Thirty six single cross hybrids with six commercial checks were evaluated for yield performance along with earliness, plant height and ear height. The mean performance for days to tasseling and days to silking were significantly different among the hybrids. Considering the yield, Kaberi 50 and Pioneer 3396 were found as the best and the second best check, respectively. None of the hybrids produced higher yield compared to the best check Kaberi 50. On the other hand, L1´T1, L3´T1, L5´T3, L6´T3, L10´T1 and L1´T3 produced higher yield compared to 2nd best check Pioneer 3396 whereas L5´T3 and L11´T3 were earlier than the checks. The seven lines involving these crosses were selected for next generation evaluation. Better performing crosses showed higher K+/Na+ and lower contents of O2•-, H2O2 and melondialdehyde (MDA) than the susceptible crosses suggesting their better stress tolerance ability and thus better performance in yield.
Salinity, a severe threat for crop growth and production, has been increasing due to global climate. It is predicted that due to salinity, each year about 12 billion US$ will be lost globally by reducing agricultural production (Flowers et al., 2010). The possibility of salinity increasing is more in arid and semi-arid regions due to water scarcity together with high temperature. Salinity stress causes morphological, physiological, biochemical and molecular changes in cell which causes functional loss of cell organelles and finally cell death (Gill and Tujeta, 2010). Accumulation of Na+ under salinity stress competes with K+ binding with proteins causing inhibition in protein synthesis and metabolic enzymes (Schachtman and Liu, 1999; Pardo and Quintero, 2002). Besides, high concentration of NaCl outside the roots reduces the water potential which hampers water up-take resulting in osmotic stress.
High salt level in leaves causes stomatal closure, impairs electron transport and damages the photosynthetic apparatus which ultimately reduces photosynthesis and productivity (Deinlein et al., 2014). The ionic imbalance, osmotic stress causes over production of reactive oxygen species (ROS) in plants (Chawla et al., 2013). A large number of studies established that tolerant plants contain lower magnitude of ROS and melondialdehyde (MDA) under salinity and other abiotic stress as compared to sensitive ones. Therefore, ROS like superoxide (O2•-), hydrogen peroxide (H2O2) and secondary metabolites of ROS like MDA can be an important biomarkers for selection of tolerant plants under abiotic stress including salinity. On the other hand, genotypes showing a high K+/Na+ are tolerance to salinity (Chen et al., 2017).
Maize (Zea mays L.) is the 3rd most important cereal crop after rice and wheat and is grown under a wide spectrum of soil and climatic conditions. It is an important C4 plant from the poaceae family and considered to have wider adaptability to growing environment (Chinnusamy et al., 2005). In Bangladesh, maize is becoming an important crop due to its high market demands with comparatively lower production cost and high yield. It continues to expand rapidly at an average rate of 20% yearly. A growing popularity for maize in the country is due to huge demand, particularly for poultry feed industry. The acreage and production of maize have an increasing tendency with the introduction of exotic hybrids due to high yield potentials.
In Bangladesh, about 1 million hectare of land is under saline coastal belt and there is an opportunity to expand maize cultivation in this area. However, the crisis of saline tolerant maize variety is a major limitation to introduce this crop in this area. Therefore, attention has been given in developing saline tolerant maize. With this objective, 36 single cross hybrids along with 6 commercial checks were evaluated in saline area of Agricultural Research Station, Benarpota, Satkhira to find suitable line for producing hybrid. For selecting suitable line for saline environment, parameters like plant height, days to tasseling, days to silking, ear height and yield were emphasized. At the same time, some biochemical parameters like K+/Na+, ROS and MDA were examined as selection criteria.
Plant materials and crop management
The materials for this experiment consisted of 36 single cross hybrids producing from 12 lines: Pac60/S4-3, 9MG/S4-6, 7074/S3-2, 7074/S3-5, 7074/S3-6, 7074/S3-13, 7074/S3-18, 7074/S3-21, 7074/S3-26, 7074/S3-30, QY11/S3-24 and CML-427 considered as L1, L3, L5, L6, L7, L8, L9, L10 and L11, L12 L13 and L14, respectively, and 3 testers: 9MS/S6-14, BIL-110, BIL-113 as T1, T2 and T3, respectively. L2 and L4 did not germinate. The produced hybrids were evaluated atBenerpota, Satkhira, a saline costal region (Latitude 21°48´-22°58´N, Longitude 88°55´-89°55´E and Altitude 16 feet from sea level), in winter 2016 to 17 following RCB design with 3 replications.
Six commercial hybrids (BHM-9, 981, Sunshine, Pioneer3396, Kaberi 50 and Pacific 99Super) were used as check variety. Each entry planted in one row of 4 m long plot. The spacing between rows was 60 cm and plant to plant distance was 25 cm. One healthy seedling per hill was kept after proper thinning.
Fertilizers were applied @ 250, 55, 110, 40, 5 and 1.5 kg/ha of N, P, K, S, Zn, B, respectively. Standard agronomic practices were followed and plant protection measures were taken as required. Ten randomly selected plants were used for recording observations on plant height, ear height, days to tasseling, days to silking and grain yield were recorded on whole plot basis. ROS and MDA were measured from flag leaves at grain filling stage.
Measurement of soil salinity
Salinity level of the maize growing field was measured by a conductivity meter (HI-993310).
Measurement of ROS and MDA
Measurement of the O2•− generation rate
Superoxide radical was determined in flag leaves according to the method of Elstner and Heupel (1976) with modifications. Leaves (0.3 g) were homogenized in 3 ml of 65 mmol phosphate buffer (pH 7.8) on an ice bath and then centrifuged at 4°C and 5,000 × g for 10 min. The supernatants (0.75 ml) were mixed with 0.675 ml of 65 mM phosphate buffer (pH 7.8) and 0.07 ml of 10 mM hydroxylamine chlorhydrate and placed at 25°C. After 20 min, 0.375 ml of 17 mM sulfanilamide and 0.375 ml of 7 mM α-naphthylamine were added, and the mixture was placed at 25°C for another 20 min before it was mixed with 2.25 ml of ether. The absorbance was measured at 530 nm and the O2•−concentration was calculated from a standard curve of NaNO2.
Measurement of H2O2
H2O2 was assayed according to the method described by Yu et al. (2003). Flag leaf tissue (0.5 g) was homogenized in 3 ml of 50 mM K-P buffer (pH 6.5) at 4°C. The homogenate was centrifuged at 11,500×g for 15 min. The supernatant (3 ml) was mixed with 1 ml of 0.1% TiCl4 in 20% H2SO4 (v/v), and the mixture was then centrifuged at 11,500×g for 15 min at room temperature. The optical absorption of the supernatant was measured spectrophotometrically at 410 nm to determine the H2O2 content (Є= 0.28 μM-1 cm-1) and expressed as micromoles per gram FW.
Measurement of MDA
The level of lipid peroxidation was measured by estimating melondialdehyde (MDA), a decomposition product of the peroxidized polyunsaturated fatty acid component of the membrane lipid, using thiobarbituric acid (TBA) as the reactive material (Heath and Packer, 1968). Briefly, flag leaf tissue (0.5 g) was homogenized in 3 ml 5% (w/v) trichloroacetic acid (TCA), and the homogenate was centrifuged at 11,500×g for 10 min. The supernatant (1 ml) was mixed with 4 ml of TBA reagent (0.5% of TBA in 20% TCA). The reaction mixture was heated at 95°C for 30 min in a water bath and then quickly cooled in an ice bath and centrifuged at 11,500 ×g for 15 min.
The absorbance of the colored supernatant was measured at 532 nm and was corrected for non-specific absorbance at 600 nm. The concentration of MDA was calculated by using the extinction coefficient of 155 mM-1 cm-1 expressed as nanomole of MDA per gram FW.
Measurement of K+/Na+
The sap was extracted from leaves and was put on compact Na+ ion meter (Horiba-731, Japan) and compact K+ ion meter (Horiba-722, Japan) to estimate the Na+ and K+ ions in leaves. The K+/Na+ ratio was measured from the estimated values.
Statistical analysis
Mean performance of different characters and comparison with 2 best checks were analyzed using Statistix 10 software by least significant difference (LSD) following RCB design.
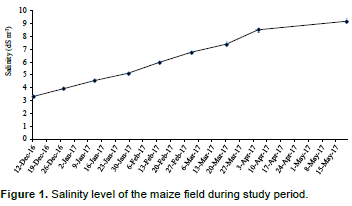
The salinity levels in coastal area of Bangladesh increased after November which increases upto May. It is related with soil moisture. After November, the land dries due to lack of rain. During this study period, the salinity started from 3.3 dSm-1 and at harvesting, the level was almost 10 dSm-1 (Figure 1). Therefore, it is clear that the crop growth and development was hampered due to salinity at salinity level >4 dSm-1 which become injurious for crop growth.
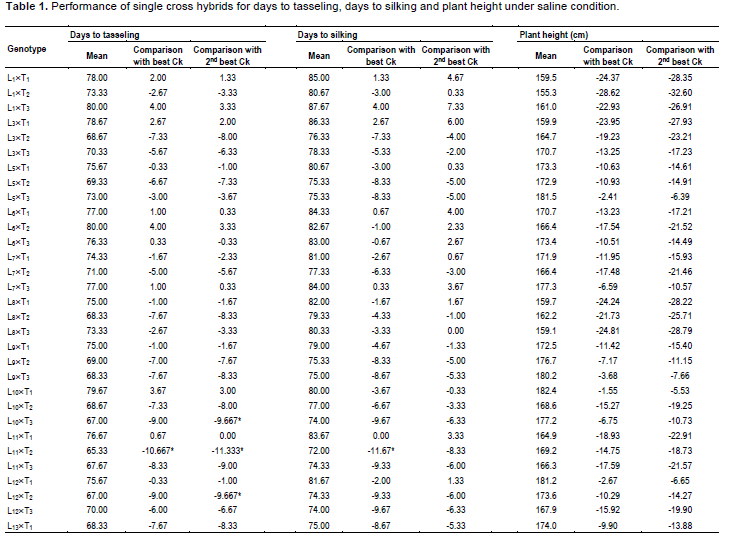
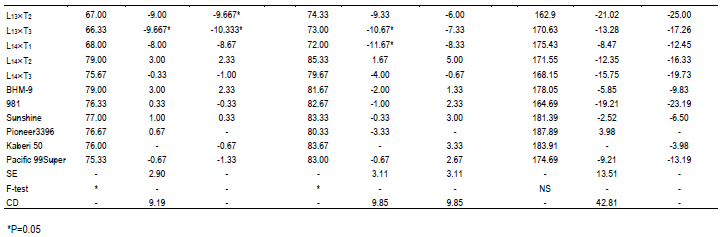
During selection of maize, usually the important traits like days to tasseling (DT), days to silking (DS), plant height (PH), ear height (EH) and yield are given importance. In comparison of these desired traits, 6 commercial hybrids (BHM-9, 981, Sunshine, Pioneer 3396, Kaberi 50 and Pacific 99 Super) were included as check (Ck) in this study. Among the Cks, Kaberi 50 was found as the best check followed by Pioneer 3396 as the second best Ck. Notably, during selection of maize genotypes, yield is considered as most important. It is assumed that genotypes with comparatively lower plant height and ear height have lower possibility to lodge.
Hence, these 2 traits were monitored carefully. On the other hand, early harvesting facilitates to escape higher salinity and traits like early tasseling and silking which are important for maize cultivation in saline area. The mean performance of the crosses was significantly different for DT and DS (Table 1). In comparison of performance for DT with best check, Kaberi 50, 2 crosses L11´T2 and L13´T3 were significantly earlier, for DS, 3 crosses L11´T2, L13´T3 and L14´T1 were found earlier. However, significant difference was not found among the genotypes for plant height (PH), ear height (EH) and yield/ha (Yld). Interestingly, most of the crosses had shorter plant height than both of the best checks, although the difference was not statistically significant.
In case of plant height, most of the genotypes were shorter than the best and 2nd best check (Table 1). On the other hand, considering the ear height genotypes, L1´T1, L3´T1, L3´T2, L6´T3 were shorter than the best check. L1´T3 and L11´T1 were considerable over the 2nd best check. Since none of the genotypes were over yielder than the best check Kaberi 50, the genotypes showing higher yield over the 2nd best check was considered for selection. Among the crosses, comparing the yield of the best check, yield of L1´T1, L3´T1, L3´T2, L5´T3, L10´T1 and L11´T3 was higher or similar. However, all the desired traits rarely correlate with higher yield.
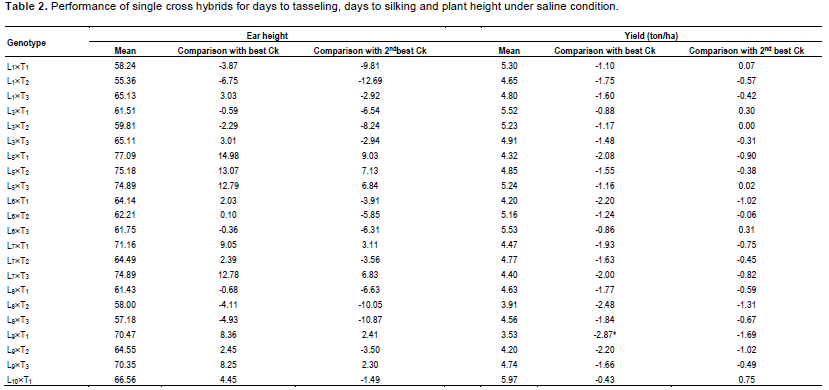
Though, considering yield (over the 2nd best check), earliness for L1´T1, L5´T3 and L10´T1 was sacrificed. During gain filling stage K+/Na+, ROS and MDA were measured. For convenience, the results were presented for higher yielder, 7 genotypes, 2 most lower yielder, 2 genotypes and 2 best checks (Figure 2). The ratio of K+ to Na+(K+/Na+) in the measured genotypes differed significantly.
The leaves of most of the tolerant genotypes contained significantly higher K+/Na+ ratio than the 2 sensitive genotypes (LT13´T2 and LT13´T3). However, the ratio in L5´T3 and L10´T1 was statistically similar to that in sensitive genotypes. Importantly, genotypes like L1´T1 contented comparatively higher K+/Na+ than the best Ck Kaberi 50, although they are statistically similar.
Saline stress eventually leads to reduction in crop yield which varies substantially in different crops. The relative yield loss which varied greatly depends on salinity levels and the degree of tolerance (Maas, 1983). In this study, a linear increase in salinity level was found upto maturity. However, it could be assumed that lower possibility to hamper germination as the salinity level was very low (<4 dSm-1). Na+ is the principal toxic ion for maize interfering with K uptake and transport, leading to alter of stomata modulations which cause water loss and necrosis (Fortmeier and Schubert, 1995; Sümer et al., 2004). Saline stress causes competition between K+ and Na+ which reduces severely K+ content in both leaves and roots of maize (Kaya et al., 2010; de Azevedo Neto and Tabosa, 2000). However, reduction of K uptake varies with genotypes where resistant maize hybrids have higher K+/Na+ ratio than the sensitive ones (Farooq et al., 2015; Akram et al., 2007; Akram et al., 2010). In this experiment, the cross with higher yield (Table 2 and Figure 2) showed comparatively higher K+/Na+ than the most sensitive genotypes, depicting their higher tolerance to salinity.
Like the ratio of potassium to sodium ion, O2•-, H2O2 and MDA differed significantly among the selected genotypes (Figure 3). Importantly, the levels of O2•-, H2O2 and MDA in susceptible genotypes were significantly higher than those in most of the higher yielding genotypes. It was also remarkable that the levels of O2•-, H2O2 and MDA seemed to be slightly lower in Ck genotypes compared to higher yielding genotypes. Plants are most sensitive to over production of ROS and MDA, a lipid peroxidative product (Gill and Tujeta, 2010). The levels of ROS, MDA and their scavenging antioxidants are used as, selection criteria of genotypes under salinity (Azooz et al., 2009). In this study, the levels of O2•-, H2O2 and MDA were compared in 7 higher yield genotypes and 2 lower yielded genotypes (L13´T2 and L13´T3) along with 2 best Ck (Kaberi 50 and Pioneer 3396) (Figure 3).
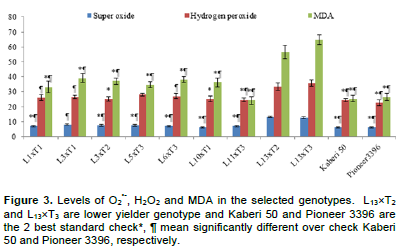
Osmotic effect due to salinity changes in general metabolic system, leads to over production of ROS and causes oxidative stress in maize (Menezes-Benavente et al., 2004). ROS are highly toxic and damages cell organelles like proteins, DNA, lipids and carbohydrates. The apparatus of photosynthesis system in chloroplast is the major target of ROS which compelled the cell organelles to functional loss and eventually cell death (Gill and Tuteja, 2010). Among the ROS, singlet oxygen 1O2, H2O2 and O2•- are very much important and cause severe damage to cell. In this study, H2O2 and O2•- were compared in flag leaves of some tolerant and susceptible genotypes (as shown in Figure 3), where the contents were higher in the sensitive genotypes. Higher concentrations of H2O2 and O2•- under salinity can modify proteins and DNA components and alter metabolic process (Gill and Tujeta, 2010) which limits growth and yield.
The higher ROS cause veins of maize to collapse due to leakage into neighboring cells (Menezes-Benavente et al., 2004). They attack cell wall by interacting with polyunsaturated fatty acid and cause peroxidation product as MDA. Therefore, sensitive genotypes are most likely to loss cell organelles due to higher possibility of cell membrane leakage, as the MDA content was higher in sensitive genotypes (Figure 3). The MDA content in sensitive maize genotypes was also reported in seedling stage in our previous studies (Rohman et al., 2016) which might be due to differential antioxidant system (Hichem et al., 2009; Rohman et al., 2016).
Considering earliness, plant height, ear height and yield above 2nd best check, L1´T1, L1´T2, L3´T1, L3´T2, L5´T3, L6´T3, L10´T1 and L11´T3 were considered as promising for saline area. Therefore, the lines involving these crosses were selected and advanced for next generational experiment. Importantly, higher yielding crosses showed lower contents of O2•-, H2O2 and MDA than the susceptible crosses suggesting their better stress tolerance ability.