ABSTRACT
Plant protection agents applied in agriculture deserve special attention, given their toxicity, accumulability, and persistence in the soil environment, where they frequently induce disturbances in biochemical processes. Assessment of the risk associated with introduction of chemical preparations to agricultural cultivation is an essential element of protection of the natural environment against the harmful effects of these substances. Therefore, a three-year field study (2010 to 2012) was carried out to assess the long-term effect of soil contamination with the Reglone 200 SL herbicide and Elastiq 550 EC preparation (limiting rapeseed loss) on the course of the ammonification and nitrification processes and soil respiratory activity. The experiment was established in the split-block design on soil classified as black earth proper (WRB-Mollic Gleysols) with pH= 6.1. Soil for the analyses was sampled in 8 periods, that is, after 2, 10, 12, 14, 22, 24, and 26 months of the experiment. The experiments demonstrated that the amount of emitted CO2 and the content of ammonium and nitrate ions depended largely on the period of the analysis and the type of the chemical agent. The optimal dose of 200 SL and Elastiq 550 EC applied caused periodic statistically significant changes in the respiratory activity and the intensity of the ammonification and nitrification processes in the tested soil.
Key words: Soil, respiratory activity, ammonification, nitrification, Reglone 200 SL, Elastiq 550 EC.
Agriculture contributes considerably to the increase of the pool of soil contaminants. This is related, among other things, with the introduction of chemical agents of plant protection, alternatively referred to as pesticides, in field cultivations. A problem encountered in the cultivation of rapeseed (Brassica napus L.) is its non-uniform ripening and yield losses resulting from excessive seed shedding in the course of harvest of that crop plant. The treatment of desiccation, performed with the use of various chemical agents commonly known as desiccants, is aimed at the preparation of a plantation for harvest through acceleration and uniformity of the process of ripening of seed. It reduces excessive cracking of pods during ripening, thanks to which it is possible to achieve the maximum yield (Markowski et al., 2003; Choszcz et al., 2005; Konopka et al., 2005).
Pesticides applied in field cultivations are not neutral for the soil environment (Chowdhury et al., 2008). Improper application of chemical preparations which often have the ability of long-term persistence is frequently the cause of their excessive accumulation in the soil. Agricultural chemicals undergo various transformations in the soil. The intensity and directions of those transformations depend largely on the kind of chemical applied, its properties, rate of decomposition, and also on the properties of the soil environment (Onet, 2009). This constitutes a serious threat to the natural environment. Such chemical may interfere with the run of microbiological and biochemical processes which play a key role in the correct functioning of ecosystems. In the study of the soil environment the utilisation of enzymatic activity permits comprehensive identification of changes taking place in that environment (Margesin et al., 2000; Zahir et al., 2001; Janvier et al., 2007; Kucharski et al., 2011) under the effect of contaminants, e.g. chemical substances (Radivojevi? et al., 2012). It permits the estimation of the ecological status of the soil, its biological activity, as well as its fertility and productivity (Kieliszewska-Rokicka, 2001; Quemada and Menach, 2001; Russel, 2005).
Microorganisms inhabiting the soil have the capability of producing enzymes, thanks to which they are involved in most of the processes taking place in the soil. They participate in the degradation and mineralisation of organic matter, in binding nitrogen, and also in the cycle of elements in nature (Calderon et al., 2000; Chowdhury et al., 2008; Jezierska-Tys and Fr?c, 2008). Moreover, the activity of soil microorganisms has also an effect on the degradation and detoxification of various substances contaminating the soil, including e.g. the chemical agents of plant protection, commonly known as pesticides (van Eerd et al., 2003; Beck et al., 2005).
Various chemical contaminants migrating into soil are of particular importance in the aspect of environmental studies. Taking into account the problem of soil contamination with chemical agents, a study based on a field experiment was performed, the objective of which was to determine the long-term effect of the chemical preparations Reglone 200 SL and Elastiq 550 EC on the respiratory activity and on the intensity of the processes of ammonification and nitrification in soil.
The chemical preparations used in the field experiment were the plant protection agents Reglone 200 SL and Elastiq 550 EC. In crop plant plantations, the preparation Reglone 200 SL is used for weed control, and also for the desiccation of aboveground parts of plants containing chlorophyll. The biologically active substance in the composition of the preparation is diquat ion, a compound from the group of pyridyls. Elastiq 550EC glues the pods together, preventing excessive seed shedding and losses prior to and during the harvest of rapeseed. Elastiq 550 EC contains two biologically active substances in its composition: synthetic latex and alkoxylated alcohol.
The study was conducted in the years 2009-2011, at the plant variety evaluation experimental station in G??bokie, Kujawsko-Pomorskie Province (52° 38'41''N, 18° 26'18''E). The three-year field experiment was set up in the split-block design on a soil classified as black earth proper (WRB-Mollic Gleysols), with pH 6.1.
In the first year of the experiment, all the treatments were sown with winter rapeseed cv. “Californium”, in the second year – with sugar beet, and in the third – barley. The basic characteristics of the soil are given in Table 1.

In the first year of the experiment spraying with the preparations Reglone 200 SL and Elastiq 550 EC was made. The preparations were applied at technological doses recommended by the manufacturer. The model of the experiment comprised the following treatments: K - control soil, with no chemical preparations; R - soil with an addition of the preparation Reglone 200 SL at the optimum dose (2 dm3 ha-1); E - soil with the preparation Elastiq 550 EC applied at the optimum dose (1 dm3 ha-1). The application of the preparations was made by means of a knapsack spraying system. In the case of Elastiq 550 EC the spraying was made 4 weeks, and of Reglone 200 SL - 10 days before the harvest of winter rapeseed. In all the treatments, the basic tillage operations were performed, and the same level of fertilisation was applied, as recommended for the crop plant cultivated.
The effect of the chemical preparations Reglone 200 SL and Elastiq on the soil environment was studied for three years. In 2009 soil samples for microbiological and biochemical assays were taken twice, that is, in August and October. In 2010 and 2011 soil samples were taken three times, in May, August and October. The analyses correspond to the following terms of analyses: term 0- immediately after the harvest of winter rapeseed (in the first decade of August), and then after 2, 10, 12, 14, 22, 24 and 26 months of the experiment. Soil samples for the analyses were taken from the arable horizon of each plot.
Respiratory activity of the soil was assayed with the method of respiration induction through the addition of substrate (glucose) to the soil, according to Rühling and Tyler (1973). Carbon dioxide evolved from the soil during incubation was bound by 0.2 M solution of NaOH used in the experiment, forming Na2CO3. Next, after the addition of BaCl2, insoluble sediment of BaCO3 was precipitated, and excess of non-bound soda lye was titrated with 0.1 M HCl. The respiratory activity expressed by the amount of evolved CO2 was assayed with the titration method.
The rate of the process of ammonification was determined on the basis of the content of NH4+ ions, with the Nessler method. For the determination of the content of N-NH4, portions of soil were weighed from the average soil samples. After 7 days of incubation at room temperature, 0.03 M acetic acid was added and, to extract ammonium ions the samples were shaken and then filtered. For the assay of N-NH4 the filtrate was collected, sodium-potassium tartrate and Nessler reagent were added, and the whole was topped up with distilled water. The solution obtained in this manner was mixed thoroughly and subjected to colorimetry at wavelength of 410 nm. The zero sample was a mixture of 0.03 M acetic acid, sodium-potassium tartrate, Nessler reagent and distilled water. The results were converted to mg N-NH4 kg-1 d.m of soil 7 day-1.
The intensity of the process of nitrification was determined on the basis of the content of NO3-, with the brucine method. For that purpose filtrate was taken, prepared in the same manner as in the assays of the process of ammonification. The filtrate was placed in test tubes to which brucine dissolved in concentrated sulphuric acid was added. After setting aside for 24 h (for the stabilisation of colour), colorimetry was performed at wavelength of 470 nm. The zero sample was a solution containing distilled water and brucine. The results were converted to mg N-NO3 kg-1 d.m. of soil 7 day -1.
The results obtained were processed statistically using the analysis of variance (ANOVA). The least significant differences were calculated with the Tukey test at significance level of α = 0.05. The statistical analyses of the results were performed with the use of the program STATISTICA 7.1.
One of the primary indices used in the estimation of the status of the soil environment is the respiratory activity (Yao et al., 2006) which indicates the level of general activity of soil microorganisms (Dutta et al., 2010). It is related with the processes of degradation and oxidation of organic compounds, in the course of which carbon dioxide is evolved. Measurement of the respiratory activity permits the estimation of potential disturbances in the processes of carbon transformations taking place in soils subjected to the effect of pesticides or other xenobiotics (Gil-Sotres et al., 2005).
The periodic respiratory activity in the soil of the experimental treatments, measured by the amount of evolved CO2, is presented in Figure 1. Based on the analyses performed, both activation and inhibition of the amount of evolved CO2 were observed in the treatments studied. This was related with the kind of chemical preparation applied and with the time of analyses. In the opinion of Johnsen et al. (2001), plant protection agents can constitute a source of energy and nutrients for certain microorganisms. With the passage of time, organic compounds contained in the preparations, introduced in the soil, were undergoing degradation and probably gradually used up by soil microorganisms. A stimulating effect of both chemical preparations was noted on the first date of analyses, that is, immediately after the harvest of winter rapeseed, and after 22 months of their effect on the soil. Elastiq 550 EC also caused a stimulation of the process in question after 14 and 24 months of the experiment. At other times of analyses, the respiratory activity in the experimental treatments oscillated at a fairly stable level. Analysis of the mean values (Figure 2) for the whole three-year-long period of the experiment revealed that only Elastiq 550 EC caused an increase in the amount of evolved carbon dioxide compared to the control treatment. The results obtained did not differ statistically significantly.

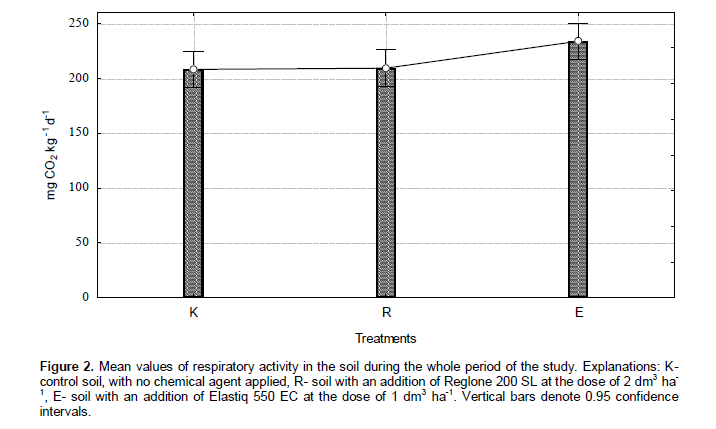
Vig et al. (2008), studying samples of soil contaminated with insecticides (triazophos and endosulphan), also did not observe their negative effect on soil respiration. There are also reports on research in which negative effects of xenobiotics on the process of evolution of CO2 was observed. Niewiadomska et al. (2009) studied the effect of plant protection agents – a herbicide with the name Fox 480 (active substance - bifenox) and a fungicide – seed primer whose active substance is flutriafol – on the intensity of CO2 evolution in soil. In that study, the authors observed a negative effect on the herbicide on the process of CO2 evolution. The fungicide under study had a positive effect on the respiratory activity of the soil, but only on the last date of analyses. In a study by Araujo et al. (2003), glyphosate caused a 10 to 15% increase in the amount of evolved CO2 in the soils studied.
Nitrogen is one of the most important biogenic elements in nature. Soil microorganisms participate actively in nitrogen transformations. Ammonification and nitrification are among those processes that provide information on nitrogen transformations in soil (Barabasz, 1992; Eemmerling et al., 2002). They also play an important role in the cycle of that element in the soil and are accepted as an important indicator of biological activity of soil. They are commonly used for the determination of the effect of various factors on the biological status of the soil environment (Jezierska-Tys, 2002; Shi et al., 2004).
Available domestic and foreign literature provides a wide range of information on the impact of pesticides on the ammonification and nitrification process in soil. The study demonstrated that throughout the whole period of the experiment the chemical preparations applied caused periodic changes in the content of ammonium ions (Figure 3). Immediately after the harvest of winter rapeseed (first date of analyses), both Reglone 200 SL and Elastiq 550 EC induced significant inhibition of the intensity of the process of ammonification in the soil studied. A statistically confirmed significant increase in the content of ammonium ions was caused by Reglone 200 SL in the 10th and 26th months of the experiment, and by Elastiq 550 EC in the 22nd and 26th months. In the 0, 14th and 24th months of the experiment, both these preparations had an inhibiting effect on the process studied. Analysing the mean values of intensity of the process of ammonification for the experimental treatments (Figure 4) for the whole three-year-long period of the study, one can conclude that the chemical agents applied did not have any significant effect on the process in question. In their investigations of the effect of contamination of soil with different doses of Harpun 500 SC, Faworyt 300 SL, Akord 180 OF, and Mocarz 75 WG herbicides on the course of the ammonification process in soil, Kucharski et al. (2009) observed that the highest inhibitory effect on the amount of N-NH4 was exerted by the Mocarz 75 WG herbicide. In turn, in an experiment with Roundup herbicide applied in a field dose, Krzy?ko-?upicka (2008) reported an increase in the concentration of ammonia nitrogen in soil. Analysis of the data presented in Figure 5 shows that the chemical preparations Reglone and Elastiq used in the experiment caused period variations in the content of nitrate ions in the soil. The preparation Reglone caused a significant increase in the content of nitrate ions in the 2nd, 14th and 22nd months as compared to the control treatment. In the final phase of the experiment a significantly the greatest drop was observed in the intensity of the process of nitrification, both in the soil with Reglone 200 SL and in that with Elastiq 550 EC, a stronger effect being noted in the treatment with Reglone 200 SL. The mean content of N-NO3 (Figure 6) for the experimental treatments (from the three-year experiment) was at a similar level. In a study by Przybulewska and Nowak (2003), the preparation Reglone 200 SL had an inhibiting effect on that process. Upon application of chlorotalonil herbicide, Lang and Cai (2009) also reported decreased nitrification in soil.
The study demonstrated that the plant protection agents Reglone 200SL and Elastiq 550 EC, applied to the soil at doses recommended by the manufacturer in the process of desiccation, did not cause any significant disturbance of the processes of ammonification and nitrification.
CONCLUSIONS AND RECOMMENDATIONS
Intensive plant protection treatments in crop fields based on application of chemical plant protection agents is now and will still be a basic method for weed, pest, and disease control in plant cultivations. The benefits of using chemical agents are undeniable, although the risk related to excessive or improper use thereof should not be underestimated. Application of these agents exerts an effect on the soil environment yielding disturbances in biochemical processes. The investigations have shown that the amount of emitted CO2 and the content of ammonium and nitrate ions depended largely on the period of the analysis and the type of the chemical agent applied.
The authors have not declared any conflict of interest.
This study was supported by grants from the Polish government - Ministry of Science and Higher Education under research project No. N N305 410538.
REFERENCES
Araujo ASF, Monteiro RTR, Abarkeli RB (2003). Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 52:799-804.
Crossref |
|
|
Beck L, Römbke J, Breure AM, Mulder C (2005). Considerations for the use of soil ecological classification and assessment concepts in soil protection. Ecotoxicol. Environ. Saf. 62:189–200.
Crossref |
|
|
Calderon FJ, Jackson LE, Scow KM, Rolston DE (2000). Microbial responses to simulated tillage in cultivated and uncultivated soils. Soil Biol. Biochem. 32:1547-1559.
Crossref |
|
|
|
Choszcz DJ, Kaliniewicz Z, Konopka S, Lipiński AJ, Markowski P, Rawa T (2005). An attempt at reducing rape seed losses during desiccation treatments. Inż. Rol. 6(66):75-83. |
|
|
Chowdhury A, Pradhan S, Saha M, Sanyal N (2008). Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian J. Microbiol. 48:114–127.
Crossref |
|
|
Dutta M, Sardar D, Pal R, Kole RK (2010). Effect of chlorpyrifos on microbial biomass and activities in tropical clay loam soil. Environ. Monit. Assess. 160:385-91.
Crossref |
|
|
Emmerling C, Schloter M, Hartmann A, Kandeler E (2002). Functional diversity of soil organisms – A review of recent research activities in Germany. J. Plant Nutr. Soil Sci. 165:408-420.
Crossref |
|
|
Gil-Sotres F, Trasar-Cepeda C, Leirós MC, Seoane S (2005). Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 37:877-887.
Crossref |
|
|
Janvier C, VilleneuveI F, Alabouvette C, Edel-Hermenn V, Mateille T, Steinberg C (2007). Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 39:1-23.
Crossref |
|
|
|
Jezierska-Tys S (2002). Transformations of nitrogenous organic matter in sulphated lessive soil amended with sewage sludge. Acta Agrophys. 70:191-200. |
|
|
|
Jezierska-Tys S, FrÄ…c M (2008). Microbiological indices of soil quality fertilized with dairy sewage sludge. Int. Agrophys. 22:215-219. |
|
|
Johnsen K, Jacobsen CS, Torsvik V, Sørensen V (2001). Pesticide effects on bacterial diversity in agricultural soils—A review. Biol. Fertil. Soils 33:443–453.
Crossref |
|
|
|
Kieliszewska-Rokicka B (2001). Soil enzymes and their importance in studies of microbiological activity of soil. In: Microorganisms of the soil environment, Ed. Dahm H, Pokojska-Burdziej A, UMK Toruń pp. 37-47. |
|
|
|
Konopka S, Choszcz DJ, Kaliniewicz Z, Lipiński AJ, Markowski P, Rawa T (2005). Effect of desiccation treatments on quality and losses of rape seeds. Inż. Rol. 11(71):227-234 |
|
|
|
KrzyÅ›ko-Åupicka T (2008). Ecological effects of phosphoorganic herbicide on soil diazotrophs in spring. Part II. Ecol. Chem. Eng. 15(4):596-602. |
|
|
|
Kucharski J, Baćmaga M, Wyszkowska J (2009). Effect of herbicides on the course of ammonification in soil. J. Elem. 14(3):477-487. |
|
|
|
Kucharski J, Wieczorek K, Wyszkowska J (2011). Changes in the enzymatic activity in sandy loam soil exposed to zinc pressure. J. Elem. 16(4):577-589. |
|
|
Lang M, Cai Z (2009). Effects of chlorothalonil and carbendazim on nitrification and denitrification in soils. J. Environ. Sci. 21(4):458-467.
Crossref |
|
|
Margesin R, Zimmerbauer A, Schinner F (2000). Monitoring of bioremediation by soil biological activities. Chemosphere 40:339-346.
Crossref |
|
|
|
Markowski P, Choszcz DJ, Kaliniewicz Z (2003). An attempt at estimation of seed losses at rapeseed desiccation with the preparations Avans and Reglone. Inż. Rol. 10(52):247-254. |
|
|
|
Niewiadomska A, Sawińska Z, Swędrzyńska D, Wolna-Maruwka A, Klama J (2009). Effect of selected plant protection agents on the activity of dehydrogenases, acid phospohatase and on evolution of CO2. Zesz. Probl. Post. Nauk Roln. 540:279-285. |
|
|
|
Onet A (2009). Study of the effect of some pesticides on soil microorganisms. Anal. Univ. din Oradea. Fascicula. Protecţia. Mediului. 14:763-765. |
|
|
|
Przybulewska K, Nowak A (2003). Effect of various systems of chemical protection applied in potato cultivation on transformations of nitrogen compounds in soil. Part I. Model experiment. Ecol. Chem. Eng. 10S2: 219-231. |
|
|
Quemada M, Menacho E (2001). Soil respiration 1 year after sewage sludge application. Biol. Fertil. Soils 33:344-346.
Crossref |
|
|
Radivojević L, Gašić S, Šantrić L, Gajić Umilendić J, Vljević DM (2012). Short-time effects of herbicide nicosulfuron on biochemical activity of Chernozem soil. J. Serb. Chem. Soc. 77:1–15.
Crossref |
|
|
Rühling A, Tyler G (1973). Heavy metal pollutions and decomposition of needle litter. Oikos 24:402- 415.
Crossref |
|
|
|
Russel S (2005). The importance of studies of enzymes in the soil environment. Acta Agrophys. Rozpr. Monogr. 3:5-9. |
|
|
Shi W, Miller BE, Stark JM, Norton JM (2004). Microbial nitrogen transformations in response to treated dairy waste in agricultural soils. Soil Soc. Am. J. 68:1867-1874.
Crossref |
|
|
Van Eerd LL, Hoagland RE, Zablotowicz RM, Hall JC (2003). Pesticide metabolism in plants and microorganisms, Weed Sci. 51:472–495.
Crossref |
|
|
Vig K, Singh DK, Agarwal HC, Dhawan AK, Dureja P (2008). Soil microorganisms in cotton fields sequentially treated with insecticides. Ecotoxicol. Environ. Saf. 69:263–276.
Crossref |
|
|
Yao X H, Min H, Lü ZH, Yuan HP (2006). Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 42(2):120-126.
Crossref |
|
|
Zahir ZA, Atteeq ur Rehman Malik M, Arshad M (2001). Soil enzymes research: A review. J. Biol. Sci. 1(5):299-307.
Crossref |