ABSTRACT
Alternaria blight (Alternaria helianthi Hansf) is one of the major diseases of sunflower during Kharif season in Maharashtra. Present Lab study was conducted at Department of Plant Pathology and field experiment at Oilseed Research Station, College of Agriculture, Latur, VNMKV, Parbhani, Maharashtra, India. Here, six fungicides were evaluated at 500, 1000, 2000 and 2500 ppm; five botanicals each at 10 and 20%) by poisoned food technique and readymade formulations of four bioagents; three fungal antagonists were evaluated in vitro and in vivo against A. helianthi- an incitant of Alternaria blight; in sunflower all the treatments were found fungistatic and significantly inhibited mycelial growth and disease intensity of the test pathogen over untreated control. Among the fungicides, maximum inhibition was observed in treatment with SAAF at 2000 ppm (90.36%), followed by Mancozeb at 2500 ppm (88.88%). Among botanicals, maximum inhibition was recorded with Neem (63.05 and 68.88%) in addition to Karanj (56.38 and 63.60%) at 10 and 20% concentrations. Fungal bioagents, T. harzianum was found most effective and recorded maximum mycelial growth inhibition (72.22%), followed by T. viride (70.27%). Bacterial antagonist P. fluorescens was found comparatively least effective with 48.60% inhibition of the test pathogen. After lab study, effective treatments was tested on field condition; results revealed that fungicide seed treatment with SAAF at 3 g/kg seed + two sprays of SAAF at 0.2% at 30 and 45 DAS recorded highest disease control (82.82%) and highest seed yield (1686 kg/ha) followed by seed treatment with SAAF 12% at 3 g/kg seed + two sprays of Mancozeb at 0.25% at 30 and 45 DAS recorded disease control (78.50%) and seed yield (1595 kg/ha) over untreated control (00.00) (792 kg/ha). Minimum disease control (45.25%) was recorded in seed treatment with Neem seed powder at 10 g/kg + two sprays of Neem extract at 10% at 30 and 45 DAS with 908 kg/ha yield.
Key words: Alternaria helianthi, sunflower, fungicides, bioagents, botanicals, management.
Sunflower (Helianthus annus L.) is one of the most important oilseed crops. The genus Helianthus is named from the Greek Helio meaning sun and anthos meaning flower. The family of sunflower is Compositae and the origin is southern U.S.A. and Mexico (Heiser, 1951). Sunflower seed is highly nutritious containing about 20% protein and 40 to 50% vegetable oil associated with a very high calorific value. The oil is considered to be of high quality due to its non-cholesterol properties and has been recommended for the patient having heart problem. It contains 60 to 73% linoleic acid, with sufficient amount of calcium, iron and vitamins like A, B, E and K (Gosal et al., 1988).
India is the largest grower of sunflower with an area of 0.90 million hectares, production of 0.62 million tonnes and the average productivity of 696 kg/ha. Important sunflower growing states in the country are Karnataka, Andhra Pradesh, Maharashtra, Tamil Nadu, Bihar, Punjab, Haryana and Uttar Pradesh. Almost 50% of area and production is accounted for by Karnataka followed by Andhra Pradesh and Maharashtra. In Maharashtra sunflower is cultivated on an area of 0.20 million hectares, and production of 0.11 million tonnes with an average productivity of 677 kg/ha (Anonymous, 2011a). Marathwada region of the state accounts for about 70% of area and production of the state. In Marathwada region sunflower is cultivated on an area of 0.13 million hectares, and production of 0.08 million tonnes with an average productivity of 633 kg/ha (Anonymous, 2011b).
Sunflower suffers from many diseases caused by fungi, bacteria, and viruses. Sunflower is the known host of more than 30 pathogens mostly fungi which under certain climatic condition may impair the normal physiology of the plant so that yield and oil quality are reduced significantly (Gulya et al., 1994). Some of the most important fungal diseases of sunflower are Alternaria leaf blight (Alternaria helianthi), Rust (Puccinia helianthi), Powdery mildew (Erysiphe cichoracearum), Downy mildew (Plasmopara halstedii), Root rot (Macrophomina phaseoli), Collar rot (Sclerotium rolfsii), Head rot (Rhizopus spp.), Verticillium wilt (Verticillium dahliae) and Leaf spot (Helminthosporium helianthi).
The yield losses due to A. helianthi varied from 48 to 57% in sunflower cultivars Morden and APSH-11 at different growth stages, respectively (Mayee and Wankhede, 1997). Sunflower cultivation throughout the country in general and particularly in the state of Maharashtra and Karnataka has been facing serious problem of the A. helianthi incidence.
Isolation
Sunflower leaves showing typical symptoms of disease were collected, washed with tap water to remove dirt, air dried and affected parts were cut into small pieces of about 2 cm in length. These pieces were disinfected with 1:1000 mercuric chloride solutions for a minute and rinsed with three changes of sterilized water to remove traces of corrosive disinfectant. These pieces were then transferred aseptically to sterilized plates poured with potato dextrose agar medium. The plates poured with sterilized medium were then incubated in inverted fashion in an incubator at 27°C±1 temperature. Profuse growth of the fungus on plates was observed after one week. Following hyphal-tip technique, test pathogen was transferred aseptically on the PDA slant in test tubes. Through frequent sub-culturing, the pathogen was purified and its pure culture was maintained on agar slant in test tubes and stored in refrigerator for further studies.
Identification
Pure culture of test pathogen obtained was inoculated aseptically on autoclaved PDA in Petri plates and plates were incubated at 27±1°C. A profuse growth of a fungus in plates was observed after one week. Cultural, morphological and microscopic characteristics of fully developed test pathogen were studied under low power as well as high power magnification of microscope.
Pathogenicity test
Ten days old culture of the organism was used for proving the pathogenicity by applying Koch’s postulates. For this purpose, seeds of sunflower hybrid KBSH-44 which is susceptible to Alternaria blight (A. helianthi) were surface sterilized with 0.1% HgCl2, and sown in the earthen pots filled with steam sterilized potting mixture of soil:sand:FYM (2:1:1). Healthy growing sunflower seedlings were maintained, watered regularly and kept in the screen house for further development. Three weeks old healthy seedlings were selected for inoculation. The spore suspension was prepared and filtered through two layers of sterile muslin cloth to remove residual mycelia. Filtrate obtained was suitably diluted with sterile distilled water to get inoculum concentration of 1×106spores/ml.
The seedlings were inoculated with 10 days old test fungus. Uninoculated seedlings of the same age sprayed with sterilized water served as control. After inoculation, the seedlings pots (both inoculated and uninoculated) were incubated in the screen house, where relative humidity (80 to 90%) and optimum temperature (27±1°C) were maintained for further development of Alternaria blight symptoms. Re-isolation was made from inoculated leaves by the isolated fungus which resembled in all respect with the original culture used for inoculation.
Efficacy of fungicides, botanicals and bioagents (in vitro)
In vitro evaluation of fungicides
Efficacy of six fungicides viz., SAAF (Mancozeb 63% + arbendazim 12%) 75 WP, Azoxystrobin 25 SC, Mancozeb 75 WP, ropiconazole 25 EC, Chlorothalonil 75 WP, Hexaconazole 5 EC were evaluated in vitro against A. helianthi, by Poisoned food technique (Nene and Thapliyal, 1993). The requisite quantity of each fungicide based on active ingredient was calculated and mixed thoroughly with autoclaved and cooled (40°C) Potato dextrose agar medium (PDA) in conical flasks to obtain desired concentrations in ppm. Plain PDA medium without fungicide served as control. Fungicide amended PDA medium was then poured aseptically in Petri plates (90 mm dia.). After solidification of the medium, all the plates were inoculated aseptically with 5 mm culture disc of the test fungus obtained from a week old actively growing pure culture of A. helianthi. The disc was placed on PDA in inverted position in the centre of the Petri plates and plates were incubated at 27±1°C. Each treatment was replicated thrice. When medium in the untreated control plates was fully covered with mycelial growth of the test fungus, radial mycelial growth was measured in all the treatment plates. Per cent inhibition of mycelial growth in treated plates was calculated by applying the formula given by Vincent (1927).
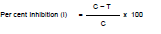
Where, C = Growth (mm) of test fungus in untreated control plate.
T = Growth (mm) of test fungus in treated plates.
In vitro efficacy of bioagents
Three fungal antagonists viz., Trichoderma viride, T. harzianum, T. hamatum, and one bacterial antagonist viz., Pseudomonas fluorescens were evaluated in vitro against A. helianthi applying dual culture technique (Dennis and Webster, 1971). Seven days old cultures of the test bioagents and test fungus (A. helianthi) grown on agar media were used for the study. Discs (5 mm dia.) of PDA along with culture growth of the test fungus and bioagents were cut out with sterilized cork borer. Then two culture discs, one each of the test fungus and bioagents were placed aseptically at equidistance and exactly opposite with each other on solidified PDA medium in Petri plates and plates were incubated at 27±1°C. PDA plates inoculated only with culture disc of test fungus were maintained as untreated control. Observations on mycelial growth of the test fungus and bioagents were recorded at an interval of 24 h and continued till untreated control plate was fully covered with mycelial growth of the test fungus. Per cent inhibition of the test fungus over untreated control was calculated by applying the formula given by Arora and Upadhyay (1978).
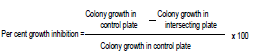
In vitro evaluation of botanicals (Plant extracts)
Plant extracts of five botanicals viz. Karang (Pongamia glabra), Neem (Azadirachta indica), Nirgudi (Vetex negundo), Mehandi (Lawsonia innermis), Dhotra (Dhatura metal) were evaluated against A. helianthi. Leaf extracts were prepared by grinding with mixture-cum grinder the 100 g washed leaves, of each plant species in 100 ml distilled water and filtered through double layered muslin cloth. The filtrates obtained were further filtered through Whatman No. 1 filter paper using funnel and volumetric flasks (100 ml cap.). The final clear extracts filtrates obtained formed the standard plant extracts of 100% concentration, which were evaluated (at 10 and 20%) in vitro against A. helianthi, applying poisoned food technique. (Nene and Thapliyal, 1993) and using potato dextrose agar as basal culture medium. An appropriate quantity of each plant extract (100%) was separately mixed thoroughly with PDA medium in conical flasks (250 ml cap.) to obtain desired concentrations of 5 and 10% after being autoclaved PDA at 15 lbs/inch2 pressure for 15 to 20 min. Sterilized and cooled PDA medium amended separately with plant extract was then poured (15 to 20 ml/plate) into sterile glass Petri plates (90 mm dia.) and allowed to solidify at room temperature. Each plant extract and its, respective concentrations were replicated thrice. The plates containing plain PDA without any plant extract were maintained as untreated control. Upon solidification of PDA, all the treatment and control plates were aseptically inoculated by placing in the centre a 5 mm mycelial disc obtained from a week old actively growing pure culture of A. helianthi. Plates containing plain PDA and inoculated with mycelial disc of the test fungus served as untreated control. All these plates were then incubated at 27±1°C temperature for a week or till the untreated control plates were fully covered with mycelial growth of the test fungus.
Observations on radial mycelial growth/colony diameter of the test fungus were recorded treatment-wise at 24 h interval and continued till mycelial growth of the test fungus was fully covered in the untreated control plates. Percent inhibition of mycelial growth over untreated control was calculated by applying the formula given by Vincent (1927).
In vivo evaluation of fungicides, botanicals and bioagents
The field experiment was conducted on the research farm of the Oilseed Research Station, Latur during Kharif, 2011, to evaluate the efficacy of fungicides, botanical and bioagent. The Neem seed kernel extract obtained from Oilseed Research Station, Latur, fungicides obtained from the Department of Plant Pathology, College of Agriculture, Latur and The talc-based formulations of the bioagent T. viride (5 × 106 cfu/g) obtain from the MKV, Parbhani:
Design: R.B.D. (Randomized Block Design)
Variety: KBSH- 44
Replications: Three
Treatments: Eight
Plot size: 4.2 m x 3.0 m
Spacing: 60 cm x 30 cm.
Gross area: 40.6 x 11 m
Treatments
T1: Seed treatment with SAFF (Mancozeb 63% +Carbendazim 12%) 75 WP at 3 g/kg seed.
T2: T1 + two spray of SAFF at 0.2%.
T3: T1 + two spray of Amistar (Azoxystrobin25 SC) at 0.05%.
T4 : T1 + two spray of Amistar ( Azoxystrobin 25 SC) at 0.1%.
T5 : T1 + two spray of Mancozeb 75 WP (Dithane M- 45) at 0.25.
T6: Seed treatment with T. viride at 10 g /kg seed + two spray of T. viride at0.5% spray.
T7 : Seed treatment with Neem seed powder at 10 g /kg seed + two spray of Neem extract at 10%.
T8 : Control (unsprayed).
The seeds of sunflower hybrid KBSH-44 susceptible to Alternaria blight were sown (20.07.2011) on the experimental field Oilseed Research Station, Latur. The crop was raised applying recommended package of practices and protective irrigation was given as and when required. Treatment sprays were undertaken as soon as the disease appeared, that is, on 30th day and second spray was undertaken 15 days after first spray, that is, (45 DAS). Observations on disease severity were recorded.
Percent disease intensity/severity
Percent intensity (severity) was calculated as per the standard area diagram developed by Mayee and Datar (1986). For recording the disease intensity at field conditions 0 to 9 disease rating scale developed by Mayee and Datar (1986) was used. For this purpose two leaves located at the bottom, two at middle and two top of the plant were chosen and scored as per scale given in Table 1.
The average intensity of each plot was worked out by using following formula.
Harvesting and threshing
Harvesting of respective sown crop was done after complete maturity of the crop. Threshing was done one week after harvesting so as to get dried seeds.
Yield
Yield of each treatment in all replications was recorded. Initially, yield of net plot was recorded on plot size basis and then converted into hectare basis.
Economics of fungicides, botanical and bioagent
To find out the most effective and economical treatment, the economics of each treatment was worked out. While calculating the cost of production, the expenditure incurred on the accounts of fungicides, botanicals and bioagents and labour charges for spraying were taken into account. Total monetary gain per treatment on hectare basis was worked out based on the existing sealing rates of the sunflower in the market and finally the B:C ratio of the treatments was worked out.
Disease management strategies
In vitro evaluation of fungicides
Effect of these fungicides on radial growth and inhibition of test pathogen were recorded. All the treatments were replicated thrice and a suitable untreated control (without fungicide) was also maintained.
Results (Tables 2 and 3) revealed that the fungicides tested (at 500, 1000, 2000 and 2500 ppm) significantly inhibited growth of the test fungus over untreated control (00.00%). Further, it was found that per cent inhibition of the test pathogen was increased with the increase in concentration of the fungicides tested. At 500 ppm, concentration maximum inhibition was recorded with Propiconazole (82.22%), this was followed by the fungicides Hexaconazole (79.99%) and Azoxystrobin (72.96%).
At 1000 ppm, concentration maximum inhibition was recorded with Propiconazole (87.03%), this was followed by the fungicides SAAF (86.29%) and Hexaconazole (82.59%). Least growth inhibition was recorded with Chlorothalonil (57.03%) and Azoxystrobin (78.88%).
At 2000 ppm, concentration maximum inhibition was recorded with SAAF (90.36%), this was followed by the fungicides Mancozeb (83.33%). Least growth inhibition was recorded with Chlorothalonil (64.81%).
At 2500 ppm, (Mancozeb) maximum per cent growth inhibition was 88.88% as compared to (00.00%) in untreated control.
From the result it is revealed that the maximum inhibition of the test pathogen recorded by combination of SAAF at 2000 ppm (90.36%); this was followed by Mancozeb at 2500 ppm (88.88%), Propiconazole at 1000 ppm (87.03%), Hexaconazole at 1000 ppm (82.59%) and minimum inhibition recorded by Chlorothalonil at 1000 ppm (57.03%); and was further followed by Azoxystrobin at 500 ppm (72.96%) as compared to (00.00%) in untreated control.
The results obtained in present studies in respect of in vitro effect of fungicides on mycelial growth inhibition of the test pathogen for the combination of SAAF, Azoxystrobin, Mancozeb, Propiconazole, Chlorothalonil and Hexaconazole fungicides effect is similar with earlier workers (Amaresh and Nargund, 2004; Akbari and Parakhia, 2007; Mathivanan and Prabavathy, 2007).
In vitro efficacy of bioagents
Three fungal (T. viride, T. harzianum and T. hamatum), and one bacterial (P. fluorescens) bioagents were evaluated in vitro against A. helianthi applying dual culture technique (Dennis and Webster, 1971) and using Potato dextrose agar (PDA) as basal medium.
Results (Table 4) revealed that all the bioagents evaluated exhibited fungistatic activity and significantly inhibited mycelial growth of A. helianthi. Of the four bioagents tested, T. harzianum was found most effective which recorded least mycelial growth (25.00 mm) and corresponding highest mycelial growth inhibition(72.22%) of the test pathogen over untreated control (90.00 mm and 00.00%, respectively), followed by T. viride (growth: 26.75 mm and inhibition: 70.27%) and T. hamatum (growth: 43.50 mm and inhibition: 51.66%). Bacterial antagonist P. fluorescens were found comparatively least effective with 46.25 mm linear mycelial growth and 48.60% inhibition of the test pathogen.
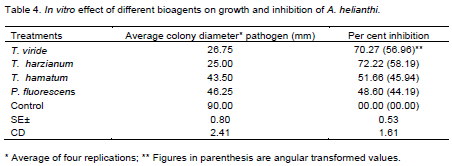
Thus, all the fungal and bacterial bioagents evaluated in vitro were found fungistatic against A. helianthi; the fungal bioagent was found effective than bacterial bioagent, for inhibition of test pathogen are in conformity to those reported earlier by several workers (Meena et al., 2004; Singh et al., 2005; Rao, 2006).
In vitro efficacy of plant extracts/botanicals
Result (Table 5) revealed that all the plant extracts, that is, Karanj, Neem, Nirgudi, Mehandi, Dhotra (at10 and 20% each), significantly inhibited growth of the test fungus over untreated control (00.00%) Further, it was found that inhibition of test pathogen was increased with increase in concentration of the botanicals tested.
At 10 and 20% concentration, maximum inhibition was recorded with Neem (63.05 and 68.88%), this was followed by Karanj (56.38 and 63.60%), Mehandi (52.49 and 60.55%). Minimum inhibition was recorded with Dhotra (40.55 and 48.60%) which was followed by
Nirgudi (49.99 and 54.16%).
Both concentrations (at 10 and 20%) of the plant extract were found effective in the inhibition of the test pathogen. However, higher concentration (at 20%) caused maximum (48.60 to 68.88%) inhibition of mycelial growth compared to lower concentration (at10%) which recorded comparatively minimum inhibition of mycelial growth in the range of 40.55 to 63.05%.
Thus, all the botanicals tested in vitro against A. helianthi (Hansf.) Tubaki and Nishihara were found effective in inhibiting the mycelial growth of the test pathogen over control. The result agree with the result of research workers (Ranjan et al., 1999; Amaresh, 2000; Rao, 2006).
In vivo evaluation of fungicides, botanicals and bioagents
Disease control, severity and seed yield: The results presented in Table 6 revealed that all treatments were found significantly superior over the control. Among all the treatments used at 30 and 45 DAS spray, treatment T2 (seed treatment with SAAF 3 g/kg and two sprays with SAAF at 0.2%) was most effective in Alternaria blight highest disease control and lowest disease severity (82.82 and 9.22%) with highest seed yield 1686 kg/ha followed by T5 (seed treatment with SAAF 3 g/kg and two sprays with Mancozeb at 0.25%) disease control and severity (78.50 and 11.55%) with 1595 kg/ha seed yield, T4 (seed treatment with SAAF 3 g/kg and sprays with Azoxystrobin at 0.1%) disease control and severity (74.12 and 13.90%) with 1468 kg/ha seed yield and T3 (seed treatment with SAAF 3 g/kg and sprays with Azoxystrobin 0.05%) disease control and severity (70.21 and 16.00%) with 1344 kg/ha seed yield.
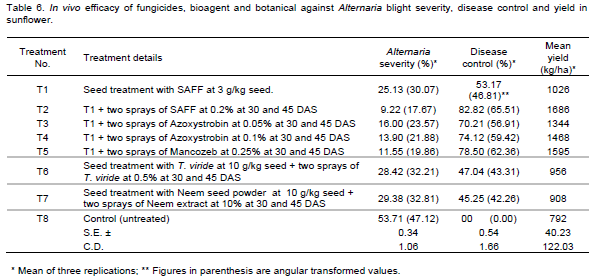
Minimum disease control and high disease severity was observed in treatment seed treatment with Neem seed powder at 10 g/ha seed + two spary of Neem extract at 10% (45.25 and 29.38%) with lowest 908 kg/ha seed yield, followed by T6 (seed treatment with T. viride 10 g/kg and sprays with T. viride 0.5%) disease control and severity (47.04 and 28.42%) with 956 seed yield and T1 (seed treatment with SAAF 3 g/kg) disease control and severity(53.17 and 25.13%) with 1026 seed yield.
Thus the new fungicides Mancozeb + Carbendazim (SAAF) evaluated against A. helianthi under field condition effectively controlled the Alternaria blight of sunflower and could be exploited on large scale for the management of the disease.
However, efficacy of fungicides, botanicals and bioagent in controlling Alternaria blight disease was reported earlier by several workers (Amaresh et al., 2000; Mathivanan and Prabavathy, 2007; Singh and Singh, 2007; Rao, 2006; Arunakumara et al., 2010).
Economics (Benefit:Cost ratio) of fungicides
The data presented in Table 7 indicated that highest B:C ratio of 4.02 was recorded in the treatment T2 (seed treatment with SAAF at 3 g/kg seed + two sprays of SAAF at 0.2% at 30 and 45 DAS). Next best treatment was treatment T5 (seed treatment with SAAF at 3 g/kg seed + two sprays of Mancozeb at 0.25% at 30 and 45 DAS) which recorded B:C ratio of 3.85 followed by the treatment T1 (seed treatment with SAAF at 3 g/kg seed) and T3 (seed treatment with SAAF at 3 g/kg seed + two sprays of Azoxystrobin at 0.05% at 30 and 45 DAS), T6 (seed treatment with T. viride 10 g/kg and sprays with T. viride 0.5% at 30 and 45 DAS), T4 (seed treatment with SAAF at 3 g/kg seed + two sprays of Azoxystrobin at 0.1% at 30 and 45 DAS), and T7 (seed treatment with Neem seed powder 10 g/kg and sprays with Neem extract 10% at 30 and 45 DAS) which recorded the B:C ratio of 2.82, 2.69, 2.39, 2.37, and 2.27 respectively over treatment T8 (untreated control) recorded lowest B : C ratio of 2.18.
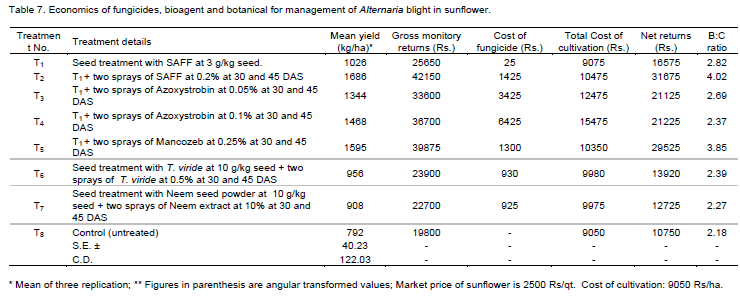
Thus all spray treatments of fungicides, were found economically effective and bioagent; botanicals were found economically less effective for management of Alternaria blight disease of sunflower. The maximum B : C ratio was recorded with T2 (seed treatment with SAAF at 3 g/kg seed + two sprays of SAAF at 0.2% at 30 and 45 DAS), that is, 4.02 and minimum 2.27 with T7 (seed treatment with Neem seed powder 10 g/kg and sprays with Neem extract 10% at 30 and 45 DAS).
These results obtained on the economics of fungicidal spraying treatments for the management of sunflower Alternaria blight disease are in conformity with those reported earlier by several workers (Singh, 2000; Mathivanan and Prabavathy, 2007).
The authors have not declared any conflict of interest.
REFERENCES
|
Anonymous (2011a). Annual report 2010-2011.Directorate of Agriculture, Andhra Pradesh. |
|
|
|
Akbari LF, Parakhia AM (2007). Management of Alternaria alternata causing blight of sesame with fungicides. J. Mycol. Plant Pathol. 37(3):426-430. |
|
|
|
Amaresh YS (2000). Epidemiology and management of Alternaria leaf blight and rust of sunflower (Helianthus annus L.). Ph.D. Thesis, Univ. Agric. Sci., Dharwad, Karnataka, India, pp. 15-16. |
|
|
|
Amaresh YS, Nargund, VB (2004). In vitro evolution of fungicides against Alternaria helianthi causing leaf blight of sunflower. Indian J. Plant Pathol. 22(1 &2):79-82. |
|
|
|
Anonymous (2011b). Annual report of sunflower Oilseeds Research Station, Latur (Maharashtra). |
|
|
Arora DK, Upadhyay RK (1978). Effect of fungal staling growth substances on colony interaction. Plant Soil 49:685-690.
Crossref |
|
|
|
Arunakumara KT, Kulkarni MS, Thammaiah N, Yashoda H (2010). Fungicidal management of early blight (Alternaria solani) of tomato. Indian Phytopathol. 63(1):96-97 |
|
|
|
Dennis C, Webster J (1971). Antagonistic properties of species group of Trichoderma and hyphal interactions. Trans. British Mycol. Soc. 57:363-369. |
|
|
|
Gosal SS, Vasiljevic L, Brar DS (1988). Plant biotechnology and sunflower improvement. Proceedings of 12th International Sunflower Conference, Novisad, Yugoslavia, July, 25-29, P. 599. |
|
|
|
Gulya T, Berlin N, Lamey A (1994). Sunflower disease in Sunflower production. Extension Bulletin (Ed. Berjlund, D. R., 1994. North Dakota Agricultural Experiment Station and North Dakota State University 98:44-62. |
|
|
|
Heiser CB (1951). The sunflower among American Indian text. Proc. Am. Philosophical Soc. 95:43. |
|
|
Mathivanan N, Prabavathy VR (2007). Effect of carbendazim and mancozeb combination on Alternaria leaf blight and seed yield of sunflower. Arachis Phytopathol. Plant Protect. 40(2):90-96.
Crossref |
|
|
|
Mayee CD, Datar VV (1986). Phytopathometry. Tech. Bull. J. Marathwada Agricultural University, Parbhani. P. 146. |
|
|
|
Mayee CD, Wankhede PB (1997). Integrated management of the Alternaria disease. IPS Golden Jubilee International Conference on Integrated Plant Disease Management for Sustainable Agril. held on 10-15 Nov. 1997. New Delhi India P. 116. |
|
|
Meena PD, Meena RL, Chattopadhyay C, Kumar A (2004). Identification of critical stage for disease development and biocontrol of Alternaria blight of Indian mustard (Brassica juncea). J. Phytopathol. 152(4):204 209.
Crossref |
|
|
|
Nene YL, Thapliyal RN (1993). Evaluation of fungicides in Fungicides for Plant Disaease Control (3rd ed.). Oxford, IHB Pub. Co. New Delhi. P. 331. |
|
|
|
Ranjan S, Lal HC, Ojha KL, Ranjan S (1999). Efficacy of plant extracts against Alternaria leaf blight of sunflower in vitro. J. Appl. Biol. 9(1):93-94. |
|
|
|
Rao MSL (2006). Studies on seed borne fungal diseases of sunflower and their management. Ph.D. Thesis, University of Agricultural Sciences, Dharwad pp. 55-90. |
|
|
|
Singh HK, Singh RB (2007). Integrated management of Alternaria blight of Rapeseed-mustard. Indian Phytopathol. 60(3):396. |
|
|
|
Singh SB, Kuwar S, Abhimanyu B (2005). Evaluation of native bio-agents against Alternaria brassicae causing Alternaria blight of mustard. Farm Sci. J. 14(2):64. |
|
|
|
Singh SN (2000). Relative efficacy of fungicides against seedling mortality and Alternaria blight of sunflower (Helianthus annuus L.). J. Mycol. Plant Pathol. 39(1):119-120. |
|
|
|
Vincent JM (1927). Distortion of fungal hyphae in the presence of certain inhibitors. Nature pp. 159-180. |