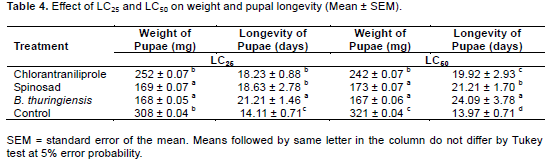ABSTRACT
Exposure of Helicoverpa armigera to sublethal concentrations of insecticides can cause physiological deficiencies manifested by reduced longevity, development, fertility and fecundity. Research on the sub-lethal effects, to identify the non-lethal negative impacts of insecticides on pests can provide practical information for integrated management. The objective of this study was to determine the lethal concentrations and sublethal effects of insecticides on larval development and reproduction of H. armigera. The insecticides were diluted in water and applied via immersion of soybean leaf discs directly in the solution for three second, subsequently provided to the larvae maintained under controlled conditions for a period of 48 h. For surviving larvae, artificial diet was provided with daily evaluation until pupation. The pupae obtained were weighed after 24 h and transferred to Petri dishes, covered with filter paper until the adult phase, to evaluate longevity and pupal viability. For the assessments related to oviposition, couples were separated in polyvinyl chloride (PVC) cages and fed with 10% honey solution; the counting of eggs was done every two days, until the end of the oviposition period. All of the insecticides tested presented lethal and sublethal effects on the parameters weight, mortality and pupal viability and reduced oviposition and can be used in pest management, representing an alternative in the product rotation for the control of the third instar of H. armigera.
Key words: Management, chemical control, lethal concentration.
Brazil is one of the largest global producers of grains, with production of 206.33 million tons in the 2014/2015 crop (Conab, 2015). The Brazilian grain production systems are characterized by being intensive, while cultural practices are extensive in relation to planted areas. These facts, coupled with inadequate farming practices, characterized by the successive planting of host plant species in contiguous areas and inappropriate handling of pesticides, have made agricultural ecosystems susceptible to attack by insect pests, due to the constant availability of food, shelter and breeding sites (Embrapa, 2013).
Among the major pests causing damage to crops are the stink bugs (Hemiptera: Pentatomidae) and the complex of defoliating caterpillars (Spodoptera spp ex, Heliothis virescens (Fabricius), Anticarsia gemmatalis Hubner, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). Recently this situation has been aggravated by the introduction of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae), a pest that has great potential for damage to various crops (Kotkar et al., 2009; Czepak et al., 2013).
The H. armigera caterpillar is a major pest of cultivated plants, having as hosts more than 181 plant species belonging to 45 families (Rajapakse and Walter, 2007). It feeds on leaves and stems, but preferably shoots, inflorescences, fruits and pods, being able to adapt to various cropping systems. Besides high polyphagia, it has a broad geographical range, potential migratory mobility, diapause (facultative), high fertility and a propensity to develop resistance to insecticides (Fitt, 1989; Mccaffery, 1998; Moral Garcia, 2006). Beginning with the 2012/2013 crop, H. armigera has caused losses to producers, especially in the North, Northeast and Mid-South, by intensely attacking different crops of economic importance in these regions, such as soybeans, cotton, corn, beans, sorghum, etc. (Fathipour and Sedaratian, 2013; Embrapa, 2013). In Bahia, the costs of infestation of the pest were estimated at about US $ 2 billion in crops such as soybeans, corn and cotton due to the increase in the number of applications of insecticides and reduced productivity (Adab, 2013).
The main control method used for H. armigera management has been almost exclusively the use of chemical insecticides. However, due to the impacts and the development of strains resistant to the products, it has become necessary to use in rotation insecticides with different modes of action, as well as techniques that can assist in integrated pest management (IPM) (Jacobson et al., 2009; Gunning and Moores, 2010; Perry et al., 2011; Shind et al., 2011; Yang et al., 2013).
Studies of the deleterious effects of insecticides on insect pests can corroborate IPM, as this combines diverse knowledge about the environment and population dynamics of the pest, using rational methods and techniques, in order to keep the pest population below the level of economic damage. Knowledge of the sublethal effects of insecticides on populations of insect pests is still very incipient. However, the few studies available have shown significant results (Desneux et al., 2007; Junior et al., 2009).
Exposure of H. armigera to sublethal concentrations of insecticides may cause physiological deficiencies, which may manifest as a reduction in longevity, development, fertility or fecundity. Research on these sub-lethal effects can provide practical information for integrated pest management. Therefore, we determined the lethal concentrations and sublethal effects of insecticides on larval development and reproduction of H. armigera.
This experiment was carried out in the laboratory of Plant Science -Professor Cinobelina Elvas Campus - UFPI, Bom Jesus, PI, during the period between January to March 2014.
Breeding and maintenance H. armigera
The population of H. armigera was obtained from the insect rearing laboratory where they were maintained on artificial diet adapted from Kasten Jr. et al. (1978). Neonate larvae (<24 h old) were isolated and transferred to 100 ml plastic containers with lids, containing artificial diet and remaining until they reached the pupal stage. The adults were transferred to polyvinyl chloride (PVC) cages (40 cm H x 30 cm Ø) lined with bond paper sheets for oviposition, fed a honey-based solution (10%) and kept under controlled conditions (25 ± 2°C, 60 ± 5% RH, 12:12 LD). Eggs were collected and stored in plastic bags and kept in laboratory conditions until the hatching of caterpillars. After hatching the larvae were transferred to pots with diet until they reached the third instar and after this period, one part was kept for maintenance, and the others used in bioassays.
Lethal concentration curves
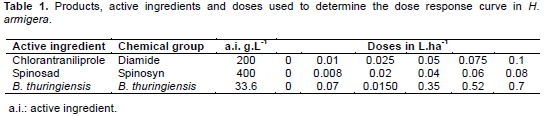
The test consisted of four treatments: spinosad (Tracer®), chlorantraniliprole (Premio®), Bacillus thuringiensis (Dipel®), and control (water). The insecticides were diluted in water, then soybean leaf discs (diameter 5 cm) were immersed for three seconds. After 30 min they were offered to 80 third instar larvae by concentration, which were kept under controlled conditions (25 ± 2°C, 60 ± 5% RH, 12:12 LD) for a period of 48 h. Subsequently, assessment of larval mortality was performed, considering dead individuals being touched with tweezers in the last abdominal segments not responding with coordinated movements. Treatments and concentrations are shown in Table 1. The determination of the concentrations corresponds to 0, 10, 25, 50, 75 and 100% of the recommended concentrations. The insecticides were diluted with distilled water to prepare each dose.Mortality was assessed daily for five days, after was corrected by the mortality in control (Abbott's formula). The mortality results were submitted to Probit analysis (Finey, 1971) through the PROC PROBIT the Statistical program (Sas Institute, 2002) generating the concentration-mortality curve (LC) LC25, LC50 and LC95.
Sublethal effects of the insecticides on H. armigera
Here, the lethal concentrations LC25 and LC50 were used to evaluate the effect of the insecticides on the surviving larvae of H. armigera. The insecticides were diluted in distilled water and soybean leaf discs (diameter 5 cm) were subsequently immersed into the solutions during three seconds. Then the leaf discs were then dried at room temperature for 30 min and offered to individual caterpillars in 100 mL plastic pots with lids. The plastic pots were kept under controlled conditions (25 ± 2°C, 60 ± 5 RH, 12:12 LD) during 48 h. After this period assessment of larval mortality was performed, considering as dead individuals when touched with tweezers in the last abdominal segments that did not respond with coordinated movements. For surviving larvae, artificial diet was supplied and survival was assessed daily until pupation. The pupae obtained were weighed after 24 h and transferred to Petri dishes covered with filter paper, separated by sex and evaluated daily until adulthood, to evaluate longevity and pupal viability.
The evaluation related to oviposition was performed using 10 replicates per concentration of each treatment, the couples being separated in PVC cages (15 cm diameter by 15 cm high) lined with bond paper and closed at the lower end with cardboard and at the upper end with "tulle" type tissue, secured with elastic. The couples were established with individuals with a maximum of two days of age and fed a 10% solution of honey furnished in coffee cups with cotton. The food was replaced every two days to prevent fermentation. The cages were randomly distributed on shelves in the laboratory; eggs were counted every two days using a stereoscopic microscope until the end of the oviposition period. The experimental design was completely randomized. Eighty third instar larvae were used per concentration, considering each individual (caterpillar, pupa), a repeat. Data were subjected to analysis of variance (ANOVA), with a significance level of 5% probability of error. The means were compared by Tukey test 5% (Sas Institute, 2002).
Lethal concentration curves
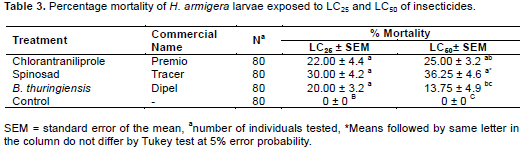
Based on the results of the toxicity bioassay there was difference between the treatments in the mortality of H. armigera. Spinosad was the product that had greater toxicity, requiring concentrations of only 0.01 and 0.05 L.ha-1 to cause mortality of 50 and 95% of subjects, respectively, compared with chlorantraniliprole and B. thuringiensis, causing LC50 mortality at higher concentrations of 0.04 and 0.22 L.ha-1 and LC95 at 0.36 and 1.5 L.ha-1, respectively (Table 2).
In assessing the lethal effects, there was no difference between treatments for LC25. The treatments spinosad, chlorantraniliprole and B. thuringiensis, showed satis-factory results in concentrations of 0.006; 0.02 and 0.10 L.ha-1, causing mortality of 30, 22 and 20% of the larvae, respectively. For LC50, the best result was obtained for the spinosad treatment that resulted in 36.25% mortality followed by chlorantraniliprole with 25% (Table 3).
Sublethal effects on the development of H. armigera
In the evaluation of sublethal effects on pupal weight, there was a difference between the treatments. Treatments that showed the best results were B. thuringiensis and spinosad in two concentrations tested, with 168 mg and 169 mg for LC25 and for LC50 167 and 173 mg, respectively (Table 4).
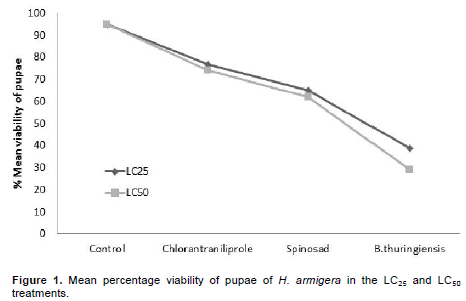
For the longevity of pupae, there were differences among the treatments. B. thuringiensis caused the greatest longevity in the two concentrations tested, presenting for LC25 longevity of 21.21 days and 24.09 days for LC50, an increase of 7 and 10 days vs. control, respectively (Table 4). As for assessing pupal viability, treatment with B. thuringiensis differed significantly compared to control. The LC25 and LC50 treatments with B. thuringiensis reduced the viability of pupae by 59.8 and 69.5%, respectively, compared to the control treatment, which showed viability of 97 and 95% (Figure 1).
Sublethal effect on oviposition of H. armigera
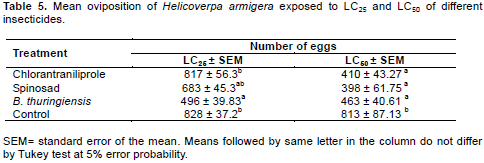
The evaluation of sublethal effects on oviposition showed significant differences between treatments. For the LC25 treatment it was B. thuringiensis that provided the lowest oviposition, averaging 496 eggs. For LC50 oviposition was reduced for all products tested compared with the control (Table 5).
Lethal concentration curves
The greatest toxicity was seen with spinosad at the concentrations of 0.006, 0.01 and 0.05 L.ha-1 for LC25 and LC50 and LC95, respectively, showing high toxicity in H. armigera (Table 2). The high toxicity of spinosad to H. armigera shows the susceptibility of this species to the product due it being the latest in use and having high insecticidal activity. High toxicity of spinosad also was reported by Wang et al. (2009) who studied the toxicity of spinosad in H. armigera and obtained an LC50 of 0.41 mg.kg-1.
For lethal effects spinosad at LC50 showed a significant difference compared to the other treatments with 36.25% mortality (Table 3). Yin et al. (2008), studying the sublethal effects of spinosad in Plutella xylostella (Lepidoptera: Yponomeutidae), found for LC25 (0.12 mg.L-1) and LC50 (0.28 mg.L-1) mortality of 24 and 51% respectively. The difference in percentage mortality of only 32.25% shown in LC50 can be explained as a function of the evaluation period. The toxicity evaluation test time was five days, while the mortality assessment was based on product exposure for only two days.
Sublethal effects on the development of H. armigera
The treatments presented sublethal effects at the different concentrations tested. Significant effects were found on pupal weight and viability in all treatments. Spinosad and B. thuringiensis were the products that most reduced the weight of the pupae (Table 4). For B. thuringiensis at LC50, the reduction in weight of the pupae was approximately 48% compared to control. Similar results were found by Lomate and Hivrale (2013), where B. thuringiensis inhibited the size and the weight of H. armigera by 56 and 54%, respectively. The weight of the pupae found with spinosad at LC25 and LC50 was 169 and 173 g, representing a decrease of approximately 46%. Yin et al. (2008), working with spinosad at LC25 and LC50, found a reduction in the weight of pupae of P. xylostella of 381 and 352 mg corresponding to 74 and 69%, respectively.
Reduced pupal weight is a consequence of reduced food intake in the larval period, or of the high metabolic cost required for detoxification which results in low weight of adults. Therefore, these factors can result in reduced fertility and may negatively affect the population growth of the next generation of H. armigera.
The longevity of the pupae differed among treatments. For both LC25 and LC50 the treatment with the greatest longevity was B. thuringiensis with an increase of 66 and 58% respectively (Table 4). The increase in longevity of pupae found in treatments is a major factor in the management of H. armigera, promoting greater time of exposure of the insect pest to biotic and abiotic factors, attenuating control by natural enemies, mechanical and physical.
A longer development period may imply a longer time of exposure to predators and parasites/parasitoids (Williams, 1999; Thaler et al., 2012.). According to Wang et al. (2009), studying the sublethal effects of spinosad on the survival, development and reproduction of H. armigera at a concentration of 0.16 mg.kg-1 found an increase of 71% in pupal mortality when compared to the control.
In assessing the viability of pupae, the best results were found for the treatment B. thuringiensis for LC25 and LC50, with 39 and 29% viability. Despite having shown a low mortality of larvae 13.75 and 20%, respectively. B. thuringiensis took longer to cause death than the neurotoxic insecticides spinosad and chlorantraniliprole. This delayed effect is due to the mode of action of the product, since the bacterial spores have to be ingested and only after reaching the mid-intestine and being solubilized, toxins are released that cause the rupture of the tissues, causing death itself. Barbosa et al. (2011), studying the effects of various insecticides on S. frugiperda, also observed a slow action of B. thuringiensis in relation to chemical products.
Spinosad presented pupal viability of 65 and 62% for the LC25 and LC50, respectively. These results are in agreement with results reported by Wang et al. (2009) when third instar larvae of H. armigera were exposed to 0.04 mg.kg-1 of spinosad, the viability of pupae found was 68.26% and control was 98.97%. Sayed and Sheikh (2014) in studies with Spodoptera littoralis (Lepidoptera: Noctuidae) found that the viability of pupae subjected to LC50 (40 µg.m-1) of spinosad was 55%. Information on the toxicity of products is important because it helps in the selection of insecticide, as some products provide longer lasting control, which reduces the number of applications and consequently entails lower costs for pest control and impacts on the environment. Effects of insecticides on the biotic potential of a pest are also relevant, especially when it comes to IPM, which aims to provide more efficient product rotation, decreased resistance and maintenance of populations for longer periods below the damage level of the crop in question, along with other control methods.
Sublethal effects on the oviposition of H. armigera
Comparing the effect of the insecticide treatments on oviposition, it was observed that B. thuringiensis and spinosad interfere with the potential for oviposition by H. armigera. The treatment with B. thuringiensis at LC25 significantly reduced oviposition by 41% compared to control (Table 4).
For the treatment spinosad at LC50 the reduction in oviposition was 51% compared to control. Similar results were found by Storch et al. (2007), studying the effect of spinosad on oviposition in Anticarsia gemmatalis, with a reduction in the number of eggs of 44.55%. Yin et al. (2008), studying the sublethal effects of spinosad on the oviposition of P. xylostella, showed a reduction of 50.4% in the number of eggs. The reduction in oviposition for chlorantraniliprole at LC50 was of approximately 50%. Zhang et al. (2013) found similar results studying the sublethal effects of the LC40 of chlorantraniliprole at a concentration of 21.57 μg.L-1 in H. armigera, obtaining a reduction in oviposition of 56% when the results were compared to control.
The results of lethal and sublethal effects are of fundamental importance, since an insecticide can act directly, causing mortality after application, as well as exerting adverse effects on development and reproduction, impacting the population dynamics of the next generation. Information on the adverse effects of the products serves as a parameter in IPM, enabling better decision-making for H. armigera control in the field.
Spinosad showed the greatest toxicity in the control of H. armigera. Spinosad and B. thuringiensis decreased in all parameters evaluated, causing negative effects on the pupal stage and oviposition.
Spinosad products chlorantraniliprole and B. thuringiensis may be used in pest management, as an alternative to product rotation in H. armigera control third instar. Further studies should be conducted to confirm these results in the field.
The authors have not declared any conflict of interest.
Thanks to FAPEP for the scholarship and Plant Laboratory of the Federal University of Piauí.
REFERENCES
|
Adab (2013). Programa de Supressão da Helicoverpa armigera. Ag. Est. Def. Agrop. Bahia. 1:1-7.
|
|
|
|
Barbosa RH, Kassab SO, Fonseca PRB, Rossoni C, Silva AS (2011). Biological and natural insecticides in the control of Spodoptera frugiperda (Smith JE, 1797) (Lepidoptera:Noctuidae) in corn cultivated under field conditions. Green J. 6(3):247-251.
|
|
|
|
|
Conab (2015). Acompanhamento da safra brasileira de grãos, safra 2014/15. Comp. Nac. Abast. 2(10):1-113
|
|
|
|
|
Czepak C, Albernaz KC, Vivan LM, Guimarães HO, Carvalhais T (2013). First reported occurrence of Helicoverpa armigera (Hubner) (Lepidoptera:Noctuidae) In Brazil. Trop. Agric. Res. 43:110-113.
|
|
|
|
|
Desneux N, Decourtye A, Delpuech JM (2007). The sublethal effects of pesticides on beneficial arthropods. Ann. Rev. Entomol. 52:81-106.
Crossref
|
|
|
|
|
Embrapa (2013). Ações emergenciais propostas pela Embrapa para o manejo integrado de Helicoverpa spp. em áreas agrícolas. 1:1-19
|
|
|
|
|
Fathipour Y, Sedaratian A (2013). Integrated Management of Helicoverpa armigera in soybean cropping systems. Soybean Pest Resistent. Chapter 9:232-280.
Crossref
|
|
|
|
|
Fitt GP (1989). The ecology of Heliothis species in relation to agroeco systems. Annu. Rev. Entomol. 34:7-52.
Crossref
|
|
|
|
|
Gunning RV, Moores GD (2010). The effects of diet on the detection of resistance to Cry1Ac toxin in Australian Helicoverpa armigera Hübner (Lepidoptera:Noctuidae). Pest. Biochem. Physiol. 97:55-59.
Crossref
|
|
|
|
|
Jacobson A, Foster R, Krupke C, Hutchison W, Pittendrigh B, Weinzierl R (2009). Resistance to Pyrethroid Insecticides in Helicoverpa zea (Lepidoptera:Noctuidae) in Indiana and Illinois. J. Econ. Entomol. 102:2289-2295.
Crossref
|
|
|
|
|
Junior HJGS, Marques EJ, Polanczyk RA, Pratissoli D, Rondelli VM (2009). Suscetibilidade de Helicoverpa zea (boddie) (Lep.:Noctuidae) a Bacillus thuringiensis Berliner (bacillaceae). Arq. Instit. Biol. 76(4):635-641.
|
|
|
|
|
Sas Institute (2002). SAS user's manual, version 9.1. In:(Ed). SAS Instit. Cary:NC.
|
|
|
|
|
Kasten Júnior P, Precetti AACM, Parra JRP (1978). Comparative biological data on Spodoptera frugiperda (J. E. Smith, 1797) on two artificial diets and natural substrate. J. Agric. 53:68-78.
|
|
|
|
|
Kotkar HM, Sarate PJ, Tamhane VA, Gupta VS, Giri AP (2009). Responses of midgut amylases of Helicoverpa armigera to feeding on various host plants. J. Inst. Physiol. 55:663-670.
Crossref
|
|
|
|
|
Lomate PR, Hivrale VK (2013). Effect of Bacillus thuringiensis (Bt) Cry1Ac toxin and protease inhibitor on growth and development of Helicoverpa armigera (Hübner). Pest. Biochem. Physiol. 105:77-83.
Crossref
|
|
|
|
|
Mccaffery AR (1998). Resistance to insecticides in Heliothine Lepidoptera:a global view. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 353(1376):1735-1750
Crossref
|
|
|
|
|
Moral Garcia FJ (2006). Analysis of the spatiotemporal distribution of Helicopverpa armigera (Hübner) in tomato field using a stochastic approach. Biosyst. Eng. Bedford. 93(3):253-259.
Crossref
|
|
|
|
|
Perry T, Batterham P, Daborn PJ (2011). The biology of insecticidal activity and resistance. Ins. Biochem. Mol. Biol. 41:411-422.
Crossref
|
|
|
|
|
Rajapakse CNK, Walter GH (2007). Polyphagy and primary host plants:oviposition preference versus larval performance in the lepidopteran pest Helicoverpa armigera, Arthropod–Plant. Int. 1:17-26.
|
|
|
|
|
Sayed AE, Sheikh E (2014). Comparative toxicity and sublethal effects of emamectin benzoate, lufenuron and spinosad on Spodoptera littoralis Boisd. (Lepidoptera:Noctuidae). Crop. Prot. 67:228-234.
|
|
|
|
|
Shinde SS, Kamtikar VN, Muley S, Nimbalkar RK (2011). LC50 for Insecticides against second instar larvae of cotton bollworm Helicoverpa armigera (Hubner) (Lepidoptera:Noctuidae) in Maharashtra. J. Ecobiot. 2(3):22-24.
|
|
|
|
|
Storch G, Loeck AE, Borba RS, Magano DA, Moraes CL, Grützmachera D (2007). Efeito de inseticidas aplicados em doses subletais sobre a dieta artificial e em lagartas de Anticarsia gemmatalis. (Lepidoptera:Noctuidae). Rev. Brasil. Agric. 13:175-179.
|
|
|
|
|
Thaler JS, Mcart SH, Kaplan I (2012). Compensatory mechanisms for ameliorating the fundamental trade-off between predator avoidance and foraging. Proceed. Nat. Acad. Sci. USA. 109:12075-12080.
Crossref
|
|
|
|
|
Wang D, Gong P, Li M, Qiua X, Wang K (2009). Sublethal effects of spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera:Noctuidae). Pest. Manage. Sci. 65:223-227.
Crossref
|
|
|
|
|
Williams IS (1999). Slow growth, high mortality – a general hypothesis, or is it? Ecol. Entomol. 24:490-495.
Crossref
|
|
|
|
|
Yang Y, Li Y, Wu Y (2013). Current Status of Insecticide Resistance in Helicoverpa armigera After 15 Years of Bt Cotton Planting in China. J. Econ. Entomol. 106:375-381.
Crossref
|
|
|
|
|
Yin XH, Wu QJ, Li XF, Zhang YJ, Xu BY (2008). Sublethal effects of spinosad on Plutella xylostella (Lepidoptera:Yponomeutidae). Crop. Prot. 27:1385-1391.
Crossref
|
|
|
|
|
Zhang RM, Dong JF, Chen JH, Ji QE, Cui JJM (2013). The Sublethal Effects of Chlorantraniliprole on Helicoverpa armigera (Lepidoptera:Noctuidae). J. Int. Agric. 12:457-466.
Crossref
|
|
|