ABSTRACT
The herbicides saflufenacil and indaziflam have recently been registered in Brazil for weed control in sugarcane crops; however, little information exists regarding their residual effects or influences on soil microorganisms. Therefore, the present study aimed: (a) to determine the effects of saflufenacil and indaziflam on soil microorganisms and (b) to evaluate the residual and dose effects of these herbicides on soybean, sunflower, sunn hemp and peanut crops. The herbicides indaziflam (100 g a.i. ha-1) and saflufenacil (120 g a.i. ha-1) were applied to dark red latosol samples, and the CO2-C released by soil basal respiration was measured at 7, 14, 21, 28, 35, 42, 49 and 56 days after treatment (DAT), in an experiment with a completely randomized design (CRD) and five replicates. The microorganisms were quantified via the use of different culture media, each replicated three times at 0, 15, 30 and 60 DAT. No significant difference occurred among the treatments for the carbon content of the microbial biomass. Regarding the basal respiration, the soils treated with saflufenacil showed a decrease in the carbon released by the soil at 49 DAT, whereas the carbon released by the soils treated with indaziflam increased until the last day of evaluation. The responses of the fungal and bacterial populations and the amylolytic and cellulolytic microorganisms differed among the treatments. The residual effect of the herbicides on the crops was evaluated via a CRD, in a 6 (doses) × 5 (sowing times) factorial arrangement with four replicates. The different indaziflam and saflufenacil doses were sprayed separately at pre-emergence. At 0, 10, 20, 40 and 60 days after the herbicide applications, soybean, sunn hemp, sunflower and peanut were sown. The phytotoxicity of saflufenacil to the crops declined throughout the evaluations for all the doses and species. Indaziflam was highly phytotoxic to all the crop species until 60 days after application, preventing the field sowing of the crops during that period.
Key words: Phytotoxicity, sugarcane, carryover, microbial degradation.
The herbicides saflufenacil and indaziflam have been recently registered in Brazil for weed control in sugarcane crops; nevertheless, little information exists regarding their effects on other crops, especially those used rotationally in sugarcane fallow areas. Saflufenacil can be pre-plant incorporated and applied pre- and post-emergence in crops such as sugarcane, maize, soybean, wheat and cotton. This herbicide is used for the control of eudicots and belongs to the pyrimidinedione chemical class, inhibiting the enzyme protoporphyrinogen oxidase (PROTOX). The main physicochemical characteristics of saflufenacil include a vapor pressure (VP) of 2 × 10-14 mm Hg at 25°C (a nonvolatile herbicide), a half-life (t1/2) of one to five weeks, a pKa of 4.3 (a weak acid) and a water solubility of 30 mg L-1 at pH 5.0 and 2100 mg L-1 at pH 7.0 (BASF, 2008). This herbicide is absorbed by both roots and leaves, with its translocation occurring mainly in the xylem and its mobility limited in the phloem. Susceptible plants show symptoms of injury within a few hours and die within one to three days (Soltani, 2010). Soil organic matter has a high affinity for the saflufenacil molecule; therefore, soils with high organic matter content have a relatively small amount of the molecule available for plant absorption (Monquero et al., 2012). Gannon et al. (2014) observed that the saflufenacil phytotoxicity to canola was dependent on the soil properties and that those soils with high contents of organic matter and clay showed relatively low toxicity. Soltani (2010) observed that saflufenacil at 100 and 200 g a.i. ha-1 caused 51 to 99% injury and reduced height by 25 to 93%, shoot dry weight by 92 to 99% and seed yield by 56 to 99% in cranberry and in adzuki, Lima, snap and white beans. Soybean and pea were the crops most tolerant to saflufenacil.
Indaziflam belongs to the alkylazine chemical class and acts on cell wall biosynthesis without affecting the synthesis of polysaccharide polymers. The action is inhibitory and most likely occurs at some point during the cross-linking stage of cellulose microfibrils. Inhibition of cell division in meristematic tissues has also been proposed as a secondary mode of action. Indaziflam is used as a pre-emergent herbicide for the control of monocotyledonous weeds and some eudicots in perennial crops, such as citrus, coffee and sugarcane. Its physicochemical characteristics are as follows: a VP of 1.875 × 10-10 mm Hg at 20°C (a nonvolatile herbicide), a half-life of approximately 150 days, a pKa of 3.5 and a water solubility of 0.044 to 0.0017 g L-1 at pH 4.0 and 0.0028 to 0.0012 g L-1 at pH 9.0 (U.S. EPA, 2010; Alonso et al., 2011). In the US, the labeled rate for indaziflam in Florida citrus ranges from 73 to 95 g a.i ha-1. Indaziflam provides three to four months of residual weed control in citrus, depending on humidity and temperature (Jhala and Singh, 2012). For the control of annual grasses sensitive to this herbicide, the doses range from 25 to 100 g ha-1, reaching up to 150 g ha-1 for more tolerant species (Kaapro and Hall, 2012). According to Guerra et al. (2014), studies conducted during the initial development of maize, millet, sorghum, soybean, sunflower, cotton, beet and cucumber crops indicated that all the species were sensitive to soil-applied indaziflam in the field. The only symptom observed in the different species after planting in soil containing this herbicide was the nonemergence of seedlings, except for sunflower. Cotton and maize did not emerge only when sown in the soil with the highest indaziflam dose (100 g ha-1).
Soybean plants emerged in the soil treated with the two lowest indaziflam doses (20 and 40 g ha-1) but died after a few days. In contrast, sorghum, millet, cucumber and beet did not emerge even in soil treated with the lowest indaziflam dose (20 g ha-1) (Guerra et al., 2014). Herbicides may directly or indirectly affect microbial activity in the soil. The direct effects include toxicity to the soil microbiota, whereas the indirect effects include damage to the crops that affects their physiology, reducing plant-microorganism interactions. For example, Arruda et al. (2001) reported that sulfentrazone application reduced root nodulation and the exudation of amino acids by soybean xylem. Note that agrochemicals may positively or negatively affect soil microorganisms. Positive effects occur when the product is metabolized by the soil microorganisms, and negative effects occur when the chemicals poison them (Santos et al., 2005; Vivian et al., 2006). Therefore, the present study aimed to determine the residual effect of saflufenacil and indaziflam and the dose-response relationship of these herbicides regarding the growth and development of Glycine max (soybean), Crotalaria juncea (sunn hemp), Helianthus annuus (sunflower) and Arachis hypogaea (peanut) together with the effects of these herbicides on amylolytic and cellulolytic microorganisms, fungi and total bacteria.
Effects of the herbicides saflufenacil and indaziflam on soil microorganisms
The herbicides saflufenacil and indaziflam were applied on August 09, 2016. The experimental units consisted of trays (28 × 43 × 4.5 cm) containing 2 kg of dark red latosol, composed of four single samples. The soil used in the experiment was collected in a native forest with no history of herbicide use, at a depth of 10 cm. The chemical analysis of the soil samples indicated the following: P = 15 mm dm-3; organic matter = 24%; pH CaCl2 = 5.1; K = 2.5 mmolc dm-3; Ca = 28%; Mg = 12%; H + Al = 0.4%; sum of bases (SB) = 42.5%; cation exchange capacity (CEC) = 82.5%; percentage of base saturation (V%) = 52; and clay, sand and silt = 600, 150 and 190 g kg-1, respectively. Herbicides were applied at doses of 120 g a.i. ha-1 of saflufenacil and 100 g a.i. ha-1 of indaziflam; the control group received no herbicide treatment. The herbicides were applied via a CO2 backpack sprayer equipped with three TeeJet DG 110.03 VS nozzles (Drift Guard), with a 0.50-m spacing and a flow rate of 200 L ha-1. At the time of application, the wind speed was 2.1 m s-1, the humidity was 56.9%, and the temperature was 30°C. The soil samples were subsequently crushed and sieved through a 2-mm mesh and homogenized, with the moisture adjusted to 60% of field capacity.
Microbial biomass carbon content
The microbial biomass was determined using the fumigation-extraction method described by Vance et al. (1987). The samples were analyzed in triplicate; that is, each soil sample was divided into six subsamples of 20 g each and placed in 100-ml glass bottles. Three subsamples were subjected to the fumigation-extraction process, and the other three were subjected to immediate extraction after weighing (non-fumigated samples). The carbon content in the soil extracts was calculated as follows:
MBC (μg C g-1 soil) = (F-NF) / Kc,
Where MBC = microbial biomass carbon; F = fumigated samples; NF = nonfumigated samples; Kc = 0.33, a correction coefficient.
Microbial activity assessed by basal respiration (respirometry)
Glass jars with lids were used as respirometers. The microbial activity was evaluated by the amount of CO2 released at 7, 14, 21, 28, 35, 42, 49 and 56 days from non-fumigated soil samples in a static system (GRISI, 1995). A completely randomized design (CRD) was used, with five replicates for each of the following treatments: blank, control (no herbicide application), indaziflam and saflufenacil. Fifty-gram samples of sieved soil within snap cap glass flasks were placed inside a jar along with another flask containing 10 ml of 1 N NaOH to capture the CO2 released by the soil. The flasks were hermetically sealed and incubated at 25 ± 2°C in the dark. Jars containing only 10 ml of 1 N NaOH were incubated as well (blanks). Every seven days of incubation, the NaOH solution was titrated with a standard solution of 0.5 N HCl, by adding 2 ml of saturated 10% BaCl2 solution to precipitate the Na2CO3 and two drops of 1% phenolphthalein solution as an indicator. Soil basal respiration was quantified using the following equation:
SBR (mg of CO2-C kg-1 soil hour-1) = (((Vb-Va)*0.5*6*1000)/Wd)/T)
where SBR = carbon from soil basal respiration, Vb = volume (mL) of hydrochloric acid used to titrate the control solution (blank), Va = volume (mL) of hydrochloric acid used for sample titration, Wd = dry weight (g) and T = time (hours).
Quantification of total bacteria, fungi and amylolytic and cellulolytic microorganisms
The bacteria and fungi present in the soil samples were quantified by counting the colony-forming units (CFU) via the serial dilution technique and plating in the following culture media: nutrient agar (NA) for bacteria, Martin’s medium for fungi, cellulose for cellulolytic microorganisms and starch agar for amylolytic microorganisms. The NA medium was prepared by adding 28 g of NA per liter of distilled water. Subsequently, the NA medium was autoclaved for 20 min at 120°C and 1 atm, and 2 ml of nystatin L-1 was added to inhibit fungal growth before pouring the medium into incubation plates. Martin’s medium consisted of 1 g of KH2PO4, 0.5 g of MgSO4∙7H2O, 5 g of peptone, 10 g of dextrose, 0.03 g of rose Bengal, 20 g of agar and 1 L of distilled water. Martin’s medium was autoclaved for 20 min at 120°C and 1 atm, and 0.2% streptomycin was added at the rate of 2 mL L-1 before the medium was poured into plates. The cellulolytic microorganisms were quantified using potato dextrose agar (PDA) medium containing 20% potato, 2% dextrose, 2% agar and tetracycline (100 mg L-1). After incubation, the plates were flooded with 10 mL of concentrated Congo red solution (2.5 g L-1) for colony quantification. The starch agar medium used to quantify amylolytic microorganisms consisted of 20 g of agar, 20 g of soluble starch and 1.0 L of distilled water.
Ten-gram samples of soil were added to 90 ml of saline (0.85% NaCl) and homogenized for 15 min with the aid of a shaker. Next, 1 ml aliquots of this suspension were transferred to test tubes containing 9 ml of saline solution. The dilution process was continued until obtaining the 10-1, 10-2 and 10-3 serial dilutions, which were used for amylolytic microorganisms, for fungi and cellulolytic microorganisms and for bacteria, respectively. Aliquots containing 100 µl of the dilutions were inoculated in triplicate onto plates containing the respective media, and the plates were incubated for 24 h at 35°C for bacteria and for 48 h at 30°C for fungi. Afterwards, the dilutions having between 30 and 300 colonies were counted, and the results are expressed as CFU g-1 of soil. The data from all the experiments with microorganisms were subjected to analysis of variance by the F test, and the means of the treatments were compared by the Tukey test at the 5% probability level.
Effects of the herbicides saflufenacil and indaziflam on agricultural crops
The experiment was conducted in a greenhouse and used a CRD in a 6 (doses) × 5 (sowing times) factorial arrangement for each species and herbicide, with four replicates. For the experiment, 5-L pots were filled with dark red latosol samples (chemical analysis as described above) and sprayed with either saflufenacil (0, 7.5, 15, 30, 60 and 120 g a.i. ha-1) or indaziflam (0, 6.5, 12.5, 25, 50 and 100 g a.i. ha-1). The herbicides were applied on February 14, 2017, via a CO2 backpack sprayer, which was equipped with a spray boom with 110.03 fan nozzles, at a constant pressure of 245.16 kPa and a flow rate of 200 L ha-1. The relative air humidity and temperature during herbicide application were monitored with a weather station, reaching 58% and 21°C, respectively. The pots were housed in a greenhouse with automatic irrigation (5 mm H2O per day). At 0, 10, 20, 40 and 60 days after herbicide application, the following species were sown: Glycine max (soybean variety 95Y52), Crotalaria juncea (sunn hemp), Helianthus annuus (sunflower variety Rajado) and Arachis hypogaea (peanut variety Runner 886). Each experimental unit consisted of six plants. The herbicide effects were evaluated at 32 days after sowing (DAS) by a percentage scale of scores, where zero (0) represents the absence of symptoms and 100 represents the death of all plants (ALAM, 1974). The following were also determined at 32 DAS: the chlorophyll concentration with a clorofiLOG chlorophyll meter (FALKER), the leaf area (by a nondestructive method based on the use of a Li-COR 3000 leaf area meter) and the dry biomass of the shoots after cutting the plants close to the ground. The dry mass was obtained by placing the plants in a forced-air oven at 65°C, until reaching a constant weight. The results were subjected to analysis of variance and regression. The regression curves were adjusted with the SigmaPlot software.
Effects of the herbicides saflufenacil and indaziflam on soil microorganisms
As seen in Table 1, no significant difference occurred among the treatments regarding the total carbon content of the microbial biomass; that is, the herbicides did not affect the microbial biomass, which is responsible for the transformation of organic matter, for nutrient cycling and for energy flow (Wardle, 1992). According to Moorman (1989), herbicides have little effect on the soil microbial biomass, but the populations and activities of certain functional groups are affected. Voos and Groffman (1997) evaluated the relationship between the microbial biomass and the dissipation of 2,4-D and dicamba by soil microorganisms and found a positive relationship between the size of the microbial biomass and the degradation of these herbicides in the soil. Thus, these results may be useful in predicting the behavior of herbicides in different ecosystems. Additionally, Moreno et al. (2007) attributed an increase in the carbon content of the microbial biomass after atrazine application to adaptation by the microorganisms, which used the atrazine as a source of carbon and energy. Hart and Brooks (1996) reported that effects of 19 years of cumulative annual field application of benomyl, chlorfenvinphos, aldicarb, triadimefon and glyphosate, either singly or in combination, therefore had no measurable long-term harmful effects on the soil microbial biomass.

Table 2 shows that at 0 and 15 days after application (DAA), significantly more CFU occurred in the saflufenacil-treated soil samples than in the control samples. Starting at 30 DAA, a decrease occurred in the microbial population compared with the populations observed during the first evaluations. This result can be explained by the product’s half-life in the soil, since herbicides can serve as nitrogen, carbon and energy sources or as a cometabolism substrate, in which microorganisms can transform a herbicide without depleting the energy needed for their development (Fournier et al., 1997). Thus, the bacteria most likely first used the carbon present in the herbicide to grow; after product degradation, the number of colonies decreased but did so without differing statistically from that of the control group. For indaziflam, the colony number increased throughout the evaluations. At 0, 15 and 30 DAA, no significant difference from the control group existed, which most likely transpired because the microorganisms were undergoing an acclimation phase. At 60 DAA, a greater number of CFU occurred in the indaziflam-treated soil samples than in the control group, perhaps due to the high persistence of indaziflam in the soil and its subsequent role as a microbial energy source, leading to an increased microbial population (Table 2).
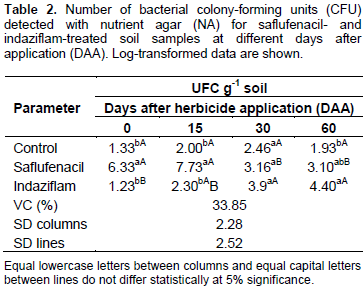
Tu et al. (1992) applied eight herbicides, atrazine, butylate, ethalfluralin, imazethapyr, linuron, metolachlor, metribuzin and trifluralin to loamy sand to determine if these materials caused any serious effects on microbial and enzymatic activities related to soil fertility. Some herbicides showed an effect on bacteria and fungi for the first week of incubation, but, subsequently, the populations returned to levels similar to those obtained in the controls. Results indicated that the herbicidal treatments at the level tested were not drastic enough to be considered deleterious to soil microbial and enzymatic activities which are important to soil fertility. Dzantor and Felsot (1991) reported the effects of simulated spills of alachlor alone or as a mixture with atrazine, metolachlor, and trifluralin on microbial activity. Simulated spills initially inhibited bacteria, but after 7 days, bacterial numbers had recovered to levels similar to those in untreated controls. Fungal populations were drastically reduced after 1 day and became undetectable after 7 and 21 days of incubation in the mixed herbicide and alachlorâ€only treatments, respectively (Dzantor and Felsot, 1991) The number of fungal colonies in the saflufenacil-herbicide-treated soil decreased throughout the study, and a statistically significant difference occurred between the treated and control groups at 30 and 60 DAA (Table3). In turn, the number of fungal colonies in the indaziflam-treated soils differed significantly from that in the control group only at 15 DAA, and an increase in the number of colonies was observed at 30 and 60 DAA.
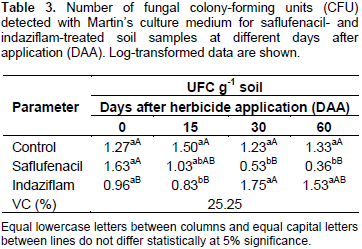
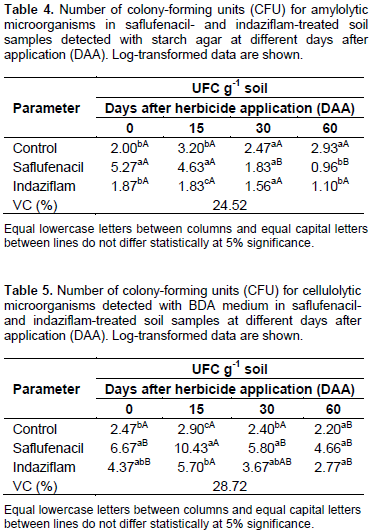
These results are consistent with those reported by Reis et al. (2008), who showed that ametryn and trifloxysulfuron-sodium, alone or in combination, and 2,4-D caused a reduction in the soil fungal population density only after 15 DAA. The density was restored in the subsequent evaluations because of either a metabolic adjustment in the subpopulations affected by the herbicide or the lower residual herbicide concentrations in the soil (Reis et al., 2008). The number of amylolytic microbial colonies observed for the saflufenacil-treated soils differed from that observed for the control group at 0, 15 and 60 DAA; the number of these colonies gradually decreased during the evaluations (Table 4). These results might be explained by an initial use of the energy contained in the herbicides to increase the microbial population, followed by subsequent decreases in population size with the dissipation of these products in the soil due to microbial action. At 15 and 60 DAA, fewer amylolytic microbial colonies occurred for the indaziflam-treated soils than for the control group soils, but no statistically significant differences existed at any of the evaluation times (Table 5). An important point to emphasize is that the starch agar medium is used to select the amylase-producing microorganisms involved in the transformation of carbon and nitrogen compounds in the soil. For cellulolytic microorganisms, the number of CFU in the saflufenacil-treated soil samples at 0, 15 and 30 DAA was statistically significantly higher compared with that in the control samples. The indaziflam-treated soils produced more CFU for cellulolytic microorganisms compared with the control soils at all the evaluation times; however, the only statistically significant difference was observed at 15 DAA (Table 5).
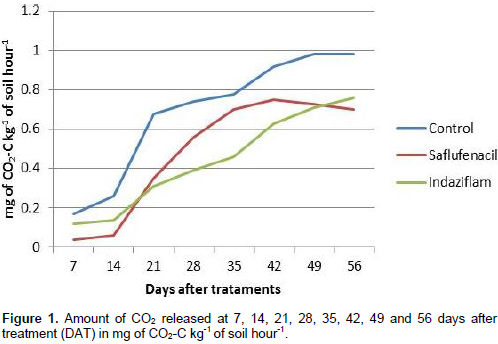
Cellulose is a polysaccharide composed of approximately 40 glycosidic chains cross-linked into compact bundles. Each chain has a degree of polymerization approximating 10,000 glucose units linked by β1-4 bonds and cannot be metabolized by most animals because most lack an enzyme that hydrolyzes these bonds. However, this material does not accumulate in the environment due to the activity of fungi and bacteria, which produce cellulolytic enzymes (Lehninger et al., 2006). Thus, the presence of microorganisms that break the cellulose chain is of extreme importance. As shown in Figure 1 for the saflufenacil-treated soils, the amount of CO2 declined at 56 DAT, in agreement with the manufacturer’s information indicating that saflufenacil is a nonvolatile herbicide (VP of 2 ×10-4 mm Hg) and has a half-life of one to five weeks (BASF, 2008). By contrast, a growing curve was observed for indaziflam. Similarly, Kaapro and Hall (2012) reported that indaziflam has a high residual period in the soil, greater than 150 days, persisting longer than other pre-emergent herbicides. Between 7 and 14 days, a phase of microbial acclimation to the herbicide is believed to have occurred, with the herbicide then released as CO2 over time, according to its persistence.
Effects of the herbicides saflufenacil and indaziflam on agricultural crops
The phytotoxicity of saflufenacil to soybean plants was above 40% when sowing was performed at 0 and 10 DAA, and a relationship existed between the plant response and the saflufenacil dose. The phytotoxicity of a commercial dose of saflufenacil was close to 20% at 20 and 40 DAA and was 15% at 60 DAA (Figure 2A). Monquero et al. (2012) studied the residual effect of saflufenacil after drought periods (0, 15, 30, 45, 60 and 90 days) in a dystrophic red latosol (clay texture) and reported that the phytotoxicity of a bioindicator (cucumber) was greater than or equal to 80% until up to 28 days of drought. The phytotoxicity of saflufenacil to crotalaria plants was less than 30% in all the doses used, except when sowing was performed on the day of herbicide application, when phytotoxicity reached 40% (Figure 2B). For sunflower plants, saflufenacil phytotoxicity exceeded or equaled 80% when sowing was performed at 0 or 20 DAA, respectively (Figure 2C). Saflufenacil phytotoxicity decreased at the other sowing times; however, even at 60 DAA, phytotoxicity close to 40% was observed for the commercial dose, which could lead to yield losses. These results corroborate those of Brighenti (2015), who reported that saflufenacil decreased the plant stand and sunflower yield (kg ha-1), with the highest dose also reducing the weight of 1000 achenes. Peanut plants presented a phytotoxicity below 20% only at 60 DAA, with their highest phytotoxicity (40%) observed at 0 DAA (Figure 2D). Soltani (2010) observed that saflufenacil at 100 and 200 g a.i ha-1 caused 51 to 99% injury and reduced height by 25 to 93%, shoot dry weight by 92 to 99% and seed yield by 56 to 99% in cranberry and in adzuki, Lima, snap and white beans.
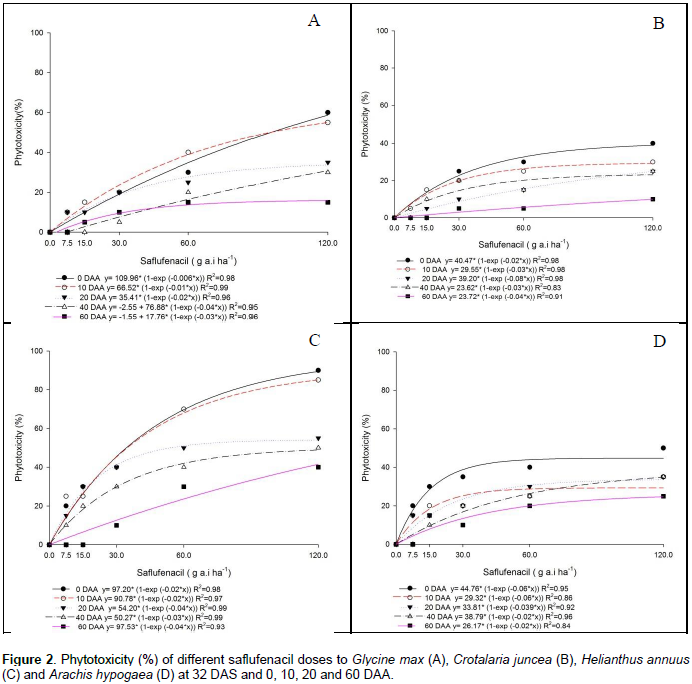
The most saflufenacil-tolerant crops were soybean and pea. In other research, Soltani et al. (2009) reported that addition of an adjuvant to saflufenacil applied POST caused 99% injury to corn at three-leaf stage and reduced yield up to 59% compared to saflufenacil applied without adjuvant. According to Papiernik et al. (2012), the half-life of saflufenacil ranges from 13 (in the arable layer) to 32 days (in the subsurface layer), with low soil sorption and rapid dissipation. However, injuries were observed in rotational crops (pumpkin, cucumber, carrots, garlic, pepper and beet) up to one year after high herbicide doses (100 to 200 g a.i. ha-1) were applied, the same doses recommended in Brazil (Robinson and Mcnaughton, 2012). Indaziflam was highly phytotoxic to soybean plants regardless of the sowing period. For example, the phytotoxicity was 70% at 60 DAA, and a direct positive relationship existed between increased dose and plant phytotoxicity (Figure 3A). These results corroborate those of Guerra et al. (2014), who reported that not only soybean but also sorghum, millet, cucumber and beet showed an indaziflam dose below 5 g ha-1 that caused 50% injury to the plants (I50 dose), with the dose commonly used in countries where this herbicide has already been registered 20 times higher than 5 g ha-1. The authors concluded that most of the species tested were highly sensitive to indaziflam (Guerra et al., 2014). In a study on the effect of simulated indaziflam drift (doses of 100, 20, 10, 5 and 2.5% of the commercial dose of 73 g a.i ha-1) on the growth of selected crops, the crops were ranked as follows according to the observed indaziflam level at which susceptibility occurred: cotton < tobacco < tomato < pumpkin < pepper < soybean. For cotton, the most sensitive crop, 2.5% of the commercial dose caused a 20% reduction in the root mass (Jeffries et al., 2014).
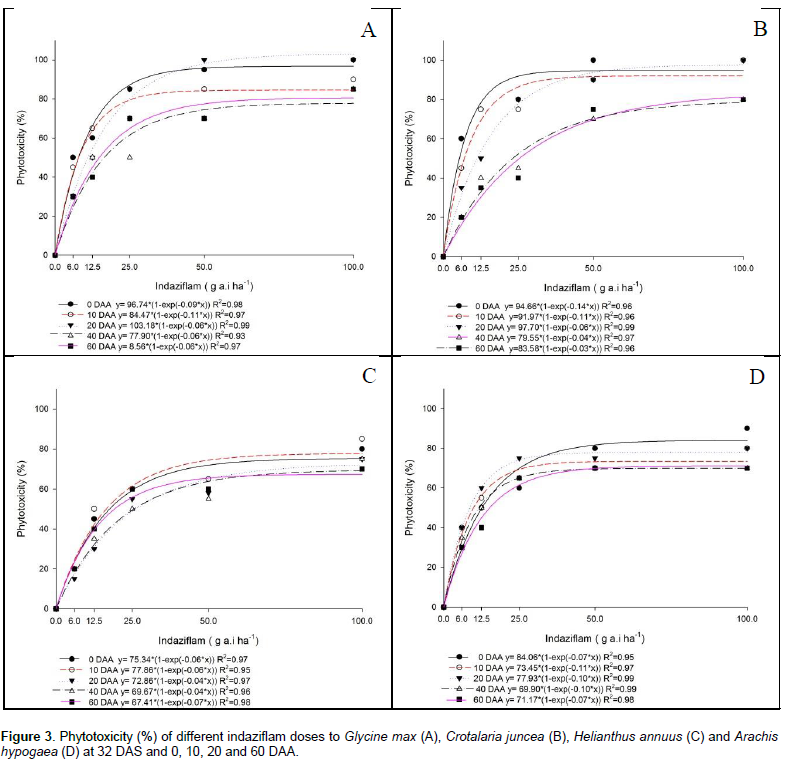
For sunn hemp, indaziflam was also highly phytotoxic. Except for sowing at 40 and 60 DAA, the phytotoxicity at all the other sowing times was equal to or greater than 80%, with a direct response to increased dose (Figure 3B). The sunflower plants presented high phytotoxicity values that ranged from 60% (sowing between 40 and 60 DAA) to 80% (sowing at the other times) (Figure 3C). These results are similar to those found by Guerra et al. (2014), who showed that sunflower was the most indaziflam-tolerant species of those evaluated, presenting only a slight decrease in the fresh weight of roots. Furthermore, even at the highest dose tested (100 g ha-1), insufficient injury occurred to reach the I50 value (the dose of herbicide required to cause a 50% reduction in the plant fresh weight, relative to the weight obtained without herbicide treatment). Guerra et al. (2014) also found that maize and cotton had an intermediate tolerance to indaziflam, with an I50 of 86 and 63.5 g ha-1, respectively. Jhala and Hanson (2011), when studying the effect of indaziflam on sunflower, cotton, maize, soybean, millet, cucumber, beet and sorghum, observed that all the species studied were sensitive to indaziflam. The only symptom observed in the different species after planting in soil containing indaziflam was the nonemergence of seedlings, except for sunflower. Cotton and maize did not emerge only when sown in the soil treated with the highest dose of indaziflam (100 g ha-1). For soybean, emergence occurred in the soil treated with the two lowest doses (20 and 40 g ha-1); however, the plants died after a few days. Additionally, sorghum, millet, cucumber and beet did not emerge, even in the soil treated with the lowest dose of this herbicide (20 g ha-1). The exact mechanisms of action of this herbicide are not yet fully understood but are believed to involve the prevention of cell wall formation in new cells, stopping plant growth.
Indaziflam cannot be considered selective for peanuts at any of the tested doses or sowing times, since peanuts presented phytotoxicity between 60 and 85% (Figure 3D). One of the options to reduce expenses during sugarcane fallow that is important to remember is the adoption of MEIOSI (Inter-Rotational Methods of Simultaneous Occurrence - Método Inter Rotacional Ocorrendo Simultaneamente). According to Rocha Neto (2013), the MEIOSI system has been gaining prominence because the simultaneous rotation of sugarcane with a legume, such as soybean or peanut, or with green manure reduces the need for nitrogen fertilization and provides better planting logistics. The MEIOSI system consists of planting the sugarcane (September/October) in a 2:8 ratio, that is, two sugarcane rows: eight crop rows first occupied by the chosen legume within a sugarcane-free area. Subsequently, at the end of the rainy period (February/March), the first-planted sugarcane is used as a seedling and is planted in the area previously occupied by the legume. If green manure is used, the manure must be incorporated into the soil to provide nutrients to the sugarcane crop. However, for the MEIOSI system to work, the herbicide must be chosen with care to avoid carryover, which can occur with indaziflam. When the soybean plants were sown in soils treated with saflufenacil, the biomass differed significantly between 0 and 10 DAA, with a lower accumulation at the highest dose used. Within each dose used, a statistically significant difference was observed among the sowing times at doses of 120, 60 and 30 g a.i. ha-1, with a higher biomass accumulation as the period between the herbicide application and the sowing time increased. For indaziflam, statistically significant differences were observed at all the doses tested, and the biomass produced declined as the herbicide dose increased (Table 6).
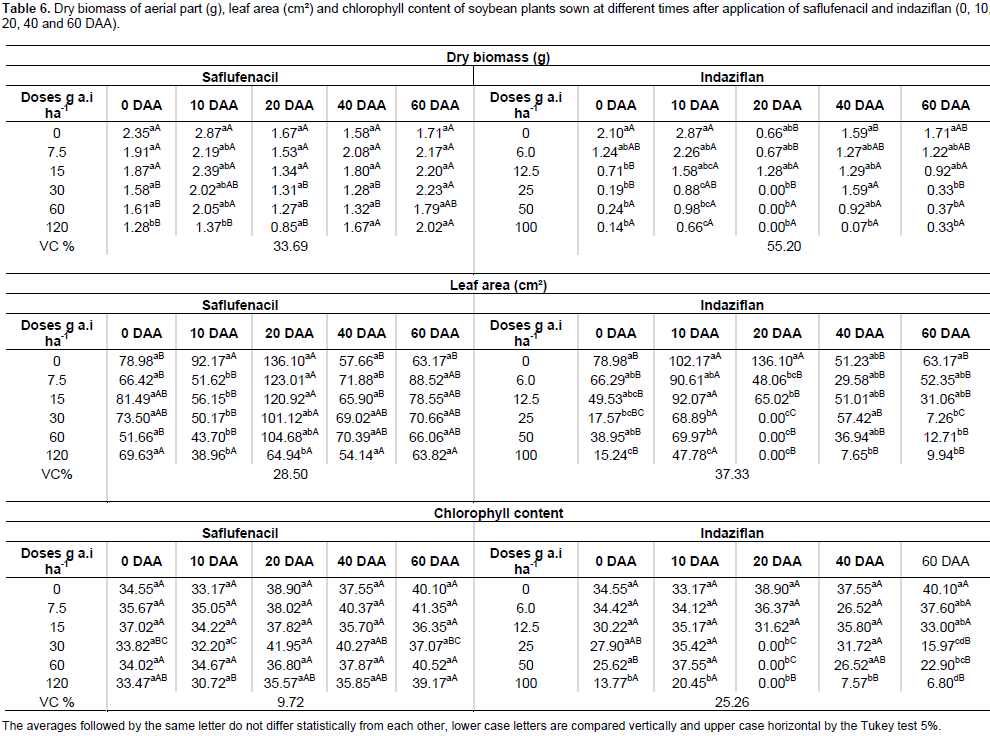
For the soils treated with saflufenacil, the leaf area of the soybean plants differed significantly compared with that of the control plants at 10 and 20 DAA, and the lowest leaf area was observed when saflufenacil was used at the commercial dose. For the soils treated with indaziflam, a statistically significant difference existed at all the sowing times, with a lower leaf area at the highest doses and reductions of up to 100% (Table 6). These results explain why Kuva and Salgado (2016), when evaluating the effect of indaziflam on weeds in a sugarcane crop, emphasized the need to consider the residual effect on rotational crops, avoiding intervals shorter than one year between indaziflam applications and the sowing of any crop. No significant decrease in the chlorophyll content occurred with any of the saflufenacil treatments. By contrast, indaziflam reduced the chlorophyll content at all the sowing times, with differences detected among the commercial dose, one-half of that dose and one-quarter of that dose. In the case of soybean, the effects of saflufenacil were only observed for the sowing times closest to the herbicide application. Soltani (2010) also reported that soybean and pea were the most tolerant crops among the several crops tested. Regarding the development of crotalaria plants, the commercial dose of saflufenacil negatively affected the biomass production of plants sown at 0, 10 and 20 DAA. At 40 DAA, the lowest biomasses were observed at the lowest doses; however, these biomasses were the same as those of the control group. Thus, the herbicide was not responsible for the reduction (Table 7). By contrast, the use of indaziflam reduced the biomass of crotalaria plants at all the sowing times, especially at the commercial dose. For the leaf area of crotalaria plants, saflufenacil negatively affected leaf expansion at 0 and 20 DAA with the use of the commercial dose and at 10 DAA with the commercial and the lowest doses. In turn, indaziflam affected the leaf area of crotalaria plants at all the sowing times (Table 7). Regarding the chlorophyll content, no statistically significant differen ce occurred between the groups treated with saflufenacil and the control group at any of the sowing times. With indaziflam, changes in the chlorophyll content were observed at 20, 40 and 60 DAA (Table 7).
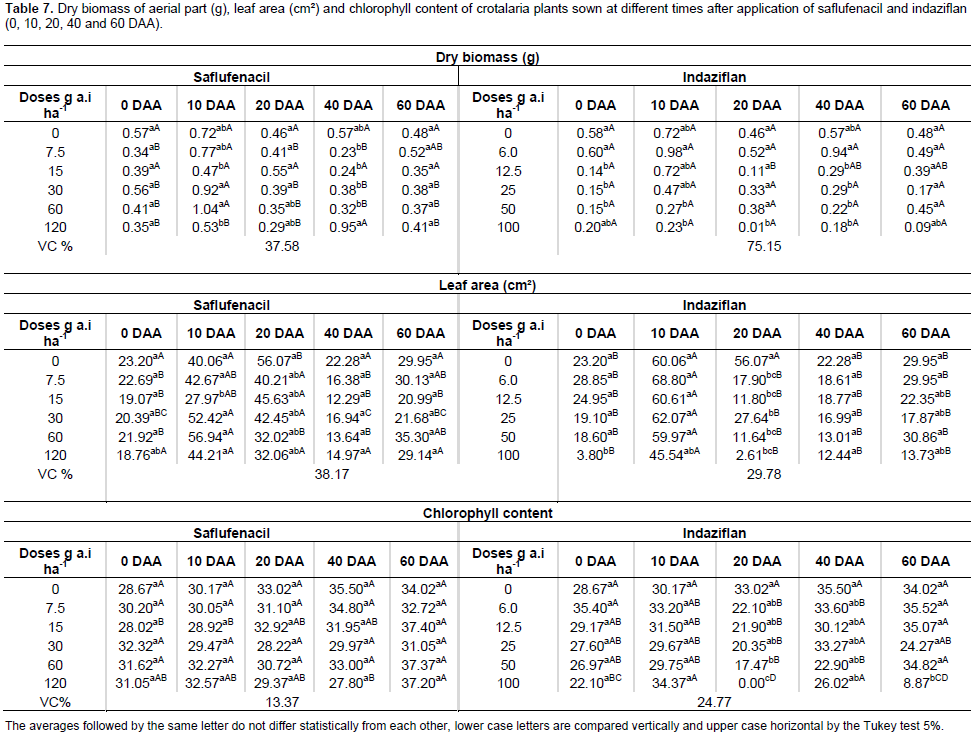
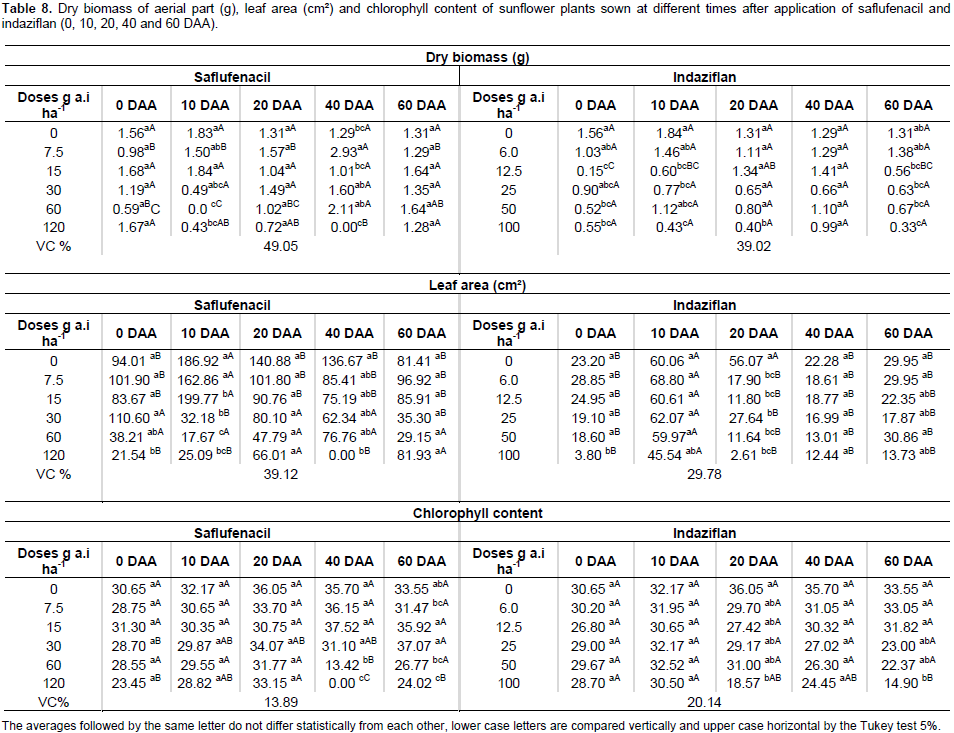
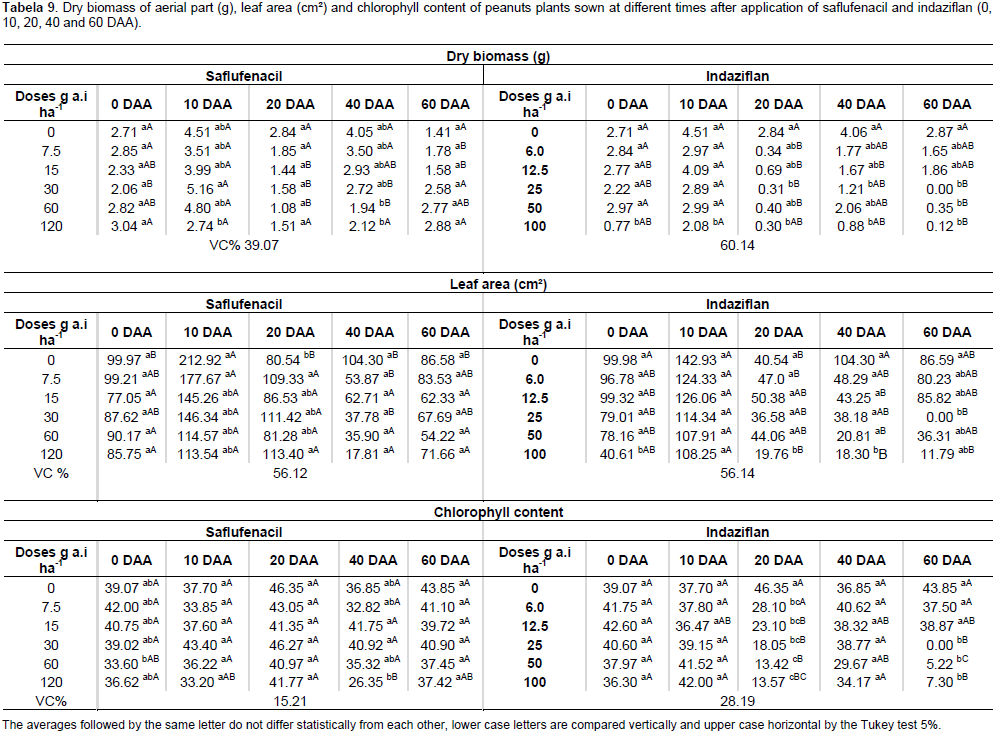
For sunflower, saflufenacil affected shoot biomass accumulation not only at 10 DAA with the one-half and the full commercial dose but also at 40 DAA with the highest dose, in which case the plants died. Indaziflam negatively affected shoot biomass accumulation at 0, 10, 20 and 60 DAA at the highest doses used (Table 8). Saflufenacil affected the leaf area of the sunflower plants at 0 and 10 DAA with the one-half and full commercial doses and at 40 DAA with the full commercial dose. Indaziflam, however, affected the leaf area of the sunflower plants at all the sowing times evaluated (Table 8). The chlorophyll content was lowest in sunflower plants with the use of one full commercial dose of saflufenacil at 40 and 60 DAA and at the commercial indaziflam dose when sowing was performed at 20 and 60 DAA (Table 8). The biomass accumulation in the peanut plants that developed in the presence of a commercial saflufenacil dose presented statistically significant differences at 10 and 40 DAA. For the peanut plants sown in the soils treated with the commercial indaziflam dose, the biomass accumulation showed significant differences at all the sowing times (Table 9). The leaf area of peanut plants was negatively affected by the highest dose of saflufenacil when sowing was performed at 10 and 20 DAA. For the commercial dose of indaziflam, a reduction in the leaf area of peanut plants was observed at all the sowing times (Table 9). For the chlorophyll content, significant differences occurred at 0 and 40 DAA in plants exposed to saflufenacil and at 20 and 60 DAA in the indaziflam-exposed plants. In general, the phytotoxicity of saflufenacil decreased starting at 40 DAA in all crop species evaluated, which was expected because this herbicide has a half-life of one to five weeks. The same was not observed for indaziflam, whose half-life exceeds 150 days. Therefore, the pre-emergent herbicide to be applied during the last sugarcane harvest must be chosen carefully when adopting crop rotation during sugarcane fallow periods.
The authors have not declared any conflict of interests.
Recognition is extended to the São Paulo Research Foundation (FAPESP) for its financial support and to members of the Agricultural Sciences Research Group (GECA) for their support.
REFERENCES
|
Alonso DG, Koskinen WC, Oliveira Jr RS, Constantin J, Mislankar S (2011). Sorption-desorption of indaziflam in selected agricultural soils. J. Agric. Food Chem. 59(4):3096-3101.
Crossref
|
|
|
|
Arruda JS, Lopes NS, Bacarin MA (2001). Nodulação e fixação do dinitrogênio em soja tratada com sulfentrazone. Pesqui. Agropecu. Bras. 36(2):325-330.
Crossref
|
|
|
|
|
Badische Anilin and Soda Fabrik (BASF) (2008). Agricultural Products KIXORTM herbicide: Worldwide Technical Brochure (GL-69288). North Carolina, NC: Agricultural Products Division, Research Triangle Park.
|
|
|
|
|
Brighenti AM (2015). Control of volunteer soybean plants in sunflower crop. Pesqui. Agropecu. Trop. 45(3):274-281.
Crossref
|
|
|
|
|
Dzantor EK, Felsot AS (1991). Microbial responses to large concentrations of herbicides in soil. Environ. Toxicol. Chem. 10(5):649-655.
Crossref
|
|
|
|
|
Fournier JC, Soulas G, Parekh NR (1997). Main microbial mechanisms of pesticide degradation in soils. In: Tarradellas J, Bitton G, Rossel D. (Eds), Soil ecotoxicology. CRC, Boca Raton. 1:85-116.
|
|
|
|
|
Gannon T, Hixson AC, Weber JB, Knezevic SZ, Yelverton FH (2014). Soil properties influence saflufenacil phytotoxicity. Weed Sci. 62(4):657-663.
Crossref
|
|
|
|
|
Grisi BM (1995). Biomassa e a atividade de microrganismos do solo: Revisão metodológica. Rev. Nordestina Biol. 10:1-22.
|
|
|
|
|
Guerra N, Júnior RSO, Constantin J, Neto AMO, Braz GBP (2014). Sensibility of plants species to herbicides aminocyclopyrachlor and indaziflam. Planta Daninha 32(3):609-617.
Crossref
|
|
|
|
|
Hart MR, Brookes PC (1996). Soil microbial biomass and mineralisation of soil organic matter after 19 years of cumulative field applications of pesticides. Soil Biol. Biochem. 28(12):1641-1649.
Crossref
|
|
|
|
|
Jeffries MD, Mahoney DJ, Gannon TW (2014). Effect of simulated indaziflam drift rates on various plant species. Weed Technol. 28(4):608-616.
Crossref
|
|
|
|
|
Jhala AJ, Hanson BD (2011). Summer weed control with glyphosate tank mixed with indaziflam or penoxsulam in California orchards and vineyards. Proc. 51st Annu. Conf. Weed Sci. Soc. Am. Portland OR. P 21.
|
|
|
|
|
Jhala AJ, Singh M (2012). Leaching of indaziflam compared with residual herbicides commonly used by Florida citrus. Weed Technnol. 26(3):602-607.
Crossref
|
|
|
|
|
Kaapro J, Hall J (2012). Indaziflam, a new herbicide for pre-emergent control of weeds in turf, forestry, industrial vegetation and ornamentals. Pak. J. Weed Sci. Res. 18(2):267-270.
|
|
|
|
|
Kuva MA, Salgado TP (2016). Interferência das plantas daninhas na cultura da cana-de-açúcar e alternativas de manejo. Rev. Can. 121(1):62-63.
|
|
|
|
|
Lehninger AL, Nelson DL, Cox MM (2006). Princípios de bioquímica. 4 ed. São Paulo: Sarvier 1202 p.
|
|
|
|
|
Monquero PA, Sabbag R, Orzari I, Hijano N, Galvani Filho M, Dallacosta V, Krolikowski V, Silva Hirata AC (2012). Lixiviação de saflufenacil e residual após períodos de seca. Plant. Dan. 30(2):415-423.
Crossref
|
|
|
|
|
Moorman TB (1989). A review of pesticide effects on microorganisms and microbial processes related to soil fertility. J. Crop Prod. 2(1):14-23.
Crossref
|
|
|
|
|
Moreno JL, Aliaga A, Navarro S, Hernández T, García C (2007). Effects of atrazine on microbial activity in semiarid soil. Appl. Soil Ecol. 35(1):120-127.
Crossref
|
|
|
|
|
Papiernik SK, Koskinen WC, Barber BL (2012). Low sorption and fast dissipation of the herbicide saflufenacil in surface soils and subsoils of an eroded prairie landscape. J. Agric. Food Chem. 60(44):10936-10941.
Crossref
|
|
|
|
|
Reis MR, Silva AA, Costa MD, Guimarães AA, Ferreira EA, Santos JB, Cecon PR (2008). Atividade microbiana em solo cultivado com cana-de-açúcar após aplicação de herbicidas. Planta Daninha 26(2):323-331.
Crossref
|
|
|
|
|
Robinson DE, Mcnaughton KE (2012). Saflufenacil Carryover Injury Varies among Rotational Crops. Weed Technol. 26(2):177-182.
Crossref
|
|
|
|
|
Rocha Neto WC (2013). Informativo para produção de leite -meiosi em cana-de-açúcar. 294 ed. Viçosa: UFV 4p.
|
|
|
|
|
Santos JB, Jakelaitis A, Silva AA, Vivian R, Costa MD, Silva A F (2005). Atividade microbiana do solo após aplicação de herbicidas em sistemas de plantio direto e convencional. Planta Daninha 23(4):683-691.
Crossref
|
|
|
|
|
Soltani N (2010). Sensitivity of Leguminous Crops to Saflufenacil. Weed Technnol. 24(2):143-146.
Crossref
|
|
|
|
|
Soltani N, Shropshire C, Sikkema PH (2009). Response of corn to pre-emergence and post-emergence applications of saflufenacil. Weed Technol. 23(3):331-334.
Crossref
|
|
|
|
|
U.S. Environmental Protection Agency (2010). Pesticide fact sheet. Conditional registration P. 108.
|
|
|
|
|
Vivian R, Reis MR, Jakelaitis A, Silva AF, Guimarães AA, Santos JB, Silva AA (2006). Persistência de sulfentrazone em Latossolo Vermelho-Amarelo cultivado com cana-de-açúcar. Planta Daninha 24(4):741-750.
Crossref
|
|
|
|
|
Voos G, Groffman PM (1997). Relationships between microbial biomass and dissipation of 2,4-D and dicamba in Soil. Biol. Fertil. Soils 24(1):106-110.
Crossref
|
|
|
|
|
Wardle DA (1992). A comparative assessment of factors wich influence microbial biomass carbon and nitrogen levels in Soil. Biol. Rev. 67(3):321-358.
Crossref
|
|