ABSTRACT
The nutrient absorption march in fig (Ficus carica L.) trees and other fruit trees has been studied only at the seedling stage, and little is known about nutrient accumulation in perennial plants when the entire life cycle of the plant is considered. To this end, the present study aimed to evaluate nutrient accumulation in the Roxo de Valinhos fig tree, cultivated with and without supplemental irrigation. The split-plot array in time was adopted, with the main factor arranged in a randomized block design with four replications. The main plot was the use of supplemental irrigation and the subplots were the eight data collection time-points. The fig plants were sampled for 0, 40, 80, 120, 160, 200, 240 and 280 days after pruning (DAP). The maximum accumulation of dry matter mass and nutrients occurred between 160 and 240 DAP in both systems. Plants in the irrigated system showed greater overall accumulation of nutrients in all the organs, more prominently in the leaves, branches, and stem. The dry matter mass and nutrient accumulation was in the order stem > leaves > branches > roots > fruit in the irrigated system and branches > stem > leaves > roots > fruit in the non-irrigated system.
Key words: Ficus carica L, dry matter mass, nutritional requirement, water irrigation.
Brazil is the second largest exporter of fresh fig globally, with Turkey being the largest. Fig production in Brazil has increased considerably in recent years, with a fig production area of approximately 2,814 ha, producing 28,253 t of figs with an average yield of 10,040 kg ha-1 (IBGE, 2014). However, the fig production for both domestic consumption and export is dependent on a single variety, Roxo de Valinhos (Costa et al., 2015). The Roxo de Valinhos variety, also called the common fig tree, is known for its high vigor and rusticity, in addition to its ability to adapt well to different environments and tolerance to drastic pruning (Boliani and Corrêa, 1999). Drastic pruning, an essential practice for fig trees cultivated in Brazil, is carried out to eliminate branches attacked by drills and rusted leaves (Penteado and Franco, 1997). However, with the almost complete removal of the branches, large amounts of nutrients are lost from the rapidly growing parts of the tree. Therefore, the lost nutrients must be sufficiently replenished to meet the nutritional demand of the tree. As for nutritional management of the fig tree, fertilization regimes for the period of seedling plantation and subsequent growth have been recommended exclusively on the basis of soil analysis. Even the analysis of the nutritional status of the plant through leaf diagnosis - although a valuable tool for perennial crops - is still incipient in fig tree culture (Brizola, 2003). Nutrient absorption march refers to the dynamics of accumulation of nutrients in the plant dry mass at the growth stage (Prado and Nascimento, 2003). Augostinho et al. (2008) emphasized that knowledge of the amount of nutrients accumulated in the plant, at each stage of development, provides valuable information that aids in the designing of a crop fertilization program.
According to Malavolta et al. (1997), nutrient absorption by the plant is influenced by the stage of development; it intensifies at the flowering stage, and during formation and growth of the fruit or organ to be harvested. In this context, these authors emphasize the importance of knowing not only the total amount of absorbed nutrients, but also their concentrations at the different stages of development of vegetable crops. Leonel and Brizola (2011) emphasized that the management practices of drastic pruning of the fig tree makes it difficult to calibrate the dosages of nutrients to be administered to this plant. According to them, it is possible to determine the quantities of such nutrients exported by the production, but it is difficult to assess the nutrient needs for the annual growth of branches, buds, and roots, and for the growth of new leaves. The accumulation of nutrients by plants is a function of the genotypic characteristics of each species, the development stage, and external factors that regulate nutrient absorption by the plants, especially water (Rozane, 2008). In this sense, with the aim of expanding information on the nutritional demand of fig trees, studies on nutrient extraction from branches, leaves, and fruits were carried out by Brizola et al. (2004) and Leonel and Techio (2009). However, studies elucidating the nutrient absorption curve for fig trees grown in field conditions remain scarce. Thus, considering the high amount of nutrients removed during pruning under different crop conditions and the need to replenish these nutrients adequately and at the appropriate phenological stages, the present study aimed to evaluate nutrient accumulation in the Roxo de Valinhos fig tree, cultivated with and without supplemental irrigation.
The study was conducted in the experimental orchard of the Horticulture Department of the Faculty of Agronomic Sciences, FCA/UNESP, Campus de Botucatu-SP. The local geographic coordinates are 22° 52 '47 "S latitude, 48° 25' 12" W longitude, and 810 m altitude. The predominant climatic type, based on the Köppen International system, according to the temperate rainy climate - Cfa, characterized as warm temperate (mesothermal) with summer rains and winter drought, with precipitation and average annual temperature of 1530 mm and 21°C, respectively (Cunha et al., 1999). The soil in the area was classified as Red Nitisol (Embrapa, 2006). The results of soil chemical analyses throughout the study are presented in Table 1. Data on rainfall and minimum, average, and maximum daily temperatures were provided by the Meteorological Office of the Natural Resources Department of the Faculty of Agronomic Sciences/UNESP. The climatic data are shown in Figure 1. Transplantation of the Roxo de Valinhos fig tree seedlings was carried out in December, 2010, adopting a spacing of 2.5 m between rows and 2.5 m between plants. The 30 cm tall seedlings were transplanted into 50 cm × 50 cm × 50 cm pits, which were previously fertilized with 1 L of corral manure, 0.5 kg of limestone, and 0.5 kg of magnesium thermophosphate containing 0.1% boron and 0.25% zinc. Cover fertilization was performed based on the recommendation of Campo Dall'Orto et al. (1996), who advocated the application of fertilizer based on the interpretations from soil chemical analysis and the plant age. Thus, in 2011 and early 2012, each plant was fertilized with 45 g of urea and 35 g of potassium chloride.
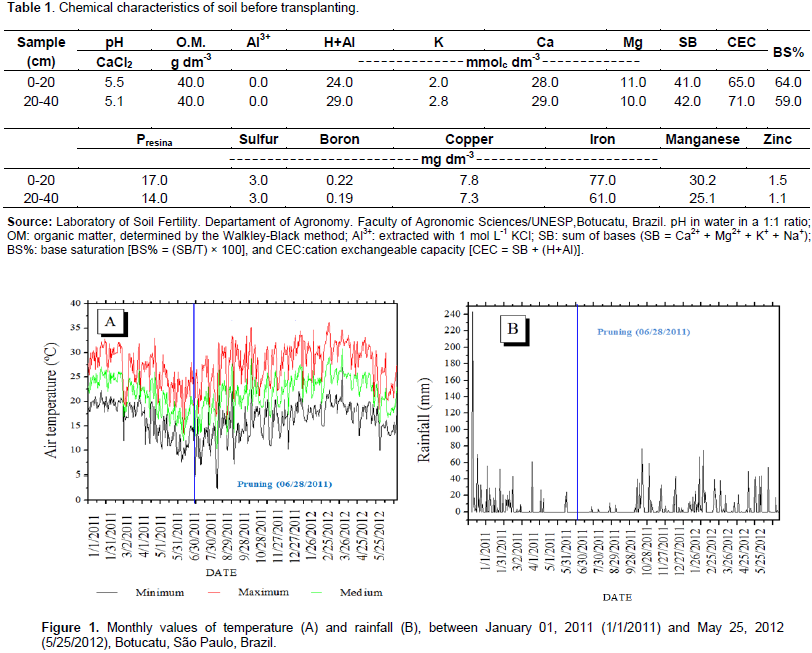
Fertilization was performed every two months. The fig plants were pruned on June 28, 2011 (6/28/2011) to ensure that each plant contains three productive branches in the first year. Further cuttings were also performed when necessary. For phytosanitary treatment, copper-based products were used to control rust caused by the fungus Cerotelium fici. The fungicides tebuconazole (Folicur®) and methyl thiophanate (Cercobin®) were also applied whenever necessary. Control of weeds between the planting lines was performed through periodic mechanical brushing, and crowning of the plants was performed through manual weeding. The soil water retention curves were experimentally obtained with samples of soil in volumetric rings and through Richardsporous plates method of matrix potential and soil moisture values. The soil density obtained was 1.4822 g cm-3 from 0 to 20 cm, and 1.3593 g cm-3 from 20 to 40 cm. It was used by the method of minimizing the squares of the deviations being adjusted using the Excel Solver optimization tool and presented coefficients of determination (r²) of 0.9974 and 0.9930, for the depths of 0 to 20 cm and 20 to 40 cm, respectively. The irrigation system adopted was drip irrigation, using two emitters with a flow of 1.5 L h-1 per plant. Irrigation management was based on the permanence of the soil matrix potential between the field capacity and a maximum value of 60 kPa, monitored by tensiometers.
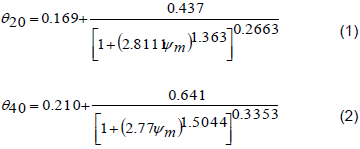
Irrigation management was performed using the tensiometry technique, with batteries of two mercury tensiometers installed in each treatment, in randomly assigned plots. The first tensiometer, installed at a 20 cm depth (relative to the center of the porous capsule of 6 cm length), was considered as the decision tensiometer, and the irrigations were performed based on these readings. The second tensiometer was considered as the control and installed at a 40 cm depth relative to the center of the porous capsule, to control the applied depth of irrigation. According to Klar (1980), the tensiometer reliably operates up to the -80 kPa range, and the variations of the readings increased when the potential becomes more negative. The tensiometer readings were taken every two days, and values for the mercury column rise were used to obtain the matrix potential. The management was aimed at limiting the soil matrix potential to approximately -30 kPa and/or volumetric moisture equal to 0.2988 cm³ water cm-3 soil. The ψm values were verified in the readings, and with these, the respective levels of volumetric humidity were calculated by the difference between both, the need to restore the water volumes was verified, respectively. The volume of water applied depended on the volume explored by the root system of the plant, which in this case was monitored by the collection of the root systems by the destructive evaluations of the growth analysis, which in this case presented measurements of equatorial and longitudinal lengths having as reference the stem of the plant and the depth reached. The rainfall distribution data and the depths of irrigation used during the experimental period are presented in Table 2.
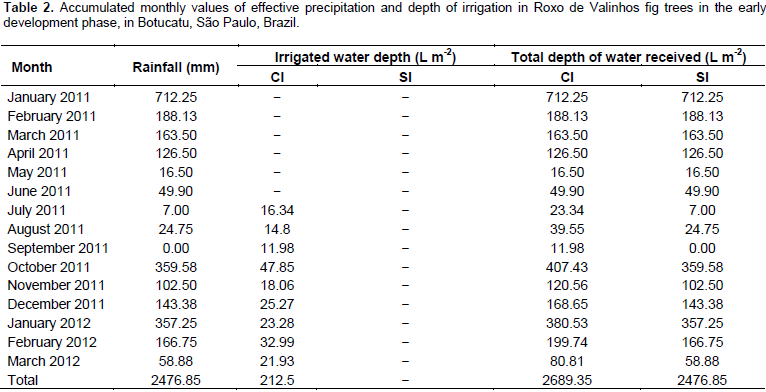
Eight collections were performed between June, 2011 and April, 2012 (June 28, August 07, September 17, October 25, December 04, 2011; and January 13, February 24, and April 03, 2012). The first collection was performed before pruning in order to characterize the plants before application of the treatments. Eight plants were harvested from the soil using a backhoe, four plants were cultivated with irrigation, and four plants were cultivated without irrigation, totaling 64 plants at the end of the experiment. The interval between two successive collections was 40 days, right from formative pruning, which was carried out on June 28, 2011. Therefore, the collections were performed at 0 (pruning time), 40, 80, 120, 160, 200, 240 and 280 days after pruning (DAP). After harvest, the plants were taken to the Fruit Production Laboratory of the Plant Production Department/FCA/UNESP, where they were sectioned into the following parts: root, stem, branches, leaves, and fruits. Each part of the plant was washed with water and detergent, and was eventually washed with distilled water. Subsequently, the plant material was packed in paper bags and dried in a hot-air oven with forced circulation at a temperature of 65°C until it reached constant weight. Finally, it was weighed to determine the mass of dry matter, and then milled in a Willey mill for determination of nutrient concentrations. The samples were taken to the Department of Natural Resources Laboratory/Soil Science/FCA/UNESP, where the macronutrient concentrations were determined, according to the method recommended by Malavolta et al. (1997). The accumulation of nutrients was determined by multiplying the nutrient concentrations by the dry mass of each organ (root, stem, branches, leaves, and fruits). The experimental design was a split-plot array in time, with the main factor arranged in randomized blocks, with four replications. The main plot was the use of supplementary irrigation, and the subplots were the eight collection time-points. When significant, the regressions (with the independent variable being the evaluation time–DAP) were adjusted by the Sisvar statistical package and the graphical representations made in the Origin 6.0 program. The differences between means were subjected to variance analysis by the F test and compared by the Tukey test at 5% significance, using the Sisvar program (Pimentel Gomes, 2009).
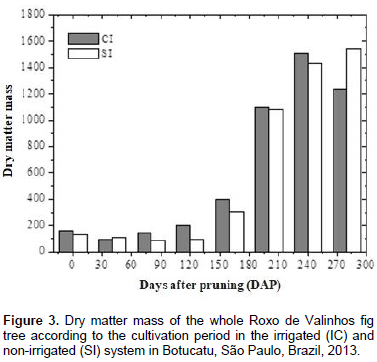
The cultivation time and water regime had a significant effect on the dry matter mass of Roxo de Valinhos fig plants (Figure 2). In all partitions, a sigmoid behavior for the growth curves was observed in both the systems. The maximum accumulation of the dry matter mass in the irrigated system was observed between 160 and 240 DAP, with the stem showing the greatest accumulation. In contrast, in the non-irrigated system, the branches showed the greatest accumulation of dry matter mass. Rozane (2008), when studying the dry matter accumulation of two cultivars of starfruit trees grown under different water regimes (irrigated and non-irrigated systems) found that the stem showed the greatest accumulation of dry matter mass in both the water regimes. Silva et al. (2011) evaluated the dry matter mass of Roxo de Valinhos fig plants in different cropping systems, which combined treatments with or without mulching and treatments with or without irrigation. The results of their study showed that, in all the treatments, the branches showed the greatest accumulation of dry matter mass, which is consistent with the findings of the present study. The findings of Da Costa et al. (2014) also corroborate these previous results and ours. They evaluated the influence of different soil cover management methods on the productivity of fig trees cultivated with and without irrigation, and reported that mulching resulted in a higher average yield of mature fruits and that under these conditions, the branches showed the highest accumulation of dry matter mass.
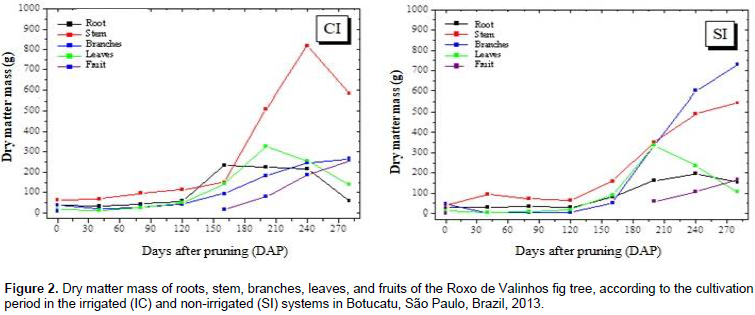
Figure 3 shows the accumulation of dry matter mass in the whole plant during the entire growth period in the two irrigation systems. In general, irrigation promoted a greater total accumulation of dry matter mass in the 3rd, 4th, and 5th harvests, which occurred at 80, 120, and 160 DAP, respectively. In this period, the third collection was mainly preceded by low rainfall, which explains the higher values for dry matter mass at this time-interval in the plants that were irrigated (Figure 1B). However, recovery of dry matter mass production in the non-irrigated plants was observed during the rainy season, even surpassing that of the plants irrigated in the last collection. This behavior may have occurred due to the cycle anticipation of the fig trees that were irrigated. The determination of dry matter mass production is of great importance, because when the nutrient accumulation curve cannot be determined, it provides information that is representative of the nutrient absorption march (Souza and Coelho, 2001). Such a comparison can be made because 5% of plant dry matter mass is composed of mineral nutrients. The accumulation of macronutrients in the roots, leaves, stem, branches, and fruits of the Roxo de Valinhos fig tree was influenced by the water regime and the cultivation period (Figures 4 and 5).
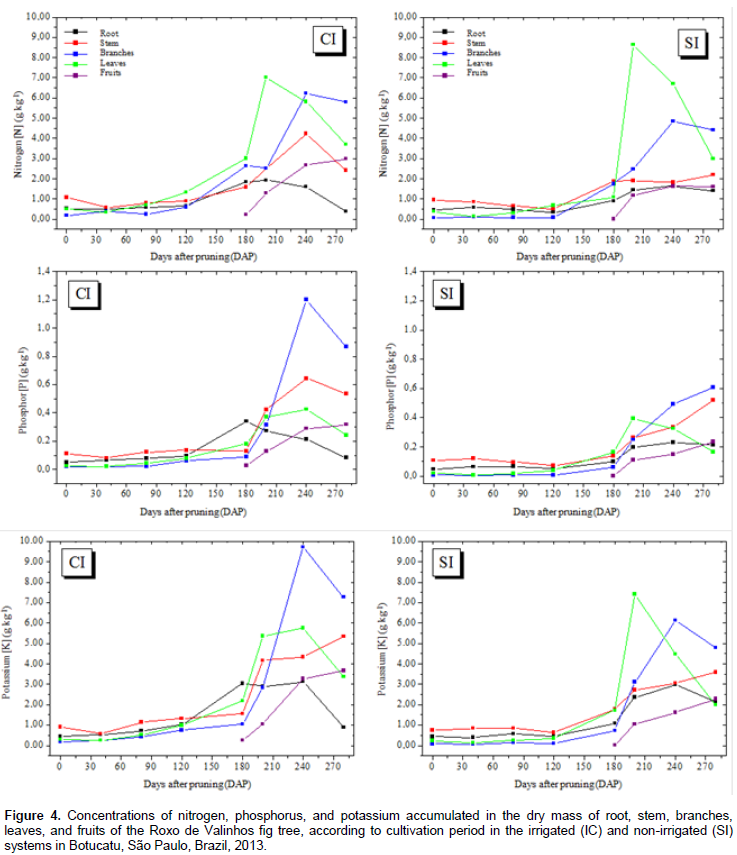
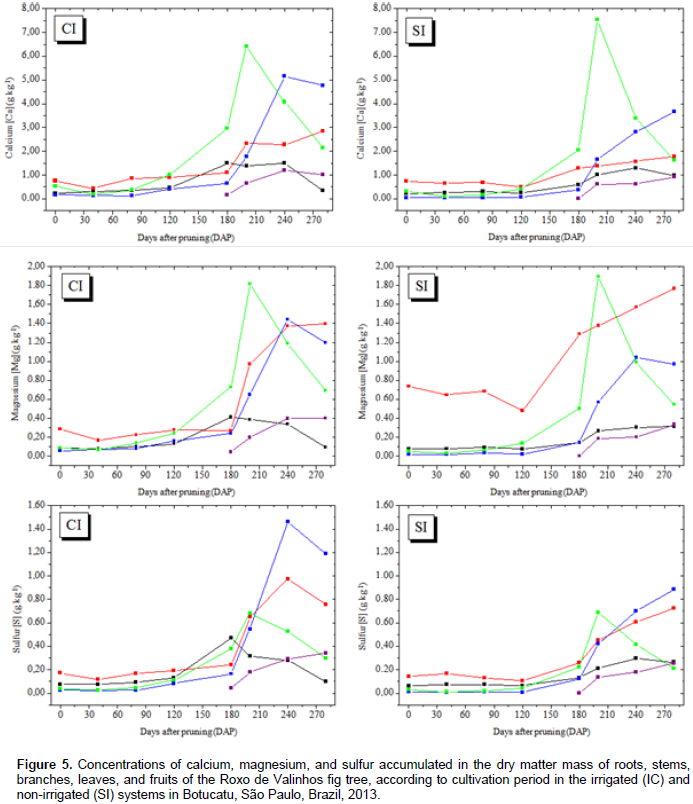
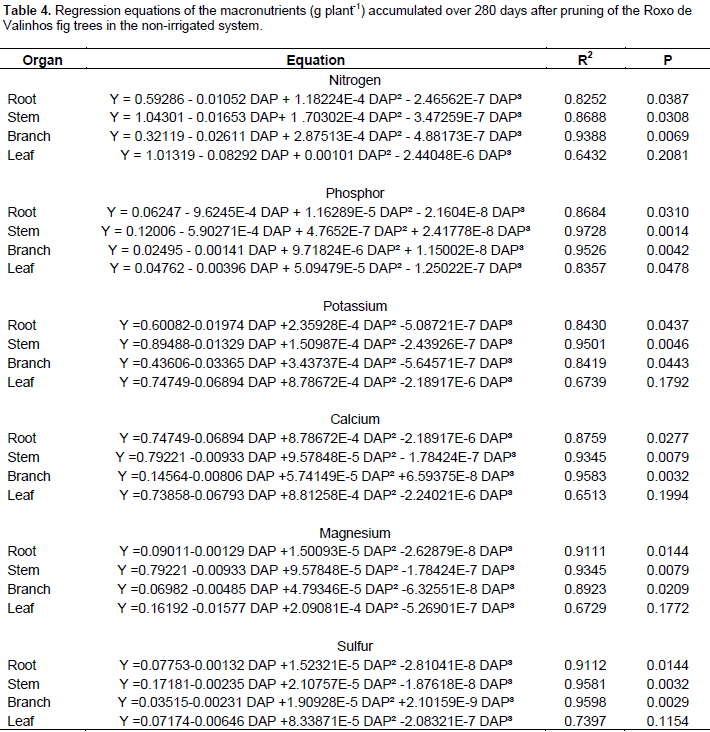
In general, in plants in both the irrigated and non-irrigated systems, the initial accumulation rate was low, reaching maximum values only between 160 and 240 DAP. The best adjusted regression model for all nutrients was the cubic polynomial. The observed sigmoid behavior for nutrient accumulation is consistent with the accumulation of dry matter mass. The highest accumulation of nitrogen, calcium, and magnesium was observed in leaf dry matter mass under both the irrigated and non-irrigated conditions (Figures 4 and 5). Phosphorus, potassium, and sulfur showed the greatest accumulation in the branch dry matter mass under both the irrigated and non-irrigated conditions. In general, irrigation favored macronutrient accumulation in most of the analyzed plant organs, with this effect being more evident in the branches and the stem. These results are consistent with those reported by Rozane (2008), who observed a greater accumulation of nutrients in star fruit trees that were irrigated than in those that were not irrigated. It should be noted that the greatest accumulation of macronutrients occurred in the aerial parts (leaves and branches) of the plant. The higher accumulation of N in leaves can be explained by the role of this element in photosynthesis. It is a part of the chlorophyll and the enzymes phosphoenolpyruvate carboxylase and ribulose 1.5 bisphosphate carboxylase/oxygenase, which are responsible for atmospheric CO2 fixation (Prado, 2008). Sete et al. (2015) reported that the highest percentages and concentrations (mg plant-1) of total N in annual organs (leaves, fruits, and branches) of the fig tree can be attributed to the fast cell division in the plant tissue and, therefore, these organs probably act like an N drain during the vegetative growth and productive cycles of the fig tree.
Celedônio et al. (2016) examined the development of the Roxo de Valinhos fig tree in different cultivation environments and with five levels of fertilization (mineral and biofertilizer). They found that the accumulation of N in fig leaves after fertigation with bovine biofertilizer, was significantly higher than that after mineral fertilization. According to Grangeiro et al. (2007), accumulation of calcium in the aerial parts is attributable to the fact that this element is absorbed by the roots and translocated to the aerial parts, but is not redistributed to the other parts of the plant owing to its low mobility in the plant Magnesium was accumulated in the aerial parts, and mainly in the leaves; this finding validates the importance of this element in the constitution of chlorophyll molecule. Depending on the level of magnesium in the plant, between 6 and 25% of the total magnesium is bound to the chlorophyll molecule and another 5 to 10% is firmly attached to pectates in the cell wall or occurs as soluble salt in the vacuole (Marschner, 1995). In the irrigated system, the branches showed the greatest accumulation of potassium, but in the non-irrigated system, the greatest accumulation of this element was observed in the leaves. These results agree with those reported by Brizola et al. (2005), which suggest increased accumulation of potassium in the aerial parts (branches and fruits) of fig trees grown under different potassium doses. The authors found that the higher dose resulted in greater accumulation of the total dry matter mass, and consequently, greater potassium accumulation.
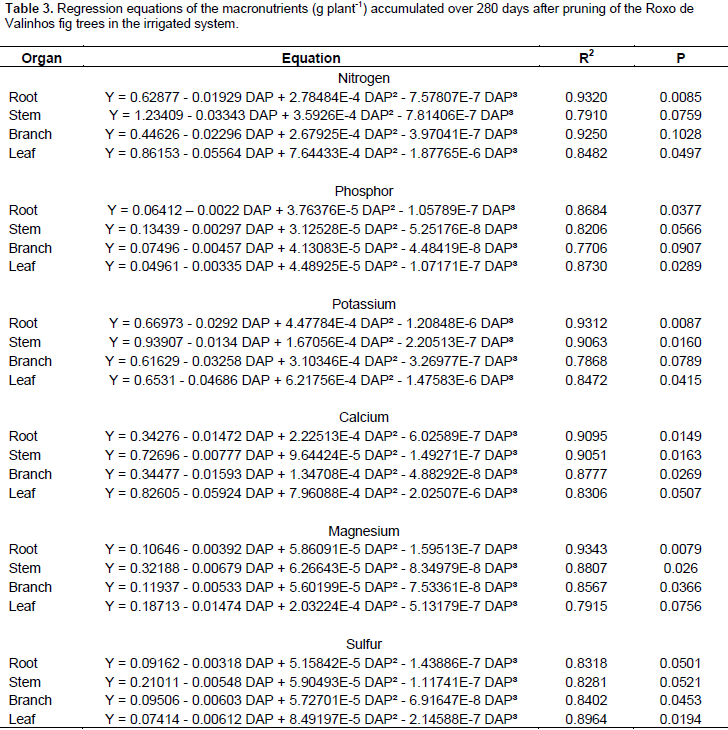
The highest levels of phosphorus were accumulated in the branches, with higher values observed for the irrigated system. Brizola et al. (2005), when examining the nutrient content in leaves and branches of the Roxo de Valinhos fig tree under different doses of potassium, reported that in the treatment without potassium fertilization, phosphorus levels were the highest in the branches. The authors observed the same trend in the accumulation of this element when they multiplied its concentration with the dry matter mass of the organ. The regression equations presented in Tables 3 and 4 represent the accumulation of macronutrients in the fig trees according to the cultivation period and water regimes. The best-fitted equations were the third-degree or cubic polynomial ones. Prado (2008) reported that for crops in their productive stages, the curve that best describes the extraction nutrients due to time is the sigmoid type, as is the curve for dry matter mass accumulation. Thus, this study suggests that, when the plant is young, the nutrient accumulation is low, and so is the dry matter mass. Thereafter, there is an increase in the dry matter mass accumulation, and absorption of nutrients increases, representing a logarithmic curve. In the final period of physiological maturation, there is a stabilization phase, in which the nutrient absorption is low or even negligible.
The results of the present study suggest that the irrigated system provided greater accumulation of nutrients in all the organs, more prominently so in the leaves, branches, and stem. The maximum accumulation of dry matter mass and nutrients occurred between 160 and 240 DAP. The organ-wise accumulation of dry matter mass in the Roxo de Valinhos fig trees was in the order stem > leaves > branches > roots > fruits for the irrigated system and branches > stem > leaves > roots > fruits for the non-irrigated system. In conclusion, the stem, branches, and leaves were the organs that showed the greatest accumulation of dry matter mass and nutrients in the Roxo de Valinhos fig tree in the two water regimes. Aiming at the continuity and improvement of this study, it would be relevant in future work to apply the nutrient absorption gait in practice, and thus to determine a fertilization program that would provide satisfactory performance of the fig tree.
The authors have not declared any conflict of interests
REFERENCES
|
Augostinho LM, Prado RM, Rozane DE, Freitas N (2008). Acúmulo de massa seca e marcha de absorção de nutrientes em mudas de goiabeira 'Pedro Sato'. Bragantia 67(3):577-585.
Crossref
|
|
|
|
Boliani AC, Corrêa LS (1999). Clima e solo para a cultura da figueira. In: Corrêa LS, Boliani AC (Ed.). Cultura da figueira do plantio àcomercialização. IlhaSolteira: Funep, pp. 37-41.
|
|
|
|
|
Brizola RMO (2003). Níveis de adubação potássica na cultura da figueira.
|
|
|
|
|
Brizola RMO, Leonel S, Tecchio MA, Da Hora RC (2004). Teores de macronutrientes em pecíolos e folhas de figueira (Ficus carica L.) em função da adubação potássica. Ciênc. Agrotec. 29:3(610-616).
|
|
|
|
|
Brizola RMO, Leonel S, Tecchio MA, Mischan MM (2005). Exportação de macronutrientespelosramos e frutos da figueiraemfunção da adubaçãopotássica. Acta Sci. Agric. 27(1):33-37.
|
|
|
|
|
Prado RM, Nascimento VM (2003). Manejo da adubação do cafeeiro no Brasil. 1.ed. Ilha Solteira: UNESP. pp. 260-273.
|
|
|
|
|
Campo Dall'Orto FA, Barbosa W, Ojima M, Raij Van B (1996). Frutas de clima temperado: II. Figo, maçã, marmelo, pêra e pêssego em pomar compacto. In: Recomendações de adubação e calagem para o Estado de São Paulo. 2 ed. Campinas: Instituto Agronômico, Fundação, Instituto Agronômico de Campinas pp. 139-140.
|
|
|
|
|
Celedônio CA, de Medeiros JF, de Araújo Viana TV, Saraiva KR, de Lima GH (2016). Área foliar da figueira em três ambientes de cultivo, sob fertirrigação de biofertilizante bovino. Revista Brasileira de Agricultura Irrigada. 10(2):586.
Crossref
|
|
|
|
|
Costa MGS, Correia ESS, Reis LL, Wilcken SRS (2015). Reação das figueiras a três espécies de nematóides das galhas. Rev. Bras. Frut. Jabot. 37(3):617-622.
Crossref
|
|
|
|
|
Cunha AR, Klosowski ES, Galvani E, Escobedo JF, Martins D (1999). Classificação climática para o município de Botucatu, SP, Segundo Köppen. In: Simpósio em Energia na Agricultura, 1999, Botucatu, SP. Anais...Botucatu: Faculdade de Ciências Agronômicas, Universidade Estadual Paulista pp. 490-491.
|
|
|
|
|
Da Costa T, Giacobbo CL, Galon L (2014). Influência da cobertura vegetal do solo na produtividade da figueira (Fícus carica). Anais do SEPE - Seminário de Ensino, Pesquisa e Extensão da UFFS. 4(1):12-24.
|
|
|
|
|
Embrapa (2006). Centro Nacional de Pesquisa de Solos. Sistema Brasileiro de Classificação de Solos. 2. ed. Rio de Janeiro: Embrapa Solos 306 p.
|
|
|
|
|
Grangeiro LC, Negreiros MZ, Souza BS, Azevedo PE, Oliveira SL, Medeiros MA (2007). Acúmulo e exportação de nutrients em beterraba. Ciênc. Agrotec. 31(2):267-273.
Crossref
|
|
|
|
|
Instituto Brasileiro de Geografia e Estatística (IBGE) (2014). Figo: área plantada e quantidade produzida. Brasília. (Produção Agrícola Municipal).
|
|
|
|
|
Klar AE (1980). A água no sistema-solo-planta-atmosfera. 2ª ed. São Paulo. Nobel.
|
|
|
|
|
Leonel S, Brizola RMO (2011). Manejo nutricional da figueira. In: Leonel S, Sampaio AC (Orgs.). A figueira. São Paulo: Editora UNESP. pp. 196-219.
|
|
|
|
|
Leonel S, Tecchio MA (2009). Teores nutricionais em folhas e frutos de figueira, submetida a épocas de poda e irrigação. Semina 30(2):347-360.
Crossref
|
|
|
|
|
Malavolta E, Vitti GC, Oliveira SA (1997). Avaliação do estado nutricional das plantas: princípios e aplicações. 2. ed. Piracicaba: Associação Brasileira de Potassa e do Fósforo P 319.
|
|
|
|
|
Marschner H (1995). Mineral nutrition of higher plants. 2nd edition. New York: Academic Press P. 889.
|
|
|
|
|
Penteado SR, Franco JAM (1997). Figo (Fícus carica L.).Manual técnico das culturas. Campinas: SAA/CATI/DCT. pp. 127-139.
|
|
|
|
|
Pimentel Gomes F (2009). Curso de estatística experimental. 15ª ed. Piracicaba: Fealq. P 451.
|
|
|
|
|
Prado RM (2008). Nutrição de plantas. Jaboticabal: EditoraUnesp P 401.
|
|
|
|
|
Rozane ED (2008). Crescimento e acúmulo de nutrients em caramboleiras nas fases de hipobioto, muda e plantas em formação.
|
|
|
|
|
Sete PB, Salume JA, Norbeto PM, Thábata I, Andrade M, Brunetto G (2015). Distribuição de nitrogênio derivado do fertilizante em figueiras (Ficus carica).
|
|
|
|
|
Silva AC, Leonel S, Souza AP, Souza ME, Tanaka AA (2011). Crescimento de figueira sob diferentescondições de cultivo. Pesqui. Agropecu. Trop. Goiânia 41(4):539-551.
|
|
|
|
|
Souza VF, Coelho EF (2001). Manejo de fertirrigação em fruteiras. In: Folegatti MV (Coord.). Fertirrigação: flores, frutas e hortaliças. Guaiba 2:71-103.
|
|