ABSTRACT
One hundred wheat (Triticum aestivum L.) accessions were selected on the basis of different geographical areas of Pakistan. Isolation and identification of seed born fungi were conducted according to standard blotter test and a total of five major seed borne fungi including Alternaria alternata, Aspergillus niger, Fusarium species, Drechslera species and Phytophthora species were isolated from the wheat seeds. The frequency of occurrence of these five seed born fungi was 49, 46, 42, 35, and 16%, respectively. Infection percentage varied from 0 to 90% in all 100 wheat accessions. Among the accessions, the highest infection (100%) of seed born fungi was recorded in 011185 and 011757 accessions while the lowest infection (10%) was recorded in 011415 accessions. Moreover, in accessions collected from Gilgit Baltistan and Azad Jammu Kashmir. Alternaria niger and Alternaria fusarium were dominant, while in Khyber Pakhtunkhawa province, A. niger was prevalent followed by A. alternata. In the case of Baluchistan province, the dominant seed born fungi was A. alternata followed by Drechslera spp. Similarly, in case of Punjab, the occurrence of A. alternata, Drechslera spp., Fusarium spp., and A. niger associated with seeds were similar. For accession collected from Sindh province, the dominant seed born fungi was A. niger and Drechslera spp. However, the Phytophthora spp. infection of wheat seeds accession of Baluchistan was the highest followed by wheat seeds accession collected from Gilgit Baltistan and AJK, Kpk and Punjab, whereas wheat seeds accessions collected from Sindh province were found to be free from Phytophthora spp.
Key words: Bread wheat, screening, wheat germplasm, seed born fungi.
Wheat (Triticum aestivum L.) is one of the most important staple foods among agricultural crops since it constitutes the basis for human nutrition and is of enormous economic importance worldwide. Wheat is used mainly for human consumption and supports nearly 35% of the world population (Schuster et al., 2009). It supplies a large fraction of the dietary protein, total food supply. It is also a principal source of carbohydrates and proteins both for human beings and animals (Ali et al., 2013).
In Pakistan, wheat (T. aestivum L.) crop is considered as the best cereal since it ranks the first among the cultivated cereals in the country and occupies about 66% of the annual food crop area (Ansari et al., 2006). Wheat contributes 10.1% to the value added in agriculture and 2.2% to gross domestic product (GDP). Area under wheat increased to 8693 thousand hectares in 2012 to 2013, from 8650 thousand hectares showing an increase of 0.5% over last year’s area. In Pakistan, the production of wheat crop stood at 24.2 million tonnes during 2012 to 2013, against the target of 25.5 million tonnes which is 5.1% decrease while an increase of 3.2% over the last year production of 23.5 million tonnes has been witnessed. The yield per hectare in 2012 to 2013 stood at 2787 (kg/ha) posted a positive growth of 2.7% as compared to negative 4.2% growth last year (PBS, 2013).
Wheat is stored for a period of time before it can be marketed or used as feed or seed. The length of time cereal can be safely stored will depend on the condition it was harvested and the type of storage facility being utilized. Conditioning of grain has the single purpose of preserving the quality of grain. Low moisture content and temperature have been shown to be essential for successful storage of grain for a long period of time (Chaudhary et al., 2000).
The quality seed of improved wheat varieties is also considered as most important input for obtaining optimum production. Only the good seed can give an economic benefit to the wheat grower. It can maintain the quality of production, which fetches higher value in the market. Therefore, availability of healthy and pure seed should be confirmed, otherwise most of the seed-borne diseases of wheat could become responsible for heavy losses (Marwat et al., 2002).
Wheat crop is subjected to a number of diseases, which reduces its overall production to a great extent, because wheat plants in all stages of growth and in all natural environments are subject to various mechanical, physiologic and biological stresses that interfere with their normal growth and development. Biotic hazards, insects, viruses, fungi, nematodes, bacteria and weeds are primary hazards to wheat production (Ahmad et al., 2003).
The actual number of wheat diseases is unknown, but nearly 200 have been reported from the wheat-growing countries in the world. Over 100 infectious diseases caused by pathogens and by weeds are transmissible
from plant to plant. Amongst these, about 50 are generally seed-borne. In Pakistan, 50 diseases are reported to occur which have great financial repercussions (Iftikhar et al., 1991). The rusts are considered the most destructive, but the problem of seed-borne diseases is also of great importance and could not be neglected. Smut, bunt, blight and root rot are some important seed-borne diseases, which are perpetuated through seeds and cause considerable losses to crops under favorable conditions (Dawson and Bateman, 2001). Seed-borne diseases have been found to affect the growth and productivity of crop plants and especially seed-borne fungi are important from the economic point of view as they render losses in a number of ways. Numerous examples exist in agriculture literature for the international spread of plant diseases as a result of importation of seeds that were infected or contaminated with pathogens (Clear and Patrick, 1993). The study of seed-borne pathogens is necessary to determine seed health and to improve germination potential of seed which finally leads to increase of the crop production (Bishaw et al., 2013).
Fungi are the principal pathogen organisms associated with crop seeds. A complex of seed-borne fungi including genera of Tiletia, Ustilago, Bipolaris, Fusarium, Alternaria, Drechslera, Stemphylium, Curvularia, Cladosporium, Rhizopus, Aspergillus and Penicillium have been convincingly reported as the most frequent seed-borne fungi of wheat throughout the world (Kumar et al., 2008). The present study was conducted to isolate the wheat seed associated fungi from seeds collected from different geographical locations of Pakistan in order to know the importance of seed borne pathogens and their effects on wheat crop in these locations.
Collection of seed samples
Wheat seeds (100 accessions) were obtained from the National Agriculture Research Center (NARC) gene bank, Islamabad. The accessions were selected on the basis of different geographical areas of Pakistan (Table 1a, b, c, d and e).
Isolation of seed associated fungi
For isolation of fungi associated with wheat seed, blotter test was used. Initially 90 mm size discs of blotting paper were moistened with autoclaved distilled water and placed at the bottom of 90 mm sterilized Petri plates. The seed were surface sterilized with 5% hypochlorite solution followed by a rinse with autoclaved water. Ten seeds of each wheat accession were placed at equal distance in separate Petri plates using a sterilized pair of forceps. The lids of the Petri plates ware held in place with parafilm. The plates were incubated at 27°C for a period of 5 to 7 days under 12 h alternating cycle of light and darkness. After the incubation period, fungi growing out from seeds were examined, identified and their percentage frequency (PF) and relative abundance of infected seeds were calculated by the following formula:
Frequency of occurrence (%) = No. of seeds on which a fungal species occurs/Total No. of seeds × 100
Purification of cultures
Pure cultures were obtained after repeated sub-culturing of fungi appearing on seeds on Potato Dextrose Agar (PDA) plates.
Identification of fungi
The pure cultures of fungi were identified on the basis of spore morphology and colony characteristics were examined using a stereo-binocular microscope (Barnett and Hunter, 1992).
Identification of seed born fungi associated with wheat seeds
After incubation, fungi infection appeared on the wheat seeds as shown in Figure 1. Pure culture of seed born fungi is as shown in Figures 3, 4, 5, 6 and 7.
Frequency of fungi in wheat seeds
The frequency of each fungus in wheat seeds is presented in Figure 2: Alternaria alternata (49%), Aspergillus niger (46%), Fusarium species (42%) Drechslera species (35%), and Phytophthora species (16%).
Isolation of fungi from wheat seeds
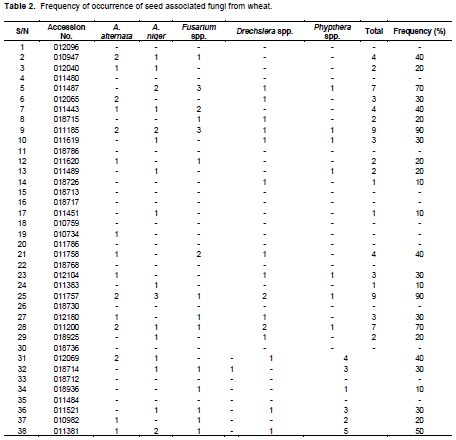
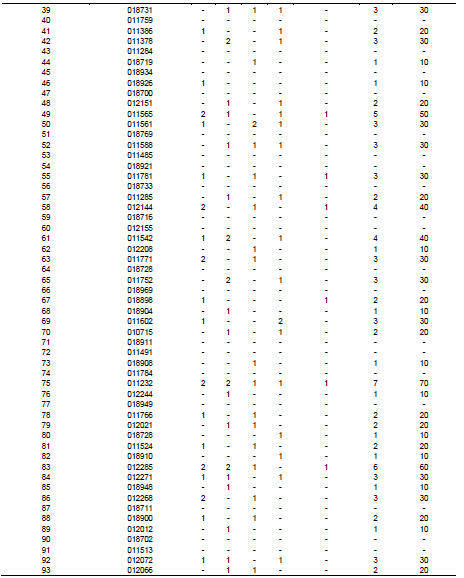
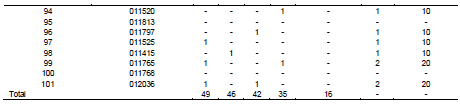
In total, five fungi including both saprophytic as well as pathogenic were isolated from wheat seeds (Table 2). Fungi isolated from wheat seeds were A. alternata, A. niger, Fusarium spp., Drechslera spp., and Phytopthra spp. Infection percentage varied from 0 to 90% in all the accessions tested with accessions no. 011185 and 011757 showing the highest 90% infection, three accessions numbers 011487, 011200 and 011232 showed 70% infection, 012285 showed 60% infection, two accessions numbers 011381 and 011565 showed 50% infection. Six accessions numbers 010947, 011443, 011758, 012069, 012144, and 011542 showed infection of 40%. Seventeen accessions 012065, 011619, 012104, 012180, 018714, 011521, 018731, 011378, 011561, 011588, 011781, 011771, 011752, 011602, 012271, 012268, and 012072 showed infection of 30%. Eighteen accessions 012040, 018715, 011620, 011489, 018925, 010982, 011386,012151, 011285, 018898, 010715, 011766, 012021, 011524, 018900, 012066, 011765, and 012036 showed infection of 20%. Eighteen accessions 018726, 011451, 011383, 018936, 018719, 018926, 012208, 018904, 018908, 011232, 018728, 018910, 018948, 012012, 011520, 011797, 011525, and 011415 showed 10% infection.
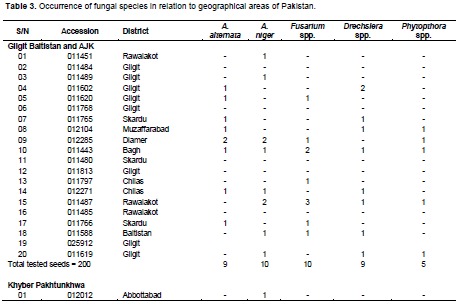
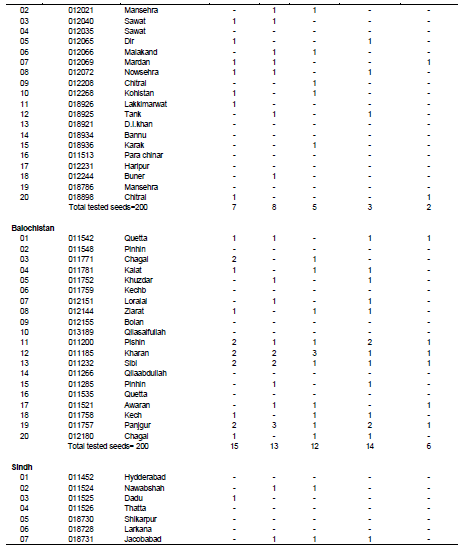
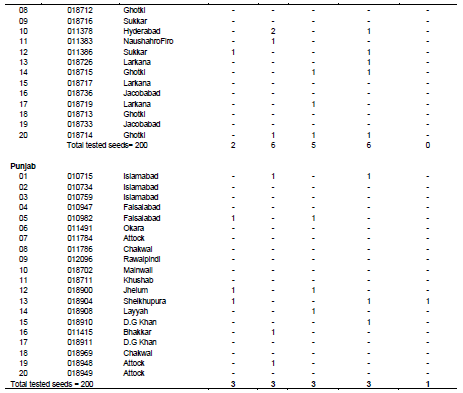
Occurrence of fungal species in relation to geographical areas of Pakistan
Table 3 reveals the occurrence of fungal species in wheat seed accessions in relation to different geographical areas of Pakistan. In the case of Gilgit Baltistan and AJK, from a total amount of 200 tested seeds, 9 were found to be infected with A. alternata, 10 with A. niger and Fusarium spp., 9 with Drechslera spp., 5 with Phytopthora spp. whereas the remaining seeds were free from seed born fungi. In case of wheat accessions for kpk, out of 200 tested seeds, 7 seeds were found to be infected with A. alternata, 8 seeds were found to be infected with A. niger, 5 were found to be infected with Fusarium spp., 3 were found to be infected with Drechslera spp. and 2 were found to infected with Phytopthora spp. whereas the rest of the seeds were found to free from seed born fungi. In case wheat accession for Baluchistan province, out of total 200 tested seeds, 15 were found to be severely infected with A. alternata, 13 seeds were recorded to be infected with A. niger, 12 seeds were found to be infected with Fusarium spp., 14 seeds were found to be infected with Drechslera spp. and 6 seeds were infected with Phytopthora spp. In case of wheat accessions from seeds, out of total 200 tested seeds, 2 seeds were infected with A. alternata, 6 seeds were found to be infected with A. niger, 5 seeds were infected with Fusarium spp., 6 seeds were found to be infected with Drechslera spp. and no seeds were found to be infected with Phytopthora spp. whereas the rest of the seeds were free from seed born fungal infections. In case of wheat accession collected from Punjab province, out of 200 tested seeds, 3 seeds exhibited infection of A. alternata, 3 seeds were found to be infected with A. niger, 3 seeds were found to be infected with Fusarium spp., 3 were infected with Drechslera spp. and 1 was found to be infected with Phytophthora.
Identification of fungi
The isolated fungi were identified on the basis of spore morphology and colony characteristics. Some features on the basis of which fungi were identified are as follows:
A. alternata
The fungus A. alternata was identified as it produced woolly or powdery chains of dark brown conidia of uneven shapes and lengths. The colony colour was dark brown (Figure 3a). The mycelium was abundant and variable in colour, usually light olive green to dark brown. Hyphae were thick, septate, dark brown and branched, conodiophores were erect and simple with septate conidia (Figure 3b).
A. niger (van Tieghem)
Colony of A. niger on seed grew slowly, consisting of a compact to fairly loose white to faintly yellow basal mycelium, which bears abundant erect and usually crowded conidial structures (Figure 4a). Conidiophores arise directly from the seeds coat and are smooth, hyaline or faintly brownish near the apex (Figure 4b).
Fusarium spp. (Sacc)
Fusarium spp. had a rapid growth on Potato dextrose agar PDA. The texture of colony was flat to wooly and pink in colour (Figure 5a). Conidia were 2 or more celled, curved, thick-walled, smooth, and canoe-shaped (Figure 5b)
Drechslera halodes (Ito) and Phytopthora spp.
Colony on PDA was dark brown (Figure 6a). Conidiophores were thick, septate, cylindrical, and paler toward the apex and were simple. Conidia were straight and slightly curved and thick walled (Figure 6b) and Phytopthora (Figure 7a and b).
Seed borne fungal pathogens transmit most of the major disease of wheat crop and reduce seed quality, nutrient contents, germination capacity as well as seedling collapse, which consequently reduce crop yield (Mushtaq and Hashmi, 2005). Over the last two decades, various studies have been carried out to identify seed born fungal pathogen of wheat crop throughout the world. For example in Canada, 35 fungal genera and 59 seed born fungi exist in association with wheat seeds. From Pakistan, Khan (1992) reported 17 genera and 45 species of seed born fungal pathogens associated with wheat seeds. In this study, the results show that a total of 5 major fungal pathogen including A. alternate, A. niger, Fusarium spp., Drechslera spp. and Phytophthora spp. were identified and isolated from the seeds of wheat crop. Zare et al. (2006) who determined the fungi species and infection rates as 15% Fusarium culmorum, 13.1% Fusarium graminearum, 4.5% Drechslera spp., 24.2% A. alternate and 5% A. niger in harvested wheat loads in different provinces of Iran. In the present study, the lowest infection rate of seed to Pytophthora was determined in seed accession collected from Sindh province and the highest infection rate was reported from Baluchistan province. The results also show that infection percentage of five major seed borne pathogens varied from 0 to 90% in all accessions collected from different geographical areas of Pakistan. Moreover, the frequencies of five mentioned major fungus were higher in wheat seed accessions collected from Baluchistan and Gilgit Baltistan and AJK as compared to other geographical regions of Pakistan. It was found that among the seed born fungi frequency, A. alternata in all accessions was the highest as compared to other fungi associated with wheat seeds. Rajput et al. (2005) tested on hundred and twenty sample of wheat seeds for the presence of fungal seed borne pathogens collected from wheat growing of Sindh. From twelve wheat varieties five seed borne fungi that are A. niger, Alternaria tenuis, Fusarium moniliformie, Stemphylium herbarum and Curvuluria lunata were isolated. Same experiment is performed by Babadost (1997) who detected some species of Fusarium fungus in wheat seeds collected from cereal fields in the North West of Iran. In the results, A. alternata was the most frequent fungi associated with the wheat seeds. The presence of weakly pathogenic or saprophytic fungi such as Helminthosporium, Curvularia, Stemphylium, Rhizopus, Cladosporium, Aspergillus, Penicillium, Alternaria, Gonatobotrys and Nigrospora has been reported from wheat seeds (Habib et al., 2011). Saberi et al. (2004) also isolated seed-borne fungi (Aspergillus species, Alternaria spp., Cladosporium species, Penicillium species and Ulocladium spp.) associated with wheat grains in Markazi province. Hussain et al. (2013) reported Bipolaris sorokiniana (11.125%), Aspergillus flavus (9.825%), A. alternata (7.15%) and A. niger (6.225%) associated with wheat seeds. It is apparent from the present research that all the accessions of wheat crop tested were contaminated by fungi. Knowing the major contribution of wheat crop in world food, its production must be enhanced to meet the nutritional requirements of a rising human populations (Oerke and Dehne, 2004). Certified and healthy seeds of wheat crop are significant input for crop production and consequently reduction of yield loss caused by these seed born fungi and is a main way to contribute to the food security in the world. Moreover, seed born fungi can be easily controlled through treatment of seeds using fungicides and biological compounds. Further, using standard storage facilities for preserving wheat seeds and developing resistant germplasm to reduce the infection level caused by these seed-borne fungi below damage threshold have been recommended (Clark et al., 2004).
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmad J, Marwat MI, Ahmad HK (2003). Effect of herbicides and row spacing on different traits of wheat (Triticum aestivum L.). Pakistan J. Weed Sci. Res. 9(1-2):33-40.
|
|
|
|
Ali S, Shumaila U, Zahida N, Naseem Z, Saima N, Ammara Y, Tehseen Y (2013). Nutritional evaluation and stabilization studies of wheat germaplasm. Pak. J. Food sci. 23:148-152.
|
|
|
|
|
Ansari MA, Meman HR, Tunio SD, Keerio SA (2006). Effect of planting pattern on growth and yield of wheat. Pak. J. Agric. Agril. Eng. Vet. Sci. 22(2):22-24.
|
|
|
|
|
Babadost M (1997). Species of Fusarium in seeds and wheat plants in West Azarbayjan and Ardabil. Iran. J. Plant Pathol. 31:88-100.
|
|
|
|
|
Bishaw Z, Struik PC, Van Gastel AJG (2013). Farmer's seed sources and seed quality: 2. Seed health. Int. J. Plant Prod. 7:637-657.
|
|
|
|
|
Chaudhary MA, Ali A, Siddique MA, Sohail R (2000). Growth and yield response of wheat to different seed rates and wild oat (Avena fatua) competition durations. Pak. J. Agric. Sci. 37:152-154.
|
|
|
|
|
Clark B, Cockerell V, Thomas J (2004). Wheat seed health and seedborne diseases-A guide. HGCA, Caledonia House, London.
|
|
|
|
|
Clear RM, Patrick SK (1993). Prevalence of some seedborne fungi on soft white winter wheat seed from Ontario, Canada. Can. Plant Dis. Surv. 73:143-149.
|
|
|
|
|
Dawson, WAJM, Bateman GI (2001). Fungal communities on roots of wheat and barley and effect on seed treatment containing fluquiconazol applied to control take-all. Plant Pathol. 50:5-82.
Crossref
|
|
|
|
|
Habib A, Sahi ST, Javed N, Ahmad S (2011). Prevalence of seed-borne fungi on wheat during storage and its impact on seed germination. Pak. J. Phytopathol. 23(1):42-47.
|
|
|
|
|
Hussain M, Muhammad UG, Muhammad IH, Raza M (2013). Seed borne mycoflora of some commercial wheat cultivars in Punjab, Pakistan. Int. J. Pathol. 2:2.
|
|
|
|
|
Iftikhar, S, Ahmad I, Aslam M (1991). Seed born diseases of wheat in Pakistan and their control. Pak. Agric. Res. Council Islamabad 11(6):14-18.
|
|
|
|
|
Khan SAJ (1992). Studies on fungi causing seed-borne diseases of wheat and rice and their control. PhD dissertation, University of Karachi, Karachi, Pakistan. Ph.D. Thesis, Department of Botany/ University of Karachi, Pakistan 261pp.
|
|
|
|
|
Kumar A, Singh US, Kumar J, Garg GK (2008). Application of molecular and immune diagnostic tools for detection, surveillance and quarantine regulation of Karnal bunt (Tilletia indica) of wheat. Food Agric. Immunol. 19:293-311.
Crossref
|
|
|
|
|
Marwat MI, Ahmad HK, Khan HH, Khan A (2002). Integrated weed management in wheat. 1. Weed density, dry weed biomass, absolute growth rate and grain yield. J. Weed Sci. Res. 8(1-2):81-93.
|
|
|
|
|
Mushtaq SNM, Hashmi MH (2005). Seed-borne mycoflora of Sunflower (Helianthus annuus L.). Pak. J. Bot. 37(2):451-457.
|
|
|
|
|
Oerke EC, Dehne HW (2004). Safeguarding production-losses in major crops and the role of crop protection. Crop Prot. 23:275-285.
Crossref
|
|
|
|
|
PBS (2013). Pakistan Bureau of statistics, Ministry of National Food Security and Research (Economic survey of Pakistan, report).
|
|
|
|
|
Rajput MA, Pathan MA, Lodhi AM, Shah GS, Khanzada KA (2005). Studies on seed-borne fungi of wheat in sindh province and their effect on seed germination. Pak. J. Bot. 37(1):181-185.
|
|
|
|
|
Saberi R, Javan-Nikkhah M, Heidarian R, Hosseni S, Soleimani P (2004). Detection of fungal infectious agent of wheat grains in storepits of Markazi province. Iran. Agric. Appl. Biol. Sci. 67:418-423.
|
|
|
|
|
Schuster I, Vieira ESN, da Silva GC, de Assis Franco F, Marchioro VS (2009). Genetic variability in Brazilian wheat cultivars assessed by microsatellite markers. Genet. Mol. Biol. 32(3):557-563.
Crossref
|
|
|
|
|
Zare L, Harsini M, Hosseini-bai S, Motieshare B (2006). Contamination of seed harvested wheat loads to a number of seed-borne fungal pathogen. In Proceeding 17th Iranian Plant Protection Congress. P 24.
|
|