa.v22i4.4875 |
|
|
|
Sadio S (1991). Pédogenèse et potentialités forestières des sols sulfatés acides salés des tannes du Sine-Saloum. ORSTOM Paris, 269p. |
|
|
|
Sanginga N, Okogun A, Vanlauwe B, Dashiell K (2002). The contribution of nitrogen by promiscuous soybeans to maize based cropping in the moist savanna of Nigeria. Plant Soil 251:1-9. |
|
|
|
Singh BB, Ehlers JD, Sharma B, Freire Filho FR. (2002). Recent progressinprogressing cowpea breeding. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production, ed. C.A Fatokun SA, Tarawali B B, Singh PM, Kormawa M, Tamo, IITA, Ibadan, Nigeria pp. 22-40. |
|
|
Shrivastava P, Kumar JR (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22(2):123-131.
https://doi.org/10.1016/j.sjbs.2014.12.001 |
|
|
|
Soumare A, Diop T, Lahcen O, Bassene G, Duponnois R, Ndoye I (2013). Impact of Eucalyptus camaldulensis on the diversity of rhizobia associated with Acacia senegal and A. seyal in Senegal. J. Appl. Biosci. 5183:5193. |
|
|
|
Tarawali SA, Singh BB, Gupta SC, Tabo R, Harris F, Nokoe S, Fernández-Rivera S, Bationo A, Manyong VM, Makinde K, Odion EC (2002). Cowpea as a key factor for a new approach to integrated crop–livestock systems research in the dry savannas of West Africa. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production, ed. C. A. Fatokun SA, Tarawali BB, Singh PM, Kormawa M. Tamo. IITA, Ibadan, Nigeria pp. 233-251. |
|
|
Tejera NA, Campos R, Sanjuan J, Lluch C (2005). Effect of sodium chloride on growth, nutrient accumulation, and nitrogen fixation of common Bean plants in symbiosis with isogenic strains. J. Plant Nutr. 28(11):1907-1921.
https://doi.org/10.1080/01904160500306458 |
|
|
Thilakarathna MS, Raizada MN (2017). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 105:177-196.
https://doi.org/10.1016/j.soilbio.2016.11.022 |
|
|
|
Timko MP, Ehlers JD, Roberts PA (2007). Pulses, Sugar and Tuber Crops. In Genome Mapping and Molecular Breeding in Plants, ed. C. Kole, Springer-Verlag Berlin Heidelberg P 23. |
|
|
|
Vincent JM (1970). A manual for the practical study of root nodule bacteria. In International Biological Programme Handbook no. 5, ed. Oxford, 73-97. Blackwell Scientific Publication Ltd. |
|
|
Wankhade S, Apte SK, Rao KK (1996). Salinity and osmotic stress regulated proteins in cowpea Rhizobium 4a (peanut isolate). Biochem. Mol. Biol. Int. 39:621-628.
https://doi.org/10.1080/15216549600201681 |
|
|
Youseif SH, Abd El-Megeed FH, Ageez A, Mohamed ZK, Shamseldin A, Saleh SA (2014). Phenotypic characteristics and genetic diversity of rhizobia nodulating soybean in Egyptian soils. Eur. J. Soil Biol. 60:34-43.
https://doi.org/10.1016/j.ejsobi.2013.10.008 |
|
|
|
Zahran HH (1999). Rhizobium-legume symbiosis and Nitrogen Fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. R. 63(4):968-989. |