ABSTRACT
Water deficit negatively affects crop development and productivity. With decreasing rainfall and shortage of arable land, there is a demand for alternative drought-tolerant species for use on non-arable land. The tree species Balanites aegyptiaca is considered as drought tolerant and a potential source of many secondary metabolites. The oil-containing seeds may also be used as biofuel. Genetic diversity was investigated amongst the B. aegyptiaca collected from different geographical regions using amplified fragment length polymorphism (AFLP) and the relationship among geographical distribution and genetic diversity was determined. Plants were grown from seedlings collected at 12 locations from 11 provenances. AFLP produced 510 bands of which 477 (93.5%) were polymorphic. Cluster and principal component analyses indicated that individual samples of B. aegyptiaca were distributed in 3 main clades and that the provenance El-Kharga represented a single clade. Several key morpho-physiological responses to water stress were examined to evaluate drought stress tolerance and to compare respective stress responses among different provenances under greenhouse conditions. Severe drought stress decreased biomass parameters in all genotypes. However, B. aegyptiaca provenances also differed in their adaptive responses to water shortage. By appropriate grouping of 2 or 3 response factors, the effects of water deficit on the various provenances could readily be distinguished. Provenance El-Kharga showed the smallest amount of biomass reduction under severe drought stress and retained the highest leaf water content.
Key words: Amplified fragment length polymorphism (AFLP), Balanites aegyptiaca, biometrical growth parameters, drought tolerance, stomatal conductance.
Water shortage is one of the major environmental limitations to plant growth and development (Harb et al., 2010; Song et al., 2012). Drought decreases crop productivity and approaching 28% of the world’s soil surface is too dry to be reliably productive (Bray et al., 2002; Ambrosone et al., 2013). Plants respond to water shortage through several regulatory pathways and adapt by reprogramming a number of metabolic and physiological mechanisms (Morison et al., 2008; Ahuja et al., 2010; Park et al., 2012; Khamis and Papenbrock, 2014). Loss of water content closes stomata resulting in slower transpiration, photosynthesis, growth and gain in biomass. Stomatal pore size controlled through guard cells is the main control point affecting water use efficiency and the intake of CO2 for photosynthesis (Song et al., 2014). Plants may respond to variation in soil water content through root signal(s) that originated from the root tips transferred via the xylem to the leaf (Cornic and Massacci, 1996) resulting in increasing the concentration of abscisic acid (ABA) up to 30-fold in the guard cell apoplast (William and Outlaw, 2003). This enhances ion outflow and leads to a reduction of solute concentration and a loss of turgor of the guard cells, finally resulting in stomatal closure (Joshi-Saha et al., 2011; Watkins et al., 2014), reduced uptake of carbon dioxide (CO2) by leaves and decreased transpiration (Cornic and Massacci, 1996). Water stress also depresses plant growth by inhibiting cell elongation, leaf expansion and thus reducing photosynthetic area. Water stress also changes the photosynthetic apparatus and pigments, such as de-epoxidised xanthophyll cycle pigments, antheraxanthin and zeaxanthin (Farooq et al., 2009).
Balanites aegyptiaca L. (Del.), with a common name desert date, belongs to Zygophyllaceae family, is a xerophytic tree, distributed in tropical and non-tropical areas in North and West Africa and West Asia (Bhandari 1995; Siddique and Anis, 2008). It is a multi-purpose tree. Kernels contain up to 46.7% oil, which has been successfully tested for biodiesel production (Chapagain et al., 2009). The kernels and various parts of the plant also contain several secondary metabolites useful in medicine and other biotechnologies (El-Tahir et al., 1998; Mohamed et al., 2002; Chapagain and Wiesman, 2008).
Across a broad range of environments and soils, B. aegyptiaca shows large phenotypic variation in crown shape, seeds, fruits, leaves, and the fruiting and flowering time (Hall and Walker, 1991; Hall, 1992; Sands, 2001; Zobel and Talbert, 2003; Elfeel et al., 2009). Its anatomical leaf characteristics (Radwan, 2007) contribute much to the notable drought tolerance of this species (Elfeel et al., 2007). B. aegyptiaca also retains an ability to produce fruits under arid conditions and mature trees resist damage by fire and flood and may thus be well-suited for cultivation in several arid desert environments in Africa and South Asia (Hall and Walker, 1991).
Little is known of the genetic variation of the species among and within its various geographic habitats. So far, genetic variability in B. aegyptiaca has only been investigated through peroxidase isozyme analysis of different germplasm pools (Chamberlain, 1992). Analysis by amplified fragment length polymorphism (AFLP) is more powerful and discriminating, and was used in the present study. It is a DNA fingerprint method where polymerase chain reaction (PCR) amplification is used to detect genomic restriction fragments without previous knowledge of the DNA sequences (Vos et al., 1995).
The main objectives of this study were to (1) investigate genetic diversity among B. aegyptiaca provenances collected from different geographical regions and determine the relationship among geographical distribution and genetic diversity, (2) investigate the morpho-physiological responses of B. aegyptiaca to different degrees of water shortage, and (3) compare responses to water stress by different provenances.
Plant
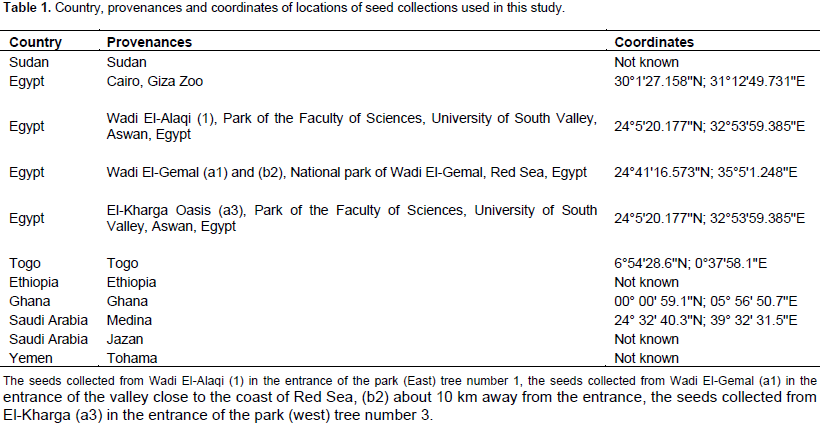
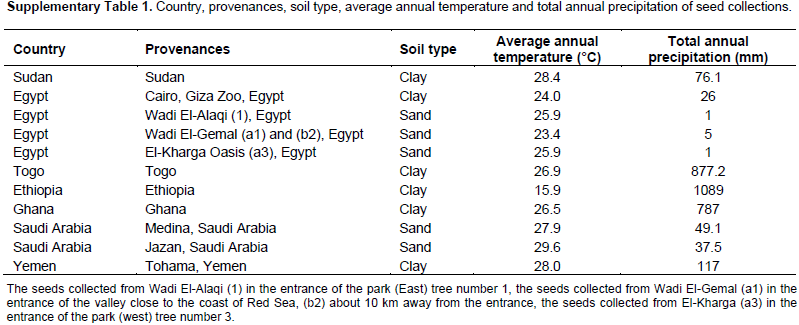
Fruits from 33 trees were collected from 11 provenances distributed at different geographical locations. The seeds were collected in the following way: from one plant of the provenances Sudan, Cairo (Egypt), Ethiopia, Saudi Arabia (Jazan and Medina) and Yemen (Table 1). Seeds from Ghana were collected from 5 trees distributed at 5 different locations, seeds from El-Kharga Oasis, Egypt, were collected from 9 trees at 3 locations: (a1, a2 and a3), (b1, b2 and b3), and (c1, c2 and c3), the seeds from Wadi El-Alaqi, Egypt, were collected from 3 trees at one location. The seeds collected from Wadi El-Gemal, Egypt were divided into 3 groups: Group a was collected from 2 trees (a1 and a2) in the entrance of the valley close to the coast of Red Sea; Group b was collected from 4 trees (b1, b2, b3 and b4) about 10 km away from the entrance of the valley; and Group c was collected from 3 trees (c1, c2 and c3) about 50 km away from the entrance of the valley. More information about soil type, annual average temperature and annual precipitation at the collection sites are summarized in the Supplementary Table 1. The seeds were germinated for 1 month and leaves from one plant were randomly selected and harvested for DNA extraction.
Molecular analysis through AFLP
DNA extraction and AFLP
DNA was extracted using the Plant Nucleospin II Kit (Macherey and Nagel, Düren, Germany) as described by the manufacturer. DNA quality was tested on agarose gels stained with ethidium bromide. A micro-plate reader with micro-volume plates (Synergy Mx Multi-Mode, BioTek, Germany) was used to measure DNA concentration. The 4 steps AFLP method was conducted according to Vos et al. (1995) with minor modifications.
(1) Restriction: The enzyme combination of 0.5 µl EcoRI (10 U µl-1) and 0.3 µl MseI (10 U µl-1) was used to digest 250 ng of genomic DNA in a total volume of 25 µl including 1 × RL buffer (10 mM Tris/HCl, 10 mM MgAc, 50 mM KAc, 5 mM DTT, pH 7.5). This mixture was incubated for 2 h at 37°C.
(2) Ligation of adaptors: The resulting restriction fragments were ligated to specific MseI (50 pmol) and EcoRI (5 pmol) adaptors (MWG Biotech Eurofins, Ebersberg, Germany) in a total volume of 5 µl reaction mixture containing 0.5 µl of EcoRI adaptor, 0.5 µl of MseI adaptor, 0.5 µl 10 × RL-buffer, 0.6 µl of 10 mM ATP, 0.05 µl of T4-DNA-ligase (1 U µl-1) and 2.85 µl H2O. The reaction mixture was incubated for 3.5 h at 37°C.
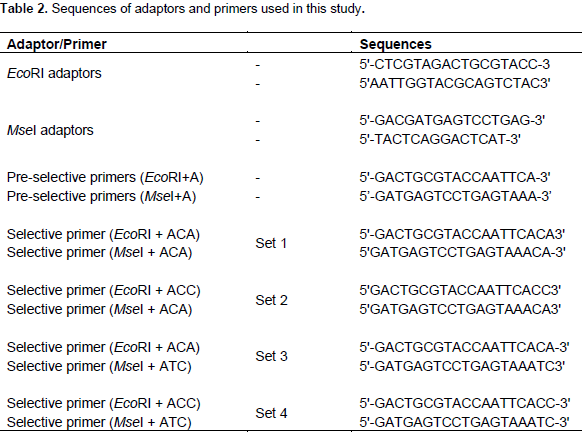
(3) Pre-amplification: Pre-amplification was performed in a total volume of 50 μl including 5 μl template of digested and ligated DNA, 1.5 μl (50 ng) of EcoRI+A and MseI+A primers (Table 2), 1 μl of Taq polymerase (MBI Fermentas, St. Leon-Rot, Germany), 1 × Williams buffer (10 mM Tris/HCl pH 8.3, 50 mM KCl, 2 mM MgCl2, 0.001% gelatine), 5 μl dNTPs (0.25 mM) and 31 μl H2O. The PCR program was run at 94°C for 5 min, then 20 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 1 min, followed by 10 min at 72°C.
(4) Amplification: The pre-amplified PCR product was diluted 1:20 with 1 TE Buffer (10 mM Tris/HCl pH 7.5, 1 mM EDTA) and 2.5 μl of the diluted pre-amplification product was used for the selective PCR amplification which was performed in a total volume of 10 μl containing of 2.5 μl EcoRI-IRD primer (2 ng μl-1) and 0.3 μl MseI primer (50 ng μl-1) through 4 primer combinations EcoRI+ACA/MseI+ATC, EcoRI+ACC/MseI+ATC, EcoRI+ACA/MseI+ACA, and EcoRI+ACC/MseI+ACA (Eurofins MWG Operon) (Table 2), 1 μl dNTPs (2 mM), 0.05 μl Taq polymerase (5 U μl-1), 1 μl 10 × William buffer and finally 2.65 μl H2O. The PCR program started with 94°C for 5 min, one cycle was performed for 94°C for 30 s, 65°C for 30 s and 72°C for 1 min. The subsequent annealing temperature was decreased by 0.7°C per cycle for the next 11 cycles, then 24 cycles starting with 94°C for 30 s, annealing temperature 56°C for 30 s and final extension at 72°C for 10 min. After final amplification, 20 μl of AFLP dye (98% formamide, 10 mM EDTA, and 0.05% pararosaniline) was added to each sample. Mixtures were warmed up to 72°C for 5 min. Following the manufacturer’s instructions, samples were loaded onto 6% AFLP gels on 4300 DNA Analyzer (LI-COR, Biosciences, Germany).
AFLP data analysis
The data was manually scored comparing with pictures where the binary data was organized and only the polymorphic fragments were scored as band present (1) and band absent (0). The 33 individuals were reduced to 12 individuals with one plant for each provenance, only Wadi El-Gemal provenance is represented by 2 individuals from 2 locations. Present and absent binary data of these 12 individuals and 477 polymorphic loci were used as the basis for the analysis.
In the present study, the band-based approach based on Bonin et al. (2007) was used for the analysis in the individual. Dice coefficient (Dice, 1945) was used to calculate the similarity among 12 individuals. Cluster analysis was prepared using unweight pair group method with the arithmetic mean (UPGMA) based on Dice index (Nei and Li, 1979). Bootstrap values (based on 1000 re-sampling) was used to estimate the reliability of the clustering pattern. The numerical taxonomy and multivariate analysis system (NTSYSpc) version 2.20 software was used to prepare the principle component analysis (PCoA) of the correlation matrix for establishing relationships among the individuals.
Investigation of water deficit tolerance
Plant and experimental conditions
Cuttings with 5 to 7 cm long were taken from the same mother plants which were propagated from seeds of 6 B. aegyptiaca populations which were collected from different countries (Table 1). Only for provenance Togo, cuttings were obtained and one cutting was grown up as mother plant and further propagated by cuttings. The cuttings were planted individually in pots (7 cm diameter) filled with CL T soil type (a soil with natural clay and peat ratio 2:1) (Einheitserde, Sinntal-Altengronau, Germany). The plants were covered by plastic boxes for 4 weeks and then plants with a good root formation were transferred to bigger pots (9 cm diameter) filled with 700 cm3 CL T soil. Plants were irrigated every 2 days, with tap water and once per week with water containing 0.25% Wuxal Top N fertilizer consist of 120 g kg-1 N, 40 g kg-1 P pentoxide, 60 g kg-1 K oxide, 0.1 g kg-1 B, 0.04 g kg-1 Cu, 0.2 g kg-1 Fe, 0.01 g kg-1 Mo, 0.04 g kg-1 Zn, and 0.12 g kg-1 Mn (Aglukon, Düsseldorf, Germany). Propagation and growth of the cuttings was done in greenhouse for 8 weeks at a temperature of 22°C and 12 h light/dark cycle. In dull weather, artificial light was given to raise the quantum fluence rate to approximately 350 µmol m-2s-1 using sodium vapour lamps (SON-T Agro 400, Philips, Amsterdam, Netherlands).
After 8 weeks, plants were subjected to a 4-week-long drought stress experiment. The plants were divided into 3 groups dependent on the target volumetric soil water content (VWC) measured with Fieldscout device based on time domain reflectometry (TDR) (Spectrum Technologies, Plainfield, USA). Each treatment comprised 5 plants per genotype. Preliminary experiments indicated that greater biomass and growth was obtained when the plants were grown in clay loam substrate compared to sand substrate. Accordingly, clay loam was used as soil substrate with an estimated maximum water holding capacity (VWC) of about 35% based on data from the Fieldscout instrument manual. This was taken as the well-watered control level. The permanent wilting point was 20% VWC and considered as a moderate stress level. To investigate the effect of severe drought stress on the plants, a target of 5% VWC was used. During the drought experiment plants were irrigated every 2 days, the water amount was calculated based on the water deficit calculation (D) of the Fieldscout; 1 mm equalling 8 ml water for each pot based on the truncated cones formula.
Plant growth analysis
Shoot length; leaf, fresh and dry weight; stem, fresh weight; and leaf water content were examined after 4 weeks drought treatment. Plant height was determined by measuring the longest shoot. Above ground plant material was harvested and weighed for fresh weight (FW) before being oven-dried at 80°C for 48 h for dry weight (DW). There were 5 plants per treatment per provenance except for provenance El-Kharga when 3 replicates were used. Leaf water content was calculated as: LWC = (FW - DW) / FW × 100.
Stomata conductance and chlorophyll fluorescence measurements
Young but fully expanded leaves were used for chlorophyll fluorescence measurements (Junior-PAM, Heinz Walz GmbH, Effeltrich, Germany) and for determination of stomata conductance measurements using a diffusion porometer (AP4 Delta-T Devices, Burwell, UK). At the 4th week, 5 leaves per treatment from each provenance were dark-adapted for 30 min at 9 am (Heinz Walz GmbH) and light curves with a ten-second frequency were used for chlorophyll fluorescence measurements. From these measurements, photochemical efficiency (∆F∕Fm’) = (Fm’- Fs) Fm’) was calculated. At the end of the experiment, the same leaves were used to estimate stomata conductance (mmol m-2 s-1).
Statistical analysis
Fresh weight, dry weight and conductance were log-transformed to achieve normal distributed residuals and variance homogeneity. To visualize potential redundancy of physiological variables of individual plants, the principal component analysis (PCA) was performed after standardization (Venables and Ripley,
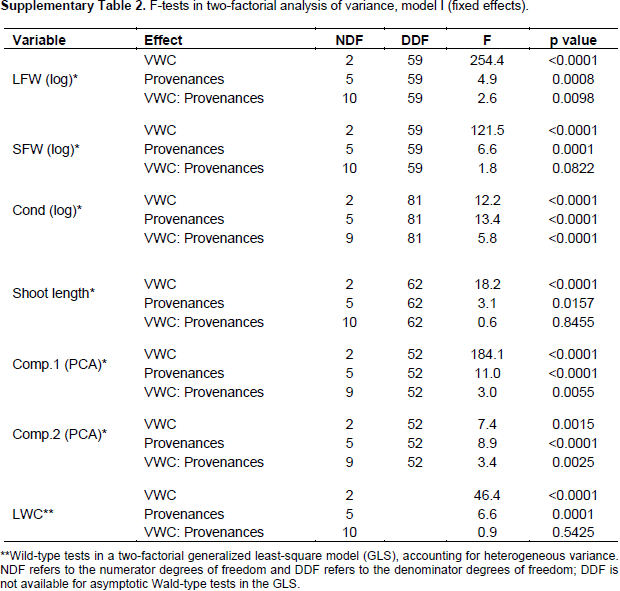
2002). To analyse significance of effects for single variables, 2 factorial analyses of variances (ANOVA) were performed, with main effects for provenance, VWC, and provenance-VWC interaction. leaf water content was analyzed in a generalized least square model (GLS) instead of ANOVA to allow different residual variances in different VWC levels, since severe heterogeneity of variances could not be removed by transformations, (Pinheiro and Bates, 2000). For detailed investigation of the provenance-VWC interactions, data were split according to provenances and all pair-wise comparisons (Tukey test) of the VWC levels were performed for each provenance separately. Likewise, data were split according to the levels of VWC and all pair-wise comparisons among provenances were tested (Tukey test) on each VWC level separately (details in supplementary information).
Further, interaction contrasts (Gabriel et al., 1973) were used to compare the magnitude of differences among VWC levels among the provenances (The effect of water stress at the level of provenances-VWC interaction through different variables). Finally, variance components of 2 factorial random effect models (provenance, VWC and provenance-VWC interaction) were estimated to visualize the proportion of the total variance due to the different factors and the residual error on a comparable scale. All computations were performed in R-3.1.0 (R Core Team, 2013), using the package multcomp (Hothorn et al., 2008) for the Tukey test and compact letter display, the packages nlme (Pinheiro and Bates, 2000) and lme4 (Bates et al., 2014) for GLS and random effect models, as well as the packages ggplot2 (Wickham, 2009) and ade4 (Dray and Dufour, 2007) for the figures. Information on all F ratios and associated numerator and denominator degrees of freedom, and p values is summarized in Supplementary Table 2
.
AFLP analysis
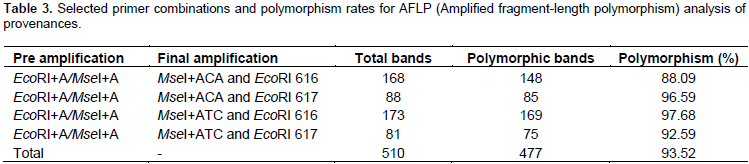
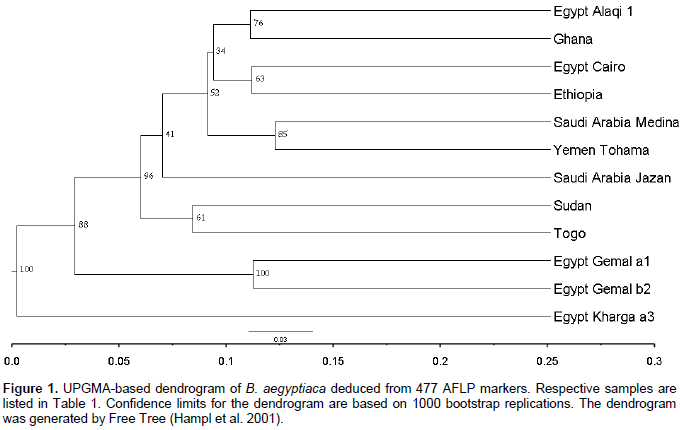
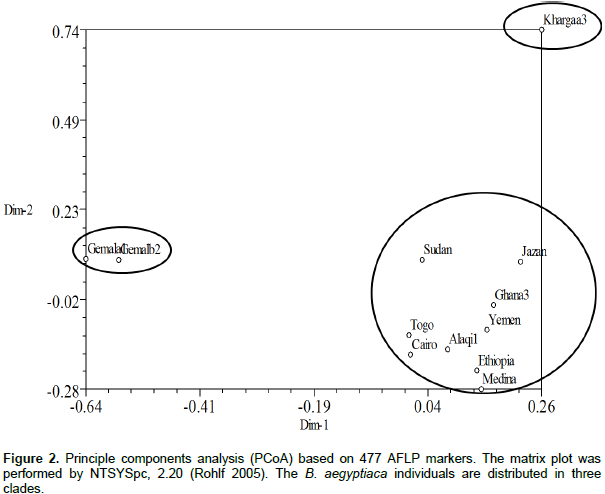
Data from 33 trees revealed that each of the individuals from Ghana, El-Kharga and Wadi El-Alaqi are distributed in one sub-clade for each provenance, also Wadi El-Gemal (b) and (c) are in one sub-clade, but Wadi El-Gemal (a) is in another sub-clade (data not shown). So the final results represent 12 individuals from 11 provenances by one plant from each provenance and only provenance Wadi El-Gemal is represented by 2 individuals (a1 and b2). A total of 510 bands were generated from the 4 AFLP primer combinations, where each primer combination gave a different number of polymorphic fragments (Table 3). The highest number of polymorphic fragments was obtained from the combination EcoRI 616 + ATC/MseI and the average number of polymorphic loci detected was about 119 per primer combination. Genetic similarity values among the 12 individual samples of B. aegyptiaca ranged from 0.246 to 0.591 based on the Dice index. Data obtained from cluster analysis indicated that individuals of B. aegyptiaca were distributed in 3 main clades; El-Kharga (a3) in one clade, the 2nd clade was Wadi El-Gemal (a1 and b2), and the remaining ones were collected in a 3rd clade (Alaqi1, Ghana3, Cairo, Ethiopia, Medina, Yemen, Jazan, Sudan and Togo) (Figure 1). This result was consistent with the data obtained by the Eigen vectors analysis of PCoA (Figure 2) where the B. aegyptiaca individuals were distributed in these same 3 clades.
Water deficit analysis
Effect of water stress on growth parameters
In provenances of Wadi Al-Alaqi, Wadi El-Gemal, Sudan and Cairo (Figure 3A), plant height was significantly reduced by decreasing soil water content (P = 0.0001; Supplementary Table 3). At severe stress (VWC 5%), the plants elongated very slowly and there was almost no increase in stem length (0, 0.32, 1.93, and 3.76%) in provenances Wadi El-Gemal, Wadi El-Alaqi, Sudan and Togo, respectively. Provenances Cairo and El-Kharga showed the highest gain in stem length at VWC 35% (59.0 and 56.1%, respectively) and at VWC 20% from provenances Cairo and Wadi El-Alaqi (69.0 and 44.4%, respectively). Also, Cairo and El-Kharga elongated the most at 5% VWC (57.5 and 11.9%, respectively)
The fresh leaf weight (FW) and dry weight (DW) were reduced by decreasing VWC (Figure 3B and C) in all provenances (P = 0.0001). Fresh leaf weight (FW) and dry weight (DW) showed significant differences between El-Kharga and Sudan at VWC 35 and 20%; there was no significant difference among the provenances at VWC 5% (Supplementary Table 4); although plants in each provenance separately represent significant decreases at different soil water contents (P = 0.0008). Provenances Sudan and Wadi El-Alaqi at VWC 35% had by far the heaviest leaf FW (9.86 ± 0.34 and 6.35 ± 0.98 g) and leaf DW (2.75 ± 0.23 and 1.86 ± 0.29 g, respectively). The lowest leaf FW was obtained with provenances Togo (0.32 g ± 0.13) at VWC 5%. The reduction in leaf FW at moderate stress was significantly larger (P = 0.0203) in Sudan than in El-Kharga (49 and 68%, respectively) compared to the control. Also, the reduction in leaf FW at severe drought stress was significantly higher (P = 0.0028, 0.121 and 0.0803) (Supplementary Table 5) in provenances Sudan, Wadi El-Alaqi, Wadi El-Gemal and Togo than in provenance El-Kharga (5, 5, 7, 7 and 23%, respectively) compared to control treatment.
At severe stress levels (5% VWC), the decrease in stem fresh weight (Figure 3D) was not significant among the provenances. Provenances Sudan and Wadi El-Alaqi at VWC 35% had the heaviest stem FW (3.84 ± 0.16 and 3.27 ± 0.66 g). Also, the reduction in stem FW at severe drought stress was higher in provenances Sudan, Wadi El-Alaqi and Wadi El-Gemal than in provenance El-Kharga (13, 9, 13 and 45%, respectively) compared to control treatment.
Beside the reduction in biomass at different soil water contents, leaf water content among provenances was decreased with increasing water limitations. There were significant differences among provenances at VWC 35% and no significant differences were observed under moderate and severe drought stress (Figure 3E). Significant difference was observed among provenances Cairo, El-Kharga, Wadi El-Gemal and Wadi El-Alaqi and no significant difference between provenances Sudan and Togo at the same soil water content (P = 0.0001). Provenance Cairo had the highest value (75.98%) at control treatment. Provenance Wadi El-Alaqi represents the lowest value at VWC 5% (14.86%). Compared to the control, the reduction in leaf water content at severe drought stress was higher in provenances Wadi El-Alaqi, Wadi El-Gemal and Togo (20, 30 and 33%, respectively) than in provenances El-Kharga, Sudan and Cairo (79, 65 and 53%, respectively).
Moreover, other morphological changes have been monitored under severe drought stress: leaves shedding in Wadi El-Gemal provenance, leaf rolling and necrosis in provenance Togo, and chlorosis of many leaves in provenance Wadi El-Alaqi. Also, under severe stress most of the plants from Wadi El-Alaqi were severely wilted or died, 2 plants survived from 5 in provenances Wadi El-Gemal, Sudan and Togo, and one plant only died from provenances Cairo and El-Kharga.
Stomatal conductance
At moderate stress levels (20% VWC), conductance (a measure of stomatal closure) was reduced (Figure 4A) to a statistically significant extent only in provenance Wadi El-Alaqi. Severe stress (5% VWC) lowered conductance in Cairo and in El-Kharga (by 10.7 and 21.8%, respectively), compared to controls. In Wadi El-Gemal and Sudan, conductance was already very low in controls and water stress did not reduce these further. In Togo, there was no statistically significant effect of stress. Readings for Wadi El-Alaqi at 5% VWC were not possible because they were severely wilted and died by the 3rd week at VWC 5%.
Principal component analysis for biomass and leaf conductance variables
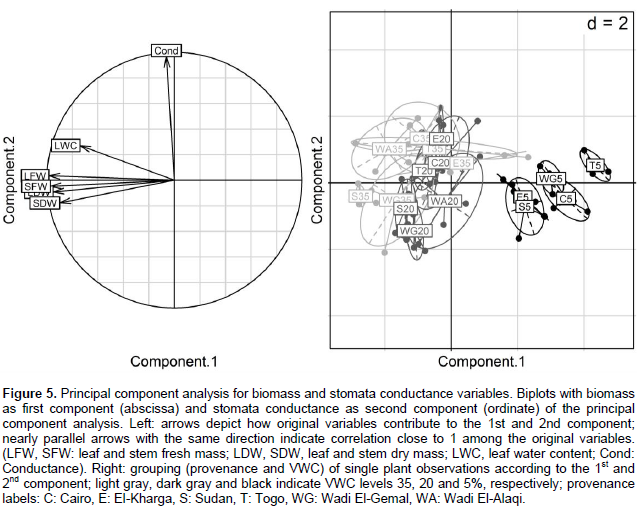
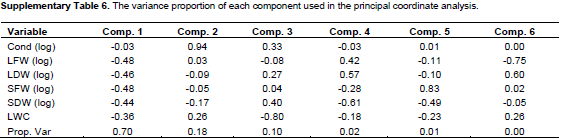
Fresh and dry mass of stem and leaves were summarized with about equal weights as the 1st component, explaining roughly 70% of variability in the data (Figure 5). Observations of fresh and dry weights were highly correlated among each other, suggesting that these variables provide mutually exchangeable information on the biomass effects of drought stress in this highly controlled experiment. The 2nd component mainly consists of conductance and explains roughly 18% of the variation. In this overall view, the variability of conductance is uncorrelated to the 4 biomass variables; overall changes in conductance do not go along with the overall changes in the biomass variables (Supplementary Table 6). The 3rd component contrasts the leaf water content with the dry mass and stomata conductance, and accounts for 10% of the variance. That is, roughly 98% of the variance can be accounted through 3 components, major parts of the joint variation in these 6 physiological parameters, may be explained by only 2 or 3 variables. The 1st component (biomass) mainly distinguishes the severe drought stress VWC 5% observations from the moderate drought stress VWC 20% and control VWC 35% observations. The 2nd component (stomata conductance) could distinguish the control treatment VWC 35% (high conductance) from the moderate drought stress VWC 20% (low conductance) observations, but to distinguish the severe drought observations from the moderate and control treatments, this was not clear through some provenances compared to the 1st component.
Chlorophyll fluorescence measurements
The impact of water limitation on chlorophyll fluorescence was assessed using photochemical efficiency (∆F∕Fm’). There were no significant differences among the genotypes within each treatment. Only in the 2nd week, there was a significant decrease in photochemical efficiency (Figure 4B). This was observed with provenance Wadi El-Alaqi at VWC 5%. The values were all similar during the 4th week and the impact of water stress was unclear among the provenances. By the 4th week, the mean photochemical efficiency for all treatments was between 0.439 and 0.603.
Molecular analysis through AFLP
This study analysed genetic diversity among B. aegyptiaca provenances collected from different geographical locations using the AFLP. The relationship among geographical distribution and genetic diversity was determined. The species is highly variable in many of its visible characters and this had led to subdivisions, ferox, pallida, quarrei, and tomentosa, classified according to features such as distribution, flowers, fruits, and spines and preferred soil type (Sands, 2001). Not surprisingly therefore, from the total number of 510 AFLP bands, 477 (93.5%) were polymorphic. The high polymorphism percentage was also observed in several other woody species. For example, 88% polymorphisms were obtained from 48 accessions of Jatropha curcas collected from 6 different regions in India (Leela et al., 2009).
Clustering and principal components analysis placed the 12 individuals from 11 provenances of B. aegyptiaca collected from different regions into 3 clades. This discrimination related to the AFLP fingerprint of each sample and was not closely related to the geographic distribution. The relation among genetic diversity and geographic locations in B. aegyptiaca has been investigated by Chamberlain (1992). The author used isoelectric focusing of peroxidase isozymes to characterize different germplasm pools of B. aegyptiaca. The results revealed that, there was a higher variability in peroxidase isozyme expression of the plants from different provenances than those from the same provenances.
The seeds of B. aegyptiaca collected from 11 provenances showed a highly significant effect of soil on the germination and growth parameters. Cluster analysis displayed a grouping of provenances that depended on overall growth parameters and the grouping was not related to soil type or rainfall (Elfeel et al., 2009).
B. aegyptiaca is considered partially auto-compatible and to exhibit large amounts of fruit abortion based on a small fruit/flower ratio. Hamrick et al. (1992) reported that woody species that are distributed in a wide geographic range and have outcrossing breeding systems through wind and insects or spreading seeds by animal feeding showed a high genetic diversity within species and populations with low variation among them.
The present study suggests no clear relation among the high genetic variation within Balanites sources under investigation and the geographic distribution of these genetic variations. Genetic variations among these sources may have been achieved by outcrossing and also by the spread of the seeds through various geographic areas. The high genetic diversity within populations with low diversity among countries has also been reported in many outcrossing woody plants species such as Uapaca kirkiana (Mwase et al., 2006) and Populus tremuloides (Yeh et al., 1995).
This is a 1st report based on AFLP fingerprint to investigate the genetic diversity among different provenances of B. aegyptiaca collected from different countries. For the future, there is a demand to increase the size of populations and collected samples. This should be combined with morphological and cytological analysis for samples under investigation to make a clear conclusion about the genetic diversity among the B. aegyptiaca populations and the relation between geographical distance and genetic diversity.
Provenances respond differently to drought conditions
Major results are that B. aegyptiaca provenances differed in their morphological and physiological responses to cope with the water shortage. Only 2 to 3 parameters could distinguish the water stress levels among the provenances.
Overall, severe drought stress drastically reduced the growth with different signs of wilting and stress and decrease in survival rate in some provenances and they were combined with the sever reduction in stomata conductance in Cairo and El-Kharga provenances at 5% VWC. The increase in plant height was almost stopped under severe drought with a reduction in leaf water content in provenances Togo, Wadi El-Gemal and Wadi El-Alaqi. This reduction in growth parameters under water stress conditions could be explained in the following way; equally these responses are likely to be the damaging consequences of water shortage, smaller leaves, leaf loss, and stomatal closure are the most likely adaptive responses to water shortage. According to Farooq et al. (2009), water deficit decreases the rate of the cell division, cell elongation and expansion, resulting in reduction of growth.
Under severe drought stress, B. aegyptiaca provenances showed different morphological responses that appear to be adaptive; Wadi El-Gemal genotype showed leaves shedding, leaf rolling, while necrosis was observed in provenance Togo. In provenance Wadi El-Alaqi, most of the leaves showed chlorosis. This is consistent with the observation of Engelbrecht and Kursar (2003) who investigated seedlings of 28 woody plant species; they defined the severely wilting as "very strong change of leaf angle or change of leaf surface structure with beginning leaf necrosis". This was also noticed in Jatropha curcas where plants adapted to water limitation by selective leaf shedding as a drought tolerance strategy (Fini et al., 2013); also in Mediterranean and tropical tree species under arid or semi-arid conditions (Sanchez-Blanco et al., 2002).
Finally, B. aegyptiaca provenances at severe drought stress (VWC 5%) did not show significant differences in growth parameters among the provenances compared to the clear significant differences at control and moderate drought treatments among the provenances. Otherwise, the reduction in biomass of plants grown in control and moderate stress conditions in comparison to the severe water stress was highly significant in some provenances (Sudan, Wadi El-Alaqi, Wadi El-Gemal and Togo) compared to the others (El-Kharga and Cairo) which might be a good indicator for the water deficit effect in these provenances. In another experiment, provenance El-Kharga showed superior response to drought stress with high potential of recovery after 4 weeks of severe drought stress compared to the other provenances under investigation (data not shown).
Under water limiting conditions, a reduction in stomata closure was observed in all B. aegyptiaca provenances where stomata closure was considered as early plant response to water stress (Cornic and Massacci, 1996). Thus, under severe drought stress, B. aegyptiaca showed water saving mechanism in provenances Cairo, Sudan and El-Kharga decreased the losses in leaf water content in the range between 40.7, 46.6 and 51.6%, respectively. The responses of different sources of the oil-producing, drought-tolerant J. curcas to water stress were investigated and the authors found that this species had several mechanisms to avoid water stress, such as early stomata closure (Fini et al., 2013). Also, the plant showed a strategy to withdraw the water stress through a decrease in plant height and stomata conductance and these reactions resulted in a decrease of the transpiration rate and reduced the losses in leaf water content (Díaz-López et al., 2012).
In provenances Sudan, Wadi El-Gemal and Togo at moderate drought stress conditions, there is a reduction in stomata conductance with high leaf water content. This reduction in stomata conductance could be more related to soil moisture, where several studies suggested that the stomata conductance is more related to soil moisture than to leaf water content. Also, provenance Sudan showed no significant differences in stomata conductance and early stomata closure at the 3 levels of soil water content. The reduction in stomata conductance at control treatments might be related to the water logging at this level which affects the root system in this provenance. Under water limitation, the dehydrated roots send a chemical signal (ABA) to the leaves which affected stomata closure with stable plant water content at high levels (Farooq et al., 2009).
In the principal component analysis, the 1st component (biomass) clearly distinguishes the observations of VWC 5% from the VWC 35 and 20% treatments where the decrease in biomass (represented by fresh and dry weights) is a clear indicator of severe stress induced by the VWC 5% treatment to all genotypes. With respect to the 1st component, there is no clear distinction between the VWC 20 and 35% treatments, suggesting that VWC 20% treatment induced mostly a moderate stress which did not lead to remarkable decrease in biomass. Differences among treatments and provenances were less clear in the 2nd and 3rd component. The 2nd component (mainly dependent on stomata conductance) shows some differences among provenances in the VWC 35 and 20% treatments; however, between group differences were less clear relative to the variation within groups of the same provenances and VWC treatment. The high correlation of leaves and stems fresh and dry mass indicated that measurement of only some of these variables might be sufficient to assess the effects of severe drought stress on biomass, at least in highly controlled experimental setups.
Chlorophyll fluorescence determinations in summary indicated that there were no remarkable changes in photochemical efficiency (∆F∕Fm’) at the 3 levels of soil water content in each provenance (Figure 4B). Only provenance Wadi El-Alaqi showed a significant decrease in the 2nd week (data not shown). This might be consistent with Fini et al. (2013), through examining the drought avoidance strategy in 3 different accessions of J. curcas; the authors found that the Suriname genotype showed a decrease in plant dry weight and total leaf area with a reduction in Fv/Fm and stomata conductance, whilst the values of Fv/Fm were not affected in the genotype from Brazil that was considered as the best drought tolerant source under those experimental conditions. In the current experiment, at the 4th week of treatment, provenance Sudan showed the highest photochemical efficiency values at control treatments (Figure 4B). In provenance El-Kharga, the values were very close at the 3 levels of soil water content. In conclusion, the measurements of photochemical efficiency (∆F∕Fm’) did not represent a remarkable change even under severe drought conditions. This was previously reported in 4 genotypes of Lablab purpureus L. investigated by Guretzki and Papenbrock (2013) to compare the response mechanism of water stress among genotypes. Under drought conditions, there was a significant decrease in growth parameters and stomata conductance among the genotypes. However, the data from chlorophyll fluorescence measurements were not strong tools to examine the plant response to mild stress.
The obtained data from the band-based approach indicated that 12 B. aegyptiaca provenances collected from different geographical locations were separated into 3 main clades. This data was confirmed through cluster analysis and the Principal Co-ordinates Analysis. The high genetic variation among the individuals of B. aegyptiaca was also observed in many of outcrossing woody plant species. B. aegyptiaca provenances differed in their range of reactions to water limitation. Dependent on the plant response to water stress and survival rate, the provenances under investigation could be divided into 4 groups.
(1) Drought sensitive: From this group, including provenance Wadi El-Alaqi under severe water stress, four plants from 5 were dead at the end of the experiment.
(2) Less drought sensitive: This group includes provenances Wadi El-Gemal and Togo; both provenances showed steep reductions in growth parameters and only 2 plants from 5 survived.
(3) Less drought tolerant: This group includes provenance Sudan where 2 plants from 5 were dead. Provenance Sudan showed early stomata closure which kept the leaf water content in save level.
(4) Drought tolerant: This group including provenances Cairo and El-Kharga, only one plant died from Cairo and El-Kharga and no remarkable change in leaf structure was recorded under severe stress conditions. Finally, based on the data provided through this experiment, provenances El-Kharga, Cairo and Sudan could be recommended for further drought stress experiment to examine the potential of these provenances to recover after severe stress and finally select the most drought tolerant provenance.
The authors have not declared any conflict of interests.
The authors would like to thank the German Academic Exchange Service (DAAD) and the Ministry of Higher Education (MoHE) of the Arab Republic of Egypt cooperation agreement for the support and for providing PhD scholarship to Galal Khamis.
REFERENCES
|
Ahuja I, De Vos RC, Bones AM, Hall RD (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15:664-674.
Crossref
|
|
|
|
Ambrosone A, Di Giacomo M, Leone A, Grillo MS, Costa A (2013). Identification of early induced genes upon water deficit in potato cell cultures by cDNA-AFLP. J. Plant Res. 126:169-178.
Crossref
|
|
|
|
|
Bates D, Maechler M, Bolker B, Walker S (2014). lme4: Linear mixed-effects models using Eigen and S4. R package version. 1:1-6. http://CRAN.R- project.org/ package =lme4
|
|
|
|
|
Bhandari MM (1995). Flora of the Indian desert. MPS Repros, Jodhpur.
|
|
|
|
|
Bray EA (2002). Classification of genes differentially expressed during water-deficit stress in Arabidopsis thaliana: an analysis using microarray and differential expression data. Ann. Bot. 89:803-811.
Crossref
|
|
|
|
|
Bonin A, Ehrich D, Manel S (2007). Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol. Ecol. 16:3737-3758.
Crossref
|
|
|
|
|
Chamberlain HC (1992). Balanites aegyptiaca: A study of its genetic variation and micropropagation. M.Sc. Thesis, Wye College, University of London.
|
|
|
|
|
Chapagain BP, Wiesman Z (2008). Metabolite profiling of saponins in Balanites aegyptiaca plant tissues using LC (RI)-ESI/MS and MALDI-TOF/MS. Metabolomics 4:357-366.
Crossref
|
|
|
|
|
Chapagain BP, Yehoshua Y, Wiesman Z (2009). Desert date (Balanites aegyptiaca) as an arid lands sustainable bioresource for biodiesel. Bioresour. Technol. 100:1221-1226.
Crossref
|
|
|
|
|
Cornic G, Massacci A (1996). Leaf photosynthesis under drought stress. N. R. Baker (Ed.) Photosynthesis and the Environment. Adv. Photo. Resp. 5:347-366.
|
|
|
|
|
Dice LR (1945). Measures of the amount of ecologic association between species. Ecology 26:297-302.
Crossref
|
|
|
|
|
Díaz-López L, Gimeno V, Simón I, Martínez V, Rodríguez-Ortega WM, García-Sánchez F (2012). Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agric. Water Manage. 105:48-56.
Crossref
|
|
|
|
|
Dray S, Dufour AB (2007). The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Soft. 22:1-20.
Crossref
|
|
|
|
|
Elfeel AA, Warrag EI, Musnad HA (2007). Response of Balanites aegyptiaca (L.) Del. seedlings from varied geographical source to imposed drought stress. Disc. Innov. 619:319-325.
|
|
|
|
|
Elfeel AA, Warrag EI, Musnad HA (2009). Effect of seed origin and soil type on germination and growth of heglig tree (Balanites aegyptiaca (Del.) L. var. aegyptiaca). J. Sci. Technol. 10:56-66.
|
|
|
|
|
El-Tahir A, Ibrahim AM, Satti GMH, Theander TG, Kharazmi A, Khalid SA (1998). Potential antileishmanial activity of some Sudanese medicinal plants. Phytoth. Res. 12:570-579.
|
|
|
|
|
Engelbrecht BMJ, Kursar TA (2003). Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136:383-393.
Crossref
|
|
|
|
|
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009). Plant drought stress: effects, mechanisms and management. Agro. Sustain. Dev. 29:185-212.
Crossref
|
|
|
|
|
Fini A, Bellasio C, Pollastri S, Tattini M, Ferrini F (2013). Water relations, growth, and leaf gas exchange as affected by water stress in Jatropha curcas. J. Arid Environ. 89:21-29.
Crossref
|
|
|
|
|
Gabriel KR, Putter J, Wax Y (1973). Simultaneous confidence intervals for product-type interaction contrasts. J. R. Stat. Soc. B. 35 234-244.
|
|
|
|
|
Guretzki S, Papenbrock J (2013). Characterization of Lablab purpureus regarding drought tolerance, trypsin inhibitor activity and cyanogenic potential for selection in breeding programmes. J. Agron. Crop Sci. 200:24-35.
Crossref
|
|
|
|
|
Hall JB, Walker DH (1991). B. aegyptiaca Del.; A monograph. School of Agricultural and Forest Science, University of Wales, Bangor.
|
|
|
|
|
Hall JB (1992). Ecology of a key African multipurpose tree species, Balanites aegyptiaca (Balanitaceae): the state of knowledge. For. Ecol. Manage. 50:1-30.
Crossref
|
|
|
|
|
Hampl V, Pavlicek A, Fleger J (2001). Construction and bootstrap analysis of DNA fingerprinting based phylogenetic trees with the freeware program Free Tree: application to trichomonad parasites. Int. J. Syst. Evol. Microbiol. 51:731-735.
Crossref
|
|
|
|
|
Hamrick JL, Godt MJW, Sherma-Broyles SL (1992). Factors influencing levels of genetic diversity in woody plant species. New For. 6:95-124.
Crossref
|
|
|
|
|
Harb A, Krishnan A, Ambavaram MMR, Pereira A (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early response leading to acclimation in plant growth. Plant Physiol. 154:1254-1271.
Crossref
|
|
|
|
|
Hothorn T, Bretz F, Westfall P (2008). Simultaneous inference in general parametric models. Biol. J. 50:346-363.
Crossref
|
|
|
|
|
Joshi-Saha A, Valon C, Leung J (2011). A brand new START: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 4(201):re4.
Crossref
|
|
|
|
|
Khamis G, Papenbrock J (2014). Newly established drought-tolerant plants as renewable primary products as source of bioenergy. Emir. J. Food Agric. 26:1067-1080.
Crossref
|
|
|
|
|
Leela T, Suhas PW, Seetha K, Naresh B, Thakur KS, David AH, Prathibha D, Rajeev KV (2009). AFLP-based molecular characterization of an elite germplasm collection of Jatropha curcas L., a biofuel plant. Plant Sci. 176:503-513.
|
|
|
|
|
Mohamed AM, Wolf W, Spiess WEL (2002). Physical, morphological and chemical characteristics, oil recovery and fatty acid composition of Balanites aegyptiaca Del. kernels. Plant Foods Hum. Nutr. 57:179-189.
Crossref
|
|
|
|
|
Morison JIL, Baker NR, Mullineaux PM, Davies WJ (2008). Improving water use in crop production. Phil. Trans. R. Soc. B. 363:639-658.
Crossref
|
|
|
|
|
Mwase WF, Bjørnstad Å, Stedje B, Bokosi JM, Kwapata MB (2006). Genetic diversity of Uapaca kirkiana Muel. Årg. Populations as revealed by amplified fragment length polymorphisms (AFLPs). Afr. J. Biotechnol. 5:1205-1213.
|
|
|
|
|
Nei M, Li WH (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Nat. Acad. Sci. USA. 76:5269-5273.
Crossref
|
|
|
|
|
Park W, Scheffler BE, Bauer PJ, Campbell BT (2012). Genome-wide identification of differentially expressed genes under water deficit stress in upland cotton (Gossypium hirsutum L.). BMC Plant Biol. 12:90.
Crossref
|
|
|
|
|
Pinheiro JC, Bates DM (2000). Mixed-Effects Models in S and S-PLUS. Springer-Verlag, New York.
Crossref
|
|
|
|
|
Radwan AAU (2007). Photosynthetic and leaf anatomical characteristics of the drought- resistant Balanites aegyptiacea (L.) Del. seedlings. Am. Eur. J. Agric. Environ. Sci. 2:680-688.
|
|
|
|
|
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria.
|
|
|
|
|
Rohlf FJ (2005). Numerical Taxonomy and Multivariate Analysis System. Version 2.2. Exeter software. New York, N.Y.
|
|
|
|
|
Sanchez-Blanco M, Rodriguez P, Morales M, Ortuno M, Torrecillas A (2002). Comparative growth and water relations of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci. 162:107-113.
Crossref
|
|
|
|
|
Sands MJ (2001). The desert date and its relatives: A revision of the genus Balanites. Kew Bull. 56:1-128.
Crossref
|
|
|
|
|
Siddique I, Anis M (2008). Direct plant regeneration from nodal explants of Balanites aegyptiaca L. (Del.): A valuable medicinal tree. New For. 37:53-62.
Crossref
|
|
|
|
|
Song Y, Wang Z, Bo W, Ren Y, Zhan Z, Zhang D (2012). Transcriptional profiling by cDNA-AFLP analysis showed differential transcript abundance in response to water stress in Populus hopeiensis. BMC Genom. 13:286.
Crossref
|
|
|
|
|
Song Y, Miao Y, Song C (2014). Tansley review behind the scenes : the roles of reactive oxygen species in guard cells. Plant Physiol. 102:1121-1140.
|
|
|
|
|
Venables WN, Ripley BD (2002). Modern Applied Statistics with S. Fourth Edition. Springer-Verlag, New York.
Crossref
|
|
|
|
|
Vos P, Hogers R, Bleeker M (1995). AFLP a new technique for DNA fingerprinting. Nucl. Acid Res. 23:4407-4414.
Crossref
|
|
|
|
|
Watkins JM, Hechler PJ, Muda GK (2014). Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 164:1707-1717.
Crossref
|
|
|
|
|
William H, Outlaw JR (2003). Integration of cellular and physiological functions of guard cells. Cri. Rev. Plant Sci. 22:503-529.
Crossref
|
|
|
|
|
Yeh FC, Chong DKK, Yang RC (1995). RAPD variation within and among natural populations of trembling aspen (Populus tremuloides) from Alberta. J. Hered. 86:454-460.
Crossref
|
|
|
|
|
Zobel B, Talbert J (2003). Applied forest tree improvement. The Blackburn Press, Caldwell New Jersey. P 505.
|
|