ABSTRACT
Salinity is an environmental stress that limits growth and development in plants. The present study was to assess the effect of salinity on dry matter and ionic contents in cowpea accessions. A pot experiment was carried out to investigate the effect of different levels of salinity on growth and ionic contents of cowpea plant. There were three levels of NaCl and Na2SO4 salinity which includes 0 (control), 50, 100 and 150 mM. Growth characters such as root and shoot dry weights decreased with increase in salinity levels. Na+, Cl-, PO4-, SO4- and Na+/K+ concentrations increased with increase in salinity but Ca2+, K+ and Mg2+ concentrations were lower as salinity levels increased. It is concluded that with increase in salinity levels there was a significant reduction in biomass production in cowpea plant. Pattern of accumulation of ions varied significantly.
Key words: Salinity, concentrations, Vigna unguiculata (L.) Walp., stress.
Salinity is an environmental stress that limits growth and development in plants. It is consider a significant factor affecting crop production and agricultural sustainability in arid and semi arid region of the world reducing the value and productivity of the affected land (Munns, 2002). Salinity causes not only differences between the mean yield and the potential yield, but also causes yield reduction from year to year. It affects the plant growth directly through its interaction with metabolic rates and pathways within the plants. In the simplest analysis of the response of a plant to salinity stress, the reduction in shoot growth occurs in two phases: A rapid response to the increase in external osmotic pressure and a slower response due to accumulation of Na+ in leaves (Munn, 2002). In the first, osmotic phase which starts immediately after the salt concentration around the root increases to the threshold 40 mM NaCl for most plant which is equivalent to 4 dSm (George, 2008). As a result, the rate of shoot growth falls significantly. The second is the ionic specific phase of plant response to salinity which start when salt accumulate to toxic concentration in the leaves causing necrosis and reducing photosynthesis area resulting in decline in growth (Munn, 2002; Bayuelo-Jimenz et al., 2012).
Cowpea (Vigna unguiculata L. Walp.) is an important grain legume crop used as a fodder crop for livestock (Zahedi et al., 2012) and as a cheap source of vegetable. It is consumed both as green pod and as dry seed. The fresh green pods of cowpea contain 85.9% moisture, 4.6% protein, 0.2% fat, 0.8% minerals, 2.0% fibre and 8.5% carbohydrates. The high protein content of cowpea makes it an important supply to the diet of many African people (Giami et al., 2001).
Many varieties are grown in tropical and sub-tropical agricultural areas of the world, where salinity is a yield-limiting factor (Zahedi et al., 2012). Cowpea is reported to have a good tolerance to heat and drought (Vasquez-Tello et al., 1990 cited in Zahedi et al., 2012), and it has a high yield potential under irrigation (Murillo-Amador et al., 2006). Salinity stress disturbs the uptake and accumulation of essential nutrients (Zhu, 2001).
In view of these studies, the principal objective to carry out the present study was to assess the effect of salinity on dry matter and ionic contents in cowpea accessions.
This study was carried out from February to April, 2013 in the green house at the Department of Botany, Faculty of Science, University of Ibadan and seeds of cowpea cultivars (namely; TVu 11711 and TVu 15245) were obtained from the International Institute of Tropical Agriculture (IITA), Oyo State, Ibadan. Cowpea seeds were planted in pots of 14 cm diameter and 18.5 cm depth; each pot was filled with 3.0 kg soil. Four seeds of each cultivar were sown in each pot. Four levels of NaCl salt (0, 50, 100 and 150 mM) were applied after 15 days of germination. The experiment was laid in completely randomized designed in triplicates. When the seedlings were well established (after 10 days) that is, when the first trifoliate leaf had reached its full size and the second trifoliate leaf was starting its development, thinning was carried out, leaving two plants per pot. Salt treatment commenced 5 days after plants were thinned. These involved the application of NaCl and Na2SO4 with varied equimolar concentration (0, 50, 100 and 150 mM). The treatments were applied twice a week except for the control (0 mM) that was watered regularly. Plants were harvested four weeks after treatment. They were uprooted carefully and washed in running tap water. Plant samples were placed in oven at 80°C. After 4-days shoot and root dry weights (g/pot) were estimated with the help of weighing balance at final harvest. The dried ground plant material (0.1 g) was digested with sulphuric acid (H2SO4) and hydrogen peroxide H2O2 according to the method of Wolf (1982). The Ionic contents (Na+, K+, Ca2+ and Mg2+) of the samples were determined by atomic absorption spectrophotometry. The PO4 and SO4 contents were estimated by colorimetric and turbidimetric methods, respectively.
The Chloride of the plant material (0. 1 g) was extracted in 10 ml distilled water at 80°C for 4 h. The Cl- content was analyzed by precipitation as AgCl and titrated according to Johnson and Ulrich (1959). Data were subjected to analysis of variance, using Statistical Analysis System (SAS). Various treatment means were compared with Duncan’s New Multiple Range (DMR) Test.
Effect of varying concentrations of NaCl and Na2SO4 on biomass of two cowpea accessions is presented in Table 1. It was observed that depending on the increasing salt concentration, the fresh and dry weights of root and shoot of both accessions decrease from 0 to 150 mM (Table 1). Salt stress significantly reduced dry matter production. Total dry matter at the highest salinity (150 mM) was lower than the dry matter of plants grown in the control pots after 4 weeks of saline treatment. At the highest salinity treatment, TVu 11711 had greater dry weight than TVu 15245. Shoot dry weight was significantly reduced by salinity at P<0.05. Shoot dry weight at the highest salinity (150 mM) had the lowest values compared to non-stressed (0 mM) plants in both accessions (Table 1).
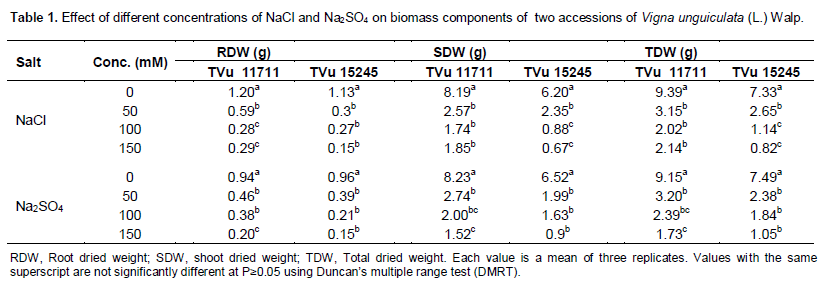
Salinity affected ions uptakes of the two cowpea accessions (Tables 2 and 3). shoot nutrient analysis of the two cowpea accessions indicated that Na+, Mg2+, Na+, Cl-, SO42-, PO43- and Na+: K+ ratio significantly increased under saline condition (p≤0.05) with increasing NaCl and Na2SO4 concentrations except K+, and Ca2+ (Tables 2 and 3). K+ uptake of salt stressed cowpea plants appeared to be differentially influenced by NaCl and Na2SO4. NaCl application reduced the uptake of K+ in both leaf and stem. Similarly, the uptake of K+ content in the accessions treated with Na2SO4 was significantly reduced with increased in concentration. The amount of Na+ uptake increased markedly in response to increasing salt levels. Besides, increased amounts of Na+, the Na+/K+ ratio rose significantly by increasing salts concentrations in both accessions.
Calcium uptake in leaf was also reduced by both salts in the accessions (Tables 2 and 3), to a great extent by Na2SO4, NaCl also followed the same trend but the rate of absorption was significantly lower than in Na2SO4 in the accessions with increased in concentrations. The uptake of magnesium was also affected by increasing concentration of salinity in the accessions in both NaCl and Na2SO4 (Tables 2 and 3). There was a decreased in the content of magnesium from 0 - 150 mM of salt treatment and this decreased were reflected in the chlorophyll contents of both accessions in both treatments since magnesium is majorly responsible for chlorophyll formation in leaf. Leaf and stem phosphate and sulfate ion concentrations from control plants did not differ significantly among the different accessions, in both salt types (Tables 2 and 3). However, the uptake of SO42- and PO43- concentrations in the shoot from salt-stressed plants increased with increase in the salts concentrations from 0 - 150 mM of Na2SO4 and NaCl.
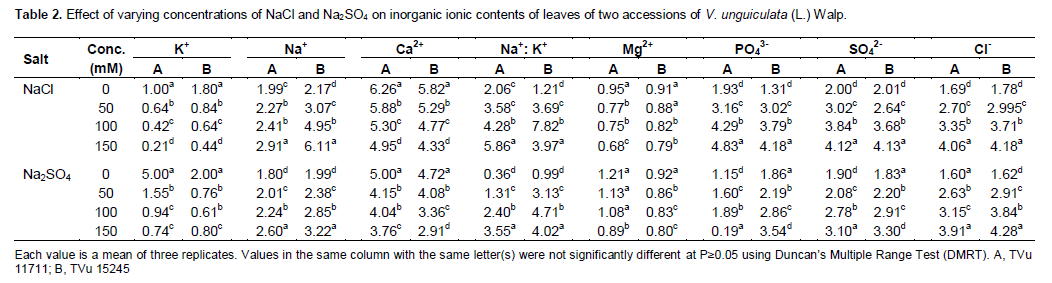
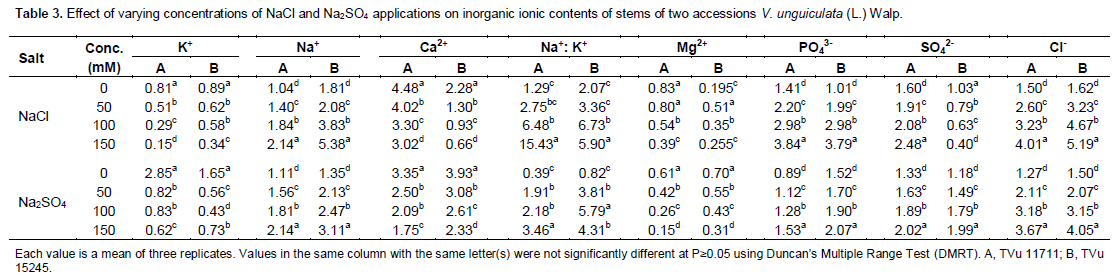
In the present study, salt stress caused a great reduction in fresh and dry weights of shoot and root of both cowpea accessions with the increase in saline contents. Reduction in plant growth as a result of salt stress had also been reported in several other plant species (Ashraf and O’leary, 1997). The increase in Na+ content and decrease in K+ uptake disturbs ionic imbalance as observed in cowpea plant exposed to salt stress. The diminution of K+ concentration in tissue may also be due to direct competition between K+ and Na+ at plasma membrane, inhibition of Na+ on K+ transport process in xylem tissues and/or Na+ induced K+ efflux from the roots. K+ and Ca2+ have been reported to be the major cautions in cell organization as well as the major contributors to osmotic adjustment under stress conditions in several plant species (Santos-Diaz and Alejo- Ochoa, 1994; Hirschi, 2004). In the present study, the level of K+, Mg2+ and Ca2+ in the salt-stressed cowpea plants gradually decreased while that of Na+ was dramatically increased. Increasing levels of NaCl induced a progressive absorption of Na and Cl in plant, agreeing with Taban et al. (1999) and Turan et al. (2007a, 2007b). Excessive Na+ concentration in the plant tissue hinders nutrient balance, osmotic regulation and causes toxicity (Amdouni et al., 2014).
Accumulation of Cl- in the root tissue is disruptive to membrane uptake mechanisms, and these results in increased translocation of Cl- to the shoots (Turan et al., 2009). When NaCl was applied to the soil, the levels of K in plant were reduced in accordance with the antagonism between Na+ and K+ (Alberico and Cramer, 1993; Azevedo and Tabosa, 2000). Mansour (1997) showed that excess NaCl leads to the loss of potassium due to membrane depolarization by sodium ions. The decrease in K+ and Ca2+ content under stress condition had been previously reported in other species particularly in the salt-sensitive lines (Lutts et al., 2004).
According to Weimberg (1987) cited in Summart et al. (2010) high levels of Na+ inhibit the K+ uptake and as a result of this it causes an increase in the Na+/K+ ratio. According to Ahmed El Sayed (2011), Na+ and Ca2+ ions probably compete much more for common uptake sites. From the result obtained, it appears that Na+ in combination with SO42- is more toxic than with Cl-. Phosphate content was increased due to salt stress in both leaf and stem. The influence of salinity on phosphorus in phosphate uptake is controversial.
A suppression of P uptake due to salt stress was been reported by Ahmed El Sayed (2011) whereas increased PO4 content due to salt stress was reported by Garg et al. (2005). Indeed symptoms of PO4- toxicity induced by salinity have been recognized by Nieman and Shannon (1976). According Ahmed El Sayed (2011) resistance to secondary salt induced stress in Glycine falcata Benth. was due to its ability to maintain a high P content in the presence of salt stress. It is possible that a high phosphate content in stem and leaf of salinized cowpea plant played similar role which corroborated with Silva et al. (2003) who reported an increase in leaf P concentration resulting from salt stress in mature and immature cowpea leaves. On the other hand, P accumulation in salt-stressed plants could be a consequence of reduced translocation associated with a decreased demand for growth.
Addition of NaCl and Na2SO4 had an adverse effect on the growth of cowpea plant. Salinity caused a significant effect on shoot fresh and dry weights. The reason for growth reduction in cowpea plant could be due to water shortage and ionic toxicity caused by salinity. Assessment of pattern of accumulation of toxic ions in a species is vital importance to understand, whether the species uses partial exclusion or inclusion mechanism for tolerating toxic ions present in its growth medium (Khalid et al., 2009).
It is concluded that salinity had adverse effect on growth of cowpea plant. Growth was reduced with the increase in salinity levels. Its ionic contents also varied significantly under salt stress. So, salinity had adverse effects on plant life cycle. However, further research should be conducted in field in other to authenticate these findings.
The authors have not declared any conflict of interest.
REFERENCES
|
Ahmed El Sayed HE (2011). Influence of salinity (NaCl and Na2SO4) Treatments on growth development of broad bean (Vicia faba L.) Plant. Am. Eur J. Agric. Environ. Sci. 10(4):600-610. |
|
|
Alberico J, Cramer GR (1993). Is the salt tolerance of maize related to sodium exclusion? Preliminary screening of seven cultivars. J. Plant Nutr. 16(11):2289–2303.
Crossref |
|
|
|
Amdouni T, Mrah S, Msilini N, Zaghdoud M, Ouerghiabidi Z, Lachaal M (2014). Physiological and biochemical responses of two maize cultivars (Corralejo and Tlaltizapon) under salt stress. J. Stress Physiol. Biochem. 10(3):246-258. |
|
|
|
Ashraf M, O'leary JM (1997). Ion distribution in leaves of salt–tolerant and salt–sensitive lines of spring wheat under salt stress. Acta Bot. Neerl. 46(2):207–217. |
|
|
Azevedo NAD, Tabosa JN (2000). Salt stress in maize seedlings: II. Distribution of cationic macronutrients and it's relation with sodium. Rev. Bras. Eng. Agric. Amb. 4:165-171.
Crossref |
|
|
|
Bayuelo-Jiménez JS, Jasso-Plata N, Ochoa I (2012). Growth and Physiological Responses of Phaseolus Species to Salinity Stress. Int. J. Agron. Article ID 527673. 13 p. |
|
|
|
Garg BK, Kathju S, Vyas SP (2005). Salinity-fertility interaction on growth, photosynthesis and nitrate reductase activity in sesame. Indian J. Plant Physiol. 10(2):162-167. |
|
|
|
George Jr. EB (2008). "Research Databases. Bibliography on Salt Tolerance," USDA-ARS. US Dep. Agric. Res. Serv. Riverside, CA. |
|
|
|
Giami S, Akosu M, Abd Emelike J (2001). Evaluation of selected food attrivutes of four advanced lines of ungerminated and germinated Nigerian cowpea (Vigna unguiculuta L. Walp). Plant Foods Hum. Nutr. 56:61-73 |
|
|
Hirschi D (2004). The calcium conundrum, both versatile nutrient and specific signal. Plant Physiol. 136:2438-2442.
Crossref |
|
|
|
Khalid H, Abdul M, Khalid N, Khizar HB, Farrukh NM (2009). Effect of Different Levels of Salinity on Growth and Ion Contents of Black Seeds (Nigella sativa L.). Curr. Res. J. Biol. Sci. 1(3):135-138. |
|
|
Lutts S, Almansouri M, Kinet JM (2004). Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in durum wheat callus. Plant Sci. 167:9-18.
Crossref |
|
|
|
Mansour MMF (1997) Cell permeability under salt stress. In: Strategies for Improving Salt Tolerance in Higher Plants. Eds. P.K. Jaiwl, R.P. Singh, A. Gulati. Science Publ., Enfield, U.S.A, 87-110. |
|
|
Munns RC (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25:239-250.
Crossref |
|
|
Murillo-Amador B, Troyo-Dieguez E, Garcıa-Hernandez JL, Lopez-Aguilar R, Avila-Serrano NY, Zamora-Salgado S, Rueda-Puente RO, Kaya C (2006). Effect of NaCl salinity in the genotypic variation of cowpea (Vigna unguiculata) during early vegetative growth. Sci. Hortic. 108:423–431.
Crossref |
|
|
|
Nieman RH, Shannon MC (1976). Screening plant for salinity tolerance. Proc., of a workshop plant adaptation to mineral stress in problem soil. Soil Publ. By Cornell Unvi. Agric. Exp. Ithaca. |
|
|
Santos-Diaz MS, Alejo-Ochoa N (1994). PEG-tolerant cell clones of chili pepper (Capsicum annum L.): growth, osmotic potentials and solute accumulation. Plant Cell Tiss. Organ. Cult. 37:1-8.
Crossref |
|
|
Silva JS, de Lacerda CF, de Costa PHA, Enéas JEF, Filho G, Prisco JT (2003). Physiological responses of NaCl stressed cowpea plants grown in nutrient solution supplemented with CaCl2. Braz. J. Plant Physiol. 15(2):87-94.
Crossref |
|
|
|
Summart J, Thanonkeo P, Panichajakul S, Prathepha P, McManus MT (2010). Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr. J. Biotechnol. 9(2):145-152. |
|
|
|
Taban S, Ozguven N, Çelik H, Katkat AV (1999). Effect of potassium on macroelements distribution in maize plant grown under salt stress. Dahlia greidinger Int. symposium nutrient management under salinity and water stress, 1-4 March, Hafia- Israel. |
|
|
|
Turan MA, Abdelkarim HAE, Taban N, Taban S (2009). Effect of salt stress on growth, stomatal resistance, proline and chlorophyll concentrations on maize plant. Afr. J. Agric. Res. 4(9):893 - 897. |
|
|
Turan MA, Katkat V, Taban S (2007b) Variations in proline, chlorophyll and mineral elements contents of wheat plants grown under salinity stress. J. Agron. 6:137-141.
Crossref |
|
|
Turan MA, Türkmen N, Taban N (2007a). Effect of NaCl on stomata resistance and proline, chlorophyll, Na, Cl and K concentrations of lentil plants. J. Agron. 6:378-381.
Crossref |
|
|
Vasquez-Tello A, Zuily-Fodil Y, Pham-Thi AT, Vieira-Da Silva JB (1990). Electrolyte and Pi leakages and soluble sugar content as physiological tests for screening resistance to water stress in Phaseolus and Vigna species. J. Exp. Bot. 41:827–832.
Crossref |
|
|
Weimberg R (1987). Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant 70:381–388.
Crossref |
|
|
Wolf B (1982). A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 13:1035-1059.
Crossref |
|
|
|
Zahedi SM, Ansari NA, Azizi M (2012). The study of the effect of salinity stress on the germination and the initial growth of cowpea (Vigna unguiculata L. Walp). J. Agric. Technol. 8(7):2353-2372 |
|
|
Zhu JK (2001) Plant salt tolerance trends. Plant Sci. 6:66-72.
Crossref |
|
|