ABSTRACT
Environmentally friendly technologies, such as the use of bacteria (e.g. Bacillus spp.) to control fungal diseases of rice (Oryza sativa L.), represent a promising alternative for the sustainability of agricultural ecosystems. The present work aimed to isolate, identify, and evaluate (in vitro) various Bacillus spp. from the rice phylloplane for their potential to control the rice fungus Magnaporthe oryzae. Samples were taken from the phylloplane of healthy young rice plants growing in commercial fields in ten municipalities of Maranhão (Brazil), and Bacillus spp. were subsequently isolated and molecularly identified. Both experiments utilized a randomized design, and data were submitted to analysis of variance as well as means comparison by the Tukey test. Twelve bacterial isolates were obtained and identified. Using a control isolate (B25), four in vitro M. oryzae-inhibition methods were compared, with the ‘circle’ method ultimately providing the highest mycelium growth inhibition. The most promising experimental isolates for biological control were then identified as B. methylotrophicus isolates B41, B31, and B22, which achieved 90.41, 69.47, and 67.55% inhibition, respectively. This study demonstrates the potential of Maranhão Bacillus spp. isolates for use as biological fungal-control agents.
Key words: biological control, pathogen, rice.
Oryza sativa L., cultivated worldwide and contributing significantly to global energy intake, is subject to attack by various fungal diseases which affect crop yield and seed quality. Notable among these, the rice panicle and leaf blast fungus Magnaporthe oryzae B. Couch is extensively distributed and has significant destructive power under the right conditions. In Brazilian agriculture (especially on highly-irrigated farmland), this fungus is one of the main factors affecting rice productivity and reproductive potential (Lobo, 2008).
Chemical antifungal agents are the most widely used countermeasures against crop disease, but the indiscriminate use of agricultural pesticides presents several environmental problems (e.g. contamination of food, soil, water, and animals; poisoning of farmers; and biological imbalance due to altered cycling of nutrients and organic matter (Bettiol and Ghini, 2001), as well as promoting pesticide resistance. Novel alternative agricultural disease control methods are required to maintain the sustainability of agricultural ecosystems. Sustainable agriculture requires strategies which increase food production without damaging the environment and public health, and which are also appropriate within the economic, ecological, social and political contexts of each region. The use of biological agents is one potential alternative which achieves these objectives. According to Filippi et al. (2012), biological agents produce chemical signals which elicit metabolic pathways involved in biomass accumulation and disease suppression, as well as increasing productivity through additional direct, indirect, and miscellaneous ecological antagonisms.
Natural plant resistance to pathogens depends on a defense system which acts in three possible ways:
(1) Constitutive resistance, which is inherited and manifests itself even in the absence of a specific pathogen aggressor;
(2) Local resistance activated at the site of aggression;
(3) Systemic acquired resistance, which protects the plant against systemic stresses (Campos, 2009).
Among the micro-organisms with high potential for the development of biocontrol agents - using in vitro production systems - are bacteria and fungi. In combating plant diseases, Bacillus subtilis and Trichoderma spp. are among the most widely-used bacteria and fungus respectively (Bettiol and Morandi, 2009). The genus Bacillus includes over 100 species (Euzeby, 2006). These bacteria are Gram positive, aerobic (facultatively anaerobic), spore-forming bacilli, sporulation provides a mechanisms of resistance to environmental changes, which result in an important aspect for the production of inoculants (Tejera et al., 2012). Various studies have employed this bacterial genus as a plant growth promoter, particularly B. subtilis and B. licheniformis (Lugtenberg and Kamilora, 2009).
The inhibition capacity of microorganisms on plant pathogens is due to different antagonistic mechanisms developed by them. Among which are the production of antimicrobial substances, competition for ecological niches, competition for nutrients, production of volatile antimicrobial compounds, detoxification, hyperparasitism, predation and parasitism, production of lytic enzymes, production of toxins, gene silencing, interference with phenomenon of Quorum Sensing, siderophore and hydrocyanic acid production (Romeiro, 2007). Some species of Bacillus can suppress fungal diseases, primarily by antagonistic action related to production of antifungal antibiotics such as iturina in B. subtilis (Araujo et al., 2005). In a study confronting Bacillus and Pyricularia grisea (Cooke) Sacc., Velusamy and Gnanamanickam (2008) observed the effect of these bacteria on biocontrol for the production of antibiotics. While Knaak et al. (2007) observed inhibition through the action of Cry 1 AB and Cry 1 AC proteins. In rice cultivation various Bacillus species in solution or microbiolized seeds have been tested against the P. grisea, Rhizoctonia. Solani Kühn, B. oryzae (Breda de Haan) Shoemaker, Curvularia sp., Fusarium oxysporum Schlecht, F. solani (Mart.) App. and Wollenw and Gerlachia oryzae (Hashioka and Yologi) W. Gams pathogens. In an experiment conducted by Remuska and Pria (2007), the bacterium B. thuringiensis proved to be very effective as an antagonist to Sclerotium rolfsii Sacc., Monilinia fruticola (G. Wint.) Honey, Sclerotinia sclerotiorum (Lib) de Bary and F. solani. The rice seeds microbiolization infected with Pseudomonas fluorescens (Flugge) Migula, B. subtilis, Bacillus sp. and S. maltophilia is considered an effective treatment in controlling of sheath blight caused by R. solani not only by the ability of these to reduce the disease, but also by the possibility of efficiency by using them associated with compounds that stimulate its activity (Ludwig and Moura, 2007).
The success of any biological control program relies on isolation and selection of pathogen-antagonistic microorganisms, which are able to exert their effects rapidly and at low cost (Mariano et al., 2000). Therefore, in order to contribute alternative biological agents for disease control, this study aimed to isolate, identify, select Bacillus spp. with the potential for use in the biological control of M. oryzae and propose the best method to verify maximum M. oryzae mycelial growth inhibition.
Isolation and identification of rice plants Phylloplane antagonists
Healthy rice plants were collected from rice plantations in the state of Maranhão, Brazil (namely: Sao Luis, Arari, Vitoria do Mearim, Miranda, Davinopolis, and Grajau cities), which present climate, temperature, similar humidity and rainfall, with some changes. The climate of São Luís, Arari, Vitória do Mearim and Pindaré is humid (B1), Miranda and Davinópolis is sub-humid (C2) and Grajaú is sub-humid dry (C1). Most of these municipalities have temperature higher than 27 ºC; only Davinópolis is between 25 and 26°C. The moisture in São Luís is more than 82%, in Arari and Vitória do Mearim is between 79-82%, and in the cities of Pindaré, Miranda and Grajaú, around 76-79%. Annual rainfall in São Luís varies from 2000 to 2400 mm; in the municipalities of Arari, Davinópolis and Grajaú, around 1200 to 1600 mm per year; in the cities of Vitória do Mearim, Pindaré and Miranda, around 1600 to 2000 mm per year (Geplan, 2002).
Plants were collected from rainfed rice cultivated in the vegetative phase with different ages and transported in paper bags to the laboratory of Plant Pathology the State University of Maranhão for frozen storage.
Isolation of Bacilli from stored plants was performed according to the methods described by Mariano and Silveira (2005) and Bettiol (1995), with modifications. Briefly, discs were punched out of healthy leaves and washed in sterile distilled water (SDW). Washed leaf discs were placed in a test glass tube with 5 ml SDW, sonicated at 10 Hz for 10 min, and heated to 80 °C for 20 min in a water-bath. The resulting suspension was serially diluted (dilution factor 10-2, or 1 in 100) in test tubes containing 4.5 mL of sterile distilled water (SDW). From the undiluted suspension and from each dilution (1 in 10, and 1 in 100), 0.1 ml was streaked onto Potato Dextrose Agar (PDA) medium solid in triplicate. After 48 h incubation under laboratory conditions (temperature of 25°C), bacterial colonies were inoculated into PDA solid medium and again plated by streaking. Colonies derived from resulting single colony-forming units (cfu) were transferred to test tubes containing Tryptone Soy Agar (TSA) solid medium.
\Molecular identification of bacteria was carried out using 16S rRNA sequencing. Briefly, the gene encoding bacterial 16S ribosomal RNA was amplified by the Polymerase Chain Reaction (PCR), and subsequently sequenced. PCR was performed on a small number of bacterial cells directly sampled from each TSA culture using an autoclaved toothpick. The bacterial cells were deposited in wells already containing specific PCR reagents, and rapidly agitated with the tip of the toothpick. The PCR reaction consisted of 10 μL 5X PCR buffer, 1 μL each of forward and reverse primers (10 mM), 1 μL of dNTP (10 mM), 0.2 μL of GoTaq DNA polymerase (5 U / uL, Promega), and 36.8 μL autoclaved MilliQ water. The forward (5 '- AGAGTTTGATCCTGGCTCAG - 3') and reverse (5 '- ACGGTTACCTTGTTACGACTT - 3') primers were described by Weisburg et al. (1991). Amplification was performed in a thermocycler (model T100, BioRad), using the following program: initial denaturation at 94 °C for 4 min; 40 cycles of 94 °C for 30 s (step 1), 60 °C for 30 s (step 2), and 72 °C for 90 s (step 3); and final extension at 72 °C for 4 min.
Amplification was verified by electrophoresis in 0.8% agarose gel plus ethidium bromide (100 ng / ml), and imaged using a gel documentation system coupled to a UV trans-illuminator. Amplified products were purified by precipitation with polyethylene glycol (the second protocol described by Schmitz and Riesner (2006). Purified products were sequenced by the chain termination reaction method (Sanger et al., 1977). The reaction consisted of 5 μL PCR product, 1 ul Big Dye 3.1 reagent (Applied Biosystems), 1.5 μL dilution buffer, 0.3 μL each forward and reverse primers (10 M), and 2.2 μL autoclaved MilliQ water. The reaction was performed in a thermocycler, using the following program: initial denaturation at 95 °C for 1 min, followed by 25 cycles of 95 °C for 5 s (step 1) and 60 °C for 4 min (step 2).
Sequencing reaction products were precipitated by adding 40 ul of isopropanol (75 %), followed by centrifugation (12,000 g for 10 min). An additional 100 ul of isopropanol (75 %) was added, and centrifugation was repeated (12,000 g for 5 min). After discarding the supernatant, the pellet was dried and resuspended in 10 ul formamide before denaturation at 95 °C for 2 min. Sequencing was performed using a 3500xL capillary sequencer Genetic Analyzer (Applied Biosystems).
The determined sequences were searched against the type of species Ribosomal Database Project, Release 10 (http://rdp.cme.msu.edu) (Cole et al., 2009). The phylogenetic tree was constructed using MEGA 6.3 program.
Evaluation of Bacillus spp. for antagonistic potential against M. oryzae
To study the antagonistic effects of isolated Bacillus spp. on M. oryzae, the 12 identified Bacillus isolates were cultured in growth chamber at a temperature of 25 °C and photoperiod on PDA solid medium for 24-48 h, while M. oryzae - isolated from Embrapa Rice and Beans (Goiânia) - was cultured in PDA solid medium for 14 days.
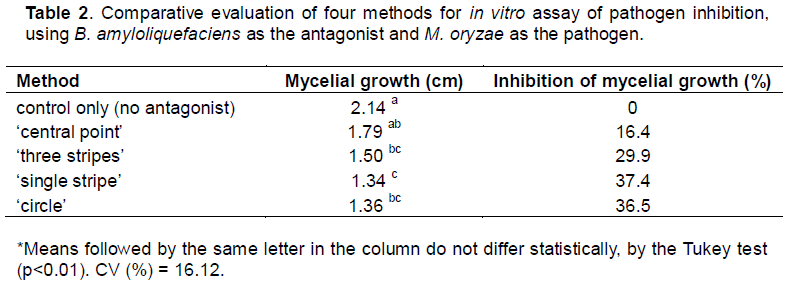
Isolate B25 (B. amyloliquefaciens), selected during pre-experimental optimization, was used to comparatively evaluate four potential in vitro antagonism assay streaking methods: ‘central stripe’, ‘the point’, ‘circle’ and ‘three stripes’.
Briefly, during the central stripe method, bacterial isolate colony material was transferred to the center of a Petri dish containing PDA. Subsequently, two 6 mm diameter discs (containing actively growing pathogen mycelium) were removed from the pathogen culture dish and deposited on either side of the central bacterial isolate, equidistant from it (Mariano and Silveira, 2005). In point method, retired, aseptically, a disc 6 mm diameter (containing actively growing pathogen mycelium), depositing it on the surface of the middle of the bioassay plate, near the edge. With the aid of platinum loop It peaked bacterial colony to the PDA medium in a diametrically opposite to that point where the mycelium disc is loaded (Mariano and Silveira, 2005). In contrast, during the circle method, a 6 mm diameter disc of pathogen-containing agar is transferred to the centre of a Petri dish containing BDA medium. Bacterial isolate is then inoculated onto the PDA in a circular pattern, with the circle diameter smaller than the dish diameter (about 5 cm) (Mariano and Silveira, 2005). In testing the above methods, a randomized experimental design with six replicates per method was employed. All methods employed aseptic technique, using a platinum loop for bacterial inoculum transfer. Assay evaluation (measurement of pathogen colony diameter) was carried out after seven days of incubation in growth chamber at 25 °C and photoperiod of 12 h.
The circle method, which yielded the best results (Table 2), was thus used to evaluated the antagonistic action of 11 Bacillus spp. isolates (B7, B7', B16, B35, B22, B22', B31, B41, B45, B25, and B46) against M. oryzae. This experiment employed a randomized design with four replicates per isolate, and pathogen colony diameter was evaluated after seven and 14 days of incubation. Colony diameter was measured in two perpendicular directions, using a millimeter ruler, and an average was calculated for each colony.
During both method selection and isolate evaluation, the control consisted of the pathogen alone. Data were analysed by means of Analysis of Variance (ANOVA) and means-comparison (Tukey test), using the Statistical Assistance Software (Assistat) version 7.7 beta (2015).
Identification of bacterial antagonists extracted from rice leaves
From the collected rice plant material, 12 Bacillus spp. isolates were obtained and identified. The identification of these isolates based on sequence of the 16 S rDNA gene indicates that the bacteria B7, B7', B16 and B35 showed similarity of 66% to B. thuringiensis serovar tolworthi Pasteur Institute standard strain (AP014864). The isolates B12, B22, B22', B31, B40, B41, B45, B57, B58 and B62 showed 100% similarity to B. methylotrophicus CBMB205 (NR_116240). The isolated B25, B46, B47, B56 and B60 showed 100% similarity to B. amyloliquefaciens NBRC15535 (NR_041455) (Figure 1).
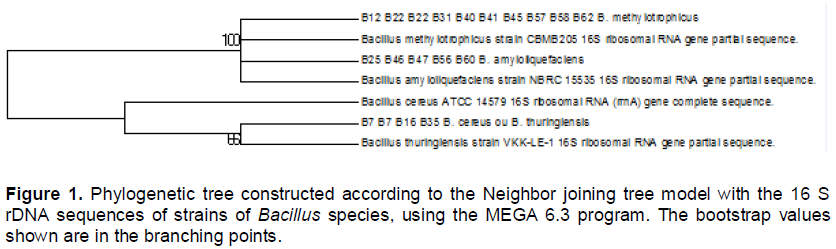
These can be divided into three species: B. thuringiensis, B. methylotrophicus, and B. amyloliquefaciens (Table 1). The diversity of Bacillus spp. can be explained by the significant environmental differences between municipalities, different varieties of rice, and differing ages of the rice plants.
The most common specie, B. methylotrophicus (6 isolates), occurred in Arari, Vitória do Mearim and Grajaú in the first two municipalities, the temperature is higher than 27°C, have high humidity, ranging from 79 to 82% with rainfall around 1200 to 1600 mm and from 1600 to 2000 mm per year, in Grajaú observed temperature range of 26 and 27°C and relative humidity of 76 to 79%.
The second most frequent, B. thuringiensis (4 isolates) occurred in São Luís and Miranda, both places with temperature higher than 27°C, relative humidity greater than 82% and ranging 76-79%, respectively. What differentiates these two environments are variations in rainfall; in the second room this is less.
The specie of minor occurrence, B. amyloliquefaciens (2 isolates) is present in the municipalities of Arari and Davinópolis, the two places have rainfall 1200-1600 mm, the cities are different in relation to climate, because one is humid and the other is sub - humid, and in the second city the relative humidity is lower. It is observed that small variations in humidity, temperature and rainfall have influence in the occurrence of one or another specie of bacterium of the genus Bacillus.
In Arari ocorred greater diversity of species, where the climate is sub-humid, temperature above 27°C and rainfall between 1200-1600 mm per year. According to Romeiro (2007), any living organism, mainly bacteria, is able to perceive changes in the environment and the presence of other living beings in their proximity, this being crucial to their survival.
Microorganisms such as bacteria, yeasts, and filamentous fungi are commonly found on rice phylloplanes. The predominant type of microorganism is dependent on the phenological stage of the plant, with bacteria being dominant during early plant development, an increasing presence of yeasts, thereafter and finally and increasing presence of filamentous fungi. This microbial succession occurs as plant development increases the level of sugars present in the leaves (Michereff, 2001). Conversely, Filippi et al. (2012) report that plants also actively respond to a variety of environmental stimuli (e.g. gravity, light, temperature, physical stresses, water and nutrient availability, and chemical stimuli), including chemical stimuli produced by microorganisms associated with the soil and the plant itself. Corroborating findings from Bettiol and Morandi (2009) demonstrate that the phyllosphere bacterial populations change with plant age, local nutrient sources, and season. In addition, cultivation can also alter composition of the microbial community.
Evaluation of the antagonistic effect of Bacillus spp against M. oryzae
Selection of the in vitro antagonism assay method revealed a significant difference between the four methods tested. Two of these methods significantly outperformed the control: central stripe and circle, achieving 37.4 and 36.5% inhibition of M. oryzae mycelial growth, respectively (Table 2). These data demonstrate the importance of choosing appropriate antagonist and pathogen cultivation and pairing methods for in vitro assays. Findings can also be influenced by the production of enzymes with activity against the fungal cell wall (Mavingui and Heulin, 1994). With regards to the circle method, the considerable inhibition of pathogen growth may be explained by the pathogen being surrounded by the antagonist, thereby blocking passage to the plate boundaries and forcing a confrontation. Lima et al. (2014), in an in vitro experiment employing the circle method with 10 antagonistic Bacillus spp. observed that at day 10 all isolates showed an inhibitory effect against the assessed pathogen (F.oxysporum f. sp. Lycopersici (Sacc.) Snyder and Hansen), by metabolite production.
During evaluation of the 11 Bacillus spp. for their potential to control M. oryzae mycelial growth, the circle method produced a significant effect (p <0.05) on pathogen growth. At day seven, isolates B41, B22, and B31 (B. methylotrophicus) had achieved 86.58, 48.16, and 43.68% inhibition (respectively) compared to the control. At day 14, eight antagonists greater inhibited mycelial growth, with inhibition ranging from 53.54 to 90.41%. At the latter time point, isolates B41, B31, and B22 (B. methylotrophicus) had achieved 90.41, 69.47, and 67.55% inhibition (respectively) compared to the control (Table 3).
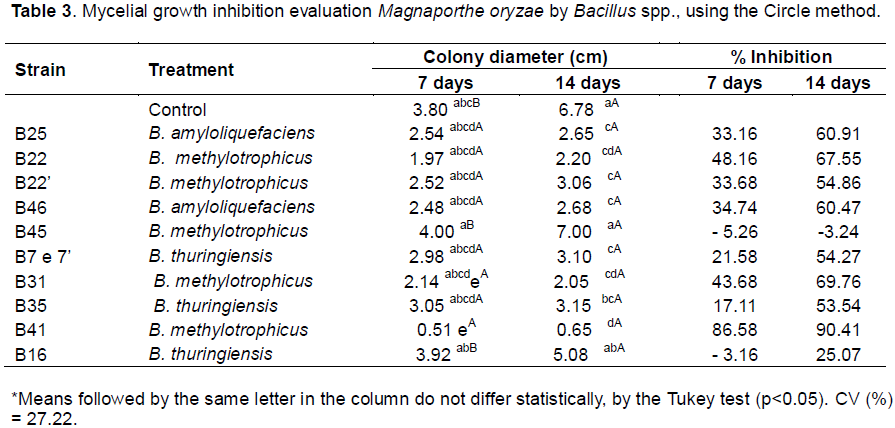
Inhibition levels observed during this experiment were thus higher than those reported previously by Remuska and Pria (2007), wherein the bacterium B. thuringiensis proved effective as an antagonist against S. rolfsii (a plant pathogen which causes fruit rot), S. sclerotiorum (a plant pathogen which causes stem rot), and F. solani., achieving 39.41, 37.97, 37.44, and 36.17% inhibition of mycelial growth, respectively.
Bacillus spp. may act through antibiosis, the production of toxic compounds capable of in vitro inhibition of mycelial growth of at least three fungi: Alternaria alternata, Bipolaris oryzae (Breda de Haan) Shoemaker, Curvularia lunata (Wakker) Boedijn Meyer, Gerlachia oryzae (Hashioka and Yologi) W. Gams, Pyricularia oryzae (Cooke) Sacc., Rhizoctonia solani, and S. rolfsii (Ludwig et al., 2009). In an in vitro bioassay, Vieira Jr. (2005) found that B. cereus (UFV-75) was capable of producing siderophores, volatile compounds, bacteriocins, and the enzyme chitinase. According to Lima et al. (2014), enzyme production by microorganisms may explain, at least in part, the biological control of plant diseases by prokaryotic agents. Reinforcing this theory, Guerrero et al. (2011) state that plant commensal bacteria promote growth and have the ability to control plant pathogens through the production of inhibitory metabolites as well as induction of plant resistance to pathogens.
Regarding the mycelial growth inhibition results at seven and 14 days post-application of Bacillus isolates, higher inhibition by B. methyltrophicus was observed at day 14. These results and the Wiwattanatapee et al. (2004), who found that 20 days post-application of different isolates of B. megaterium de Bary, the inhibitory effect on rice-sheath burning had been lost and suggest that the inhibitory effect of Bacillus may increase to a maximum out of 14 and 20 days after the application, and then decrease.
Studies on greenhouse-grown rice plants have also demonstrated successful Bacillus spp. biological control of pathogens. Silva et al. (2014) found that each of nine Bacillus spp. isolates (especially isolates B41 and B35) reduced the extent of rice plant leaf damage caused by C. lunata. Ludwig et al. (2009) had previously found that treatment of rice plants with rhizobacteria reduced the relative frequency of stained grains, and that isolates FDs 416, FDs 418 (Bacillus spp.), and FDs 223 (Pseudomonas fluorescens Migula) provided significant levels of pathogen reduction (up to71.4, 57.1, and 50%, respectively). In scald-controls, isolates FS 416 and FS 418 achieved pathogen reduction of 63.2 and 60% respectively (Ludwig et al., 2009).
Several researchers, including Remuska and Pria (2007), Kupper et al. (2003), Ludwig and Moura (2007), Navon (2000), Ludwig et al. (2009), Silva et al. (2014), and Lima et al. (2014) have demonstrated the effectiveness of these bacteria, in corroboration with the results reported here.
Therefore, additional research in this area is warranted. Availability of a greater number of Bacillus spp. with the potential for biological control of pathogens, as well as an improved understanding of the antagonist-pathogen relationship, would contribute greatly to development of sustainable agricultural practices. Pursuing related novel lines of research (such as inoculation of antagonists into the greenhouse, evaluation of antagonist efficacy in inhibiting the formation of fungal aspersoria, investigation into production of volatile compounds, pairing of antagonists with other rice plant pathogens, and the influence of in vitro methods on defensive control efficiency) may also be helpful.
The rice phylloplane supports a diverse and dynamic Bacillus spp. Population; it was isolated and three species were identified: B. thuringiensis, B. methylotrophicus, and B. amyloliquefaciens. They were impacted by a variety of culture and environmental conditions. This study demonstrates the potential of Maranhão Bacillus spp. isolates for use as biological fungal-control agents and the most promising experimental isolates for biological control were identified as B. methylotrophicus isolates B41, B31, and B22. In vitro single stripe and circle methods are the best method to verify maximum M. oryzae mycelial growth inhibition.
The authors have not declared any conflict of interests.
We would like to thank the Maranhão Foundation for the Protection of Research and Scientific and Technological Development (FAPEMA), the Coordination of Improvement of Higher Education Personnel (CAPES), and the National Research Council (CNPq) for grants, project resources, and scholarships provided. LastEdit - English editing and proofreading of scientific manuscripts (email:
[email protected]) - is also acknowledged.
REFERENCES
|
Araujo FF, Henning A, Hubngria M (2005). Phytohormones and antibiotics produced by Bacillus subtilis and their on seed pathogenic fungi and on soybean root development. World J. Microbiol. Biotechnol. Dordrecht 21:1639-1645.
Crossref
|
|
|
|
ASSISTAT (2015). ASSIAT software, version 7.5 beta,
View.
|
|
|
|
|
Bettiol W (1995). Isolamento seletivo de Bacillus, pp35-36. In: Métodos de seleção de microorganismos antagônicos a fitopatógenos (Eds Melo, I S, Sanhueza, R M.V) EMBRAPA – CNPMA, Jaguariúna.
|
|
|
|
|
Bettiol W, Ghini R (2001). Proteção de plantas em sistemas agrícolas alternativos, In: Proteção de plantas na agricultura sustentável (Eds Michereff, S.J, Barros, R) UFRPE, Imprensa Universitária, Recife. pp. 1-13.
|
|
|
|
|
Bettiol W, Morandi MAB (2009). Biocontrole de doenças de plantas: uso e perspectivas. Embrapa Meio Ambiente, Jaguariúna. 341 p.
|
|
|
|
|
Campos AD (2009). Considerações sobre indução de resistência a patógenos em plantas. Embrapa Clima Temperado, Pelotas. 28 (Embrapa Clima Temperado. Documento, 264).
|
|
|
|
|
Cole JR, Wang R,Gardenas E, Fish J,Chai B, Farris RJ,Kulam-Syed-Mohideen, AS, Mcgarrell DM, Marsh T, Garrity GM,Tiedje JM (2009).The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. J. 37:141-145.
Crossref
|
|
|
|
|
Euzeby JP (2006). List of prokariotic names with standing nomenclature. Disponível em:
View
|
|
|
|
|
Filippi MC C, Silva GB, Côrtes MVB, Lobo VLS, Prabhu AS (2012). Indução de resistência e promoção de crescimento em arroz por agentes biológicos. In: Indução de resistência em plantas a patógenos (Eds Rodrigues FA Fortunato AA Resende RS). Universidade Federal de Viçosa, Viçosa.
|
|
|
|
|
GEPLAN (2002). Gerencia de Planejamento e Desenvolvimento Econômico, Atlas do Maranhão. Laboratório de Geoprocessamento – UEMA. São Luís. 38 p.
|
|
|
|
|
Guerrero YA, Rodriguez AH, Rodriguez NR, Valle MGV, Hernández-Lauzardo NA (2011). Perspectivas del uso de bactérias rizosféricas em el control de Pyricularia grisea (Cooke Sacc) em el cultivo del arroz (Oryza sativa L.). Rev. Colomb. Biotecnol. XIII(1):16-22.
|
|
|
|
|
Knaak N, Rohr AA, Fuiza LM (2007). In vitro effect of Bacillus thuringiensis strains and cry proteins in phytopathogenic fungi of paddy-rice-field. Braz. J. Microbiol. 38:526-530.
Crossref
|
|
|
|
|
Kupper KC, Nelson Gimenes-Fernandes N, de Goes A (2003). Controle biológico de Colletotrichum acutatum, agente causal da queda prematura dos frutos cítricos. Fitopatol. Bras. 28(3):251-257.
Crossref
|
|
|
|
|
Lima ODR, Oliveira LJMG, Silva MSBS, Rodrigues AAC (2014). Ação antifúngica in vitro de isolados de Bacillus spp sobre Fusarium oxysporum f. sp. Lycopersici. Revista Caatinga. 27(4):57-64.
|
|
|
|
|
Lobo VLS (2008). Efeito do tratamento químico de sementes de arroz no controle da brusone nas folhas e na qualidade sanitária e fisiológica das sementes. Trop. Plant Pathol. 33:162-166.
Crossref
|
|
|
|
|
Ludwig J, Moura AB (2007). Controle biológico da queima-das-bainhas em arroz pela microbiolização de sementes com bactérias antagonistas. Fitopatol. Bras. 32:381-386.
Crossref
|
|
|
|
|
Ludwig J, Moura AB, Santos AS, Ribeiro AS (2009). Microbiolização de sementes para o controle da mancha parda e da escaldadura em arroz irrigado. Trop. Plant Pathol. 34(5):322-328.
Crossref
|
|
|
|
|
Lugtenberg B, Kamilova F (2009). Plant-Growth-Promoting Rhizobacteria. Ann. Rev. Microbiol. 63:541-546.
Crossref
|
|
|
|
|
Mariano RLR, Silveira EB, Gomes AMA, Rodrigues VJLB, Assis SMP (2000). Biocontrole de doenças de plantas. In: Desafios do manejo integrado de pragas e doenças (Eds Torres JB Michereff SJ) UFRPE, Recife. pp. 77-109.
|
|
|
|
|
Mariano RLR, Silveira EB (2005). Manual de Práticas em Fitobacteriologia. UFRPE, Recife.
|
|
|
|
|
Mavingui P, Heulin T (1994). In vitro chitinases and antifungal activity of a soil, hizosphere and rhizoplane population of Bacillus polymyxa. Soil Biol. Biochem. 26:801-803.
Crossref
|
|
|
|
|
Michereff SJ (2001). Fundamentos de Fitopatologia. UFRPE, Recife.
|
|
|
|
|
Navon A (2000). Bacillus thuringiensis application in agriculture, In: Entomopathogenic bacteria: from laboratory to field application (Eds Charles JF et al) Kluwer Academic Publishers, Netherlands. pp. 355-367.
Crossref
|
|
|
|
|
Remuska AC, Pria MD (2007). Efeito de Bacillus thuringiensis no crescimento de fungos fitopatogênico. Ciências Exatas Terra. Ciênc. Agrárias. 13:31-36.
|
|
|
|
|
Romeiro RG (2007). Controle biológico de enfermidades de plantas: fundamentos. Viçosa: UFV.
|
|
|
|
|
Sanger F, Nicklen, S, Coulson AR (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the Nation. Acad. of Sci. 74(12):5463-5467.
Crossref
|
|
|
|
|
Schmitz A, Riesner D (2006). Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Analyt. Biochem. 354:311-313.
Crossref
|
|
|
|
|
Silva MSBS, Rodrigues AAC, Oliveira LJMG, Silva EKC, Pereira TS (2014). Sanidade de sementes de arroz, biocontrole, caracterização e transmissão de Curvularia lunata em semente-plântula de arroz. Rev. Ceres. 61(4):511-517.
Crossref
|
|
|
|
|
Tejera B, Heydrich M, Rojas MM (2012). Antagonismo de Bacillus spp. frente a hongos fitopatógenos del cultivo del arroz (Oryza sativa L.) Rev. Protec. Veg. 27 (2):117-122.
|
|
|
|
|
Velusamy P, Gnanamanickan SS (2008). The effect of bacterial ande fungal pathogens of rice. Soil Biol. 14:93-106.
Crossref
|
|
|
|
|
Vieira Jr JR (2005). Procariotas residentes do filoplano do feijoeiro como agentes de biocontrole de enfermidades da parte aérea da cultura. 146 f. Tese (Doutorado em Fitopatologia), Universidade Federal de Viçosa, Viçosa.
|
|
|
|
|
Weisburg WG, Barns SM, Pelletier DK, Lane DJ (1991). 16S ribosomal DNA amplificatton for phylogenetic study. J. Bacteriol. 173:697-703.
|
|
|
|
|
Wiwattanatapee R, Pengnoo A, Kanjanamaneesathian M, Matchavanich W, Nilratana L, Jantharangsri A (2004). Floating pellets containing bacterial antagonist for control sheath blight or rice: formulations, viability and bacterial release studies. J. Contr. Rel. 95:455-462.
Crossref
|
|