ABSTRACT
Maize is grown in most regions of Uganda. Aflatoxin contamination of maize occurs in Uganda but there is lack of information on the distribution of Aspergillus flavus in major maize production regions of the country. The objective of this study was to determine the incidence and severity of Aspergillus flavus in major maize growing regions of Uganda. Hierarchical sampling procedure was used to randomly collect maize samples from all different fields in 16 districts in two cropping seasons of 2013. Samples were assayed for A. flavus incidence and severity. Results revealed significant (P<0.001) variation among regions and districts within regions for A. flavus incidence and severity. The highest A. flavus incidence and severity were recorded for Pallisa (74.2% and 4.8, respectively), one of the leading maize producing districts in Uganda. Among regions, the highest A. flavus incidence and severity were registered in eastern region at 62.4% and 4.6 respectively. These results reveal the presence of A. flavus and provide information on its distribution in the key maize producing regions in Uganda. There is need for regular monitoring of aflatoxin levels in maize grain from the major maize production districts in Uganda in order to establish proper aflatoxin management guidelines.
Key words: Aspergillus flavus, incidence, severity, Uganda, maize.
Aspergillus ?avus is a saprophytic fungus that belongs to Aspergillus section Flavi. A. flavus produces a diverse array of secondary metabolites, among which the most important are mycotoxins. A type of mycotoxin produced by A. flavus that occurs frequently and is of agricultural and health significance is aflatoxin. Maize infection by A. ?avus and subsequent aflatoxin accumulation, poses a serious threat to both human and animals because of aspergillosis and aflatoxicosis effects (Krishnan et al., 2009). Aspergillus flavus can invade several types of crops which include cereals, legumes, cotton, and nut trees as well as other crops either before or after harvest hence results into food and feed contamination due to aflatoxin accumulation (Cotty et al., 1994).
Losses due to aflatoxin contamination are attributed directly to crop and livestock and from the cost of regulatory programs which are designed to moderate risks to human and animal health. Estimates by Food and Agricultural Organisation (FAO) indicate that up to 25% of the world’s crops are affected by mycotoxins, with aflatoxins being the most notorious (Moreno and Kang, 1999, FAO, 2002). Aflatoxin contamination in feed for livestock and poultry may result into death, suppression of immune system, growth rates reduction, and losses in feed efficiency (Sisson, 1987, Kaaya and Warren, 2005). In crops, aflatoxin contamination results in lower yields for food and fiber crops (Simyung et al., 2013).
Maize is a major crop grown in most geographic regions of Uganda. In 2011, maize occupied 19% of the total land area under food crops in Uganda (Simyung et al., 2013). In 2011/12 the total maize production was 3,150,000 MT with total export of 787,000 MT totaling USD 46.9 million (MFPED, 2014). Marketing of maize in Uganda is hampered by contamination with different mycotoxins that reduce quality (Rodrigues and Naehrer, 2002). Uganda is characterized by diverse climate in the different agro ecologies, and this may result into variability among mycotoxin causing pathogens, including A. flavus.
In Uganda, aflatoxin contamination of maize has been reported (Kaaya and Warren, 2005, Simyung et al., 2013) but there is lack of information on the distribution of either A. flavus or other fungi of Aspergillus section Flavi across the major maize growing regions in Uganda. Comparisons of aflatoxin-producing potential among Aspergillus section Flavi communities from different maize growing regions is important for understanding population dynamics and suitable control measures for field reduction of pre-harvest aflatoxin contamination (Cotty, 1997; Horn and Dorner, 1999; Calvo et al., 1999). This study was conducted to establish the incidence and severity of A. flavus in all the major maize growing regions of Uganda.
Survey and sampling strategy
Field survey were conducted to collect samples and determine the occurrences of the potential toxigenic strain of aspergillus flavus form maize in different maize growing districts in different regions of Uganda (Figure 1). The surveys were carried out during two cropping seasons: 2013A (March to July) and 2013B (August to December), which are characterized by longer and shorter rainy periods, respectively. Five districts in the western region (Hoima, Masindi, Kiryandongo, Kyenjojo, and Kabarole), four districts in the central region (Wakiso, Luwero, Mityana, and Mubende), two districts in the northern region (Lira and Oyam) and five districts in the eastern region (Iganga, Bugiri, Kumi, Pallisa, and Soroti) were surveyed. Districts were chosen to capture the different maize growing areas in Uganda with diverse cropping patterns and environmental conditions which have an influence on the occurrence of the fungus.
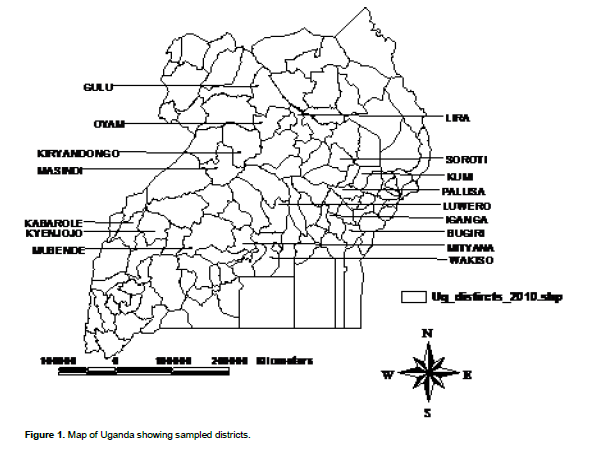
A three-level hierarchical sampling method was used to collect infected maize cob samples. This sampling method was used to facilitate capture of diversity of the fungus in different maize cobs by stratifying and focusing on collecting enough samples in fields within a districts and also representative districts with different regions. The samples were collected from different maize fields in each district within different regions in the country. Within each district 30 fields were selected. Within each fields a quadrant of 20*20 m was drawn and then on average 5 maize cobs were randomly sampled within the quadrant. All diseased maize cob samples were taken from all varieties in each farmer’s fields in cases where farmers planted a mixture because of farmer’s lack of knowledge of the varieties they cultivate. Climate data during the sampling period was accessed from the website (http://me.awhere.com/) (Table 1).
Fungal infection assessment
Ears in a sample collected from each field were put in the paper bags to reduce the evaporation and also absorb all the moisture from the samples, then they were sun dried with in their respective paper bags (Figure 2) for 7 days and then shelled as a bulked. One hundred sun dried kernels from each sample from each location were assayed for fungal mold using direct plating technique for internal infestation (Zhang et al., 1997; Moreno and Kang, 1999). All the Kernels were surface sterilized for 1 min in 2.5% NaOCl, washed three times in sterile distilled water, and fifty kernels were plate Standard 90mm Petri Dish in two replicates on the surface of 1/4 strength Potato Dextrose Agar (PDA) containing 9.75 g/l Potato Dextrose Broth (Difco) and 20 g/l agar, amended with 2 ml/l lactic acid to suppress bacterial contamination.
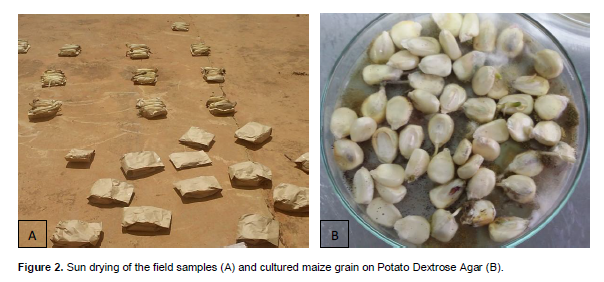
Plated kernels were incubated at 31°C for 3 days. All cultures that developed from the kernels were identified on the plates based macromorphological features which included; conidial and mycelial colour, colony diameter, reverse colony colour, presence of sclerotia (Klich, 2002). Aspergillus ear rot disease incidence and severity were assessed using percentage kernel infection method (Zhang et al., 1997). The number of infected kernels was counted and the percent kernel infection calculated Incidence of A. flavus in each sample was determined as:
Statistical analysis
Incidence and severity data were subjected to analysis of variance using MINITAB version 14 (www.minitab.com.). The general linear model (GLM) option was used to ascertain the influence of region, district and field on incidence and severity of A. flavus.
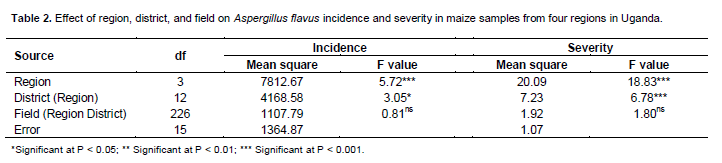
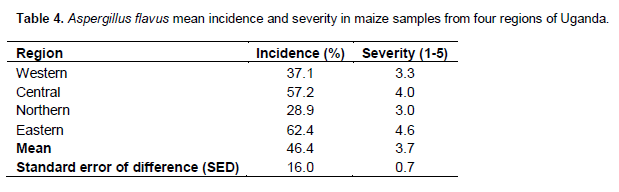
There was highly significant (P < 0.001) variation among regions for incidence and severity of Aspergillus flavus (Table 2). Incidence and severity of Aspergillus flavus differed significantly (P < 0.05 or P < 0.001) among districts within regions but not among fields with regions and districts. The mean incidence and severity of A. flavus in the districts were 48.3% and 3.9, respectively (Table 3). Among the districts, Pallisa had the highest incidence (74.2%) followed by Bugiri (73.9%) among the districts surveyed. These two districts also recorded some of the highest severity values of A. flavus. Kumi and Soroti districts had significantly lower incidence than both Pallisa and Bugiri that are in the same region (Table 3). Both Masindi and Hoima districts had significantly lower incidence than other districts in the same region. Wakiso district had the lowest incidence (7%) and least severity (2.0) of A. flavus among the districts in central region and overall. At the regional level, the highest A. flavus incidence (62.4%) was registered in the eastern region (Table 4). The eastern region had the two districts with the highest incidence. The northern region registered the lowest incidence (28.9%) but this did not differ significantly from the incidence recorded for the western region. Severity of A. flavus infection was highest in the eastern region (4.6) and lowest in the northern region (3.0).
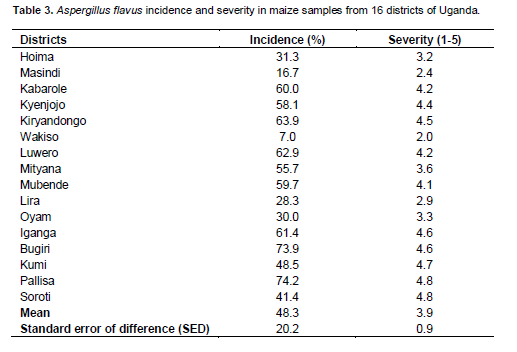
This study was conducted in the major maize growing regions and samples analyzed revealed occurrence of A. flavus. A. ?avus was identified in all the samples collected in different districts of Uganda. These results indicate that A. flavus is widely distributed in the country and that most of the commonly grown maize varieties are susceptible to fungal spoilage and a?atoxin contamination. These results corroborate earlier reports that aflatoxin accumulation in different food and feed commodities are a major threat to not only maize production in the country but also to both human and animal health (Kaaya and Warren, 2005). In a study by Simyung et al. (2013), about 11% of samples collected from different districts of Uganda were contaminated with aflatoxin that ranged from 12.7 to 123.5 µg/Kg. In addition, 9% of samples exceeded the range of 4 µg/kg to 10 or 15 µg/kg as per the new European Union aflatoxin maximum level guidelines (EU, 2010). Because we could not obtain the names of the varieties planted by the farmers, it was not possible to ascertain whether high disease incidence was related to adoption of elite maize varieties or the use of older maize varieties in the study areas. The variability of both incidence and severity of A. flavus observed among district and regions may be attributed to the different weather patterns prevailing during the sampling periods.
During the study period, annual rainfall ranged from 356.3 to 1120 mm (Table 1) which was low compared to the normal rainfall amounts characteristic of Uganda which range from 1000 to 1400 mm, but the mean temperature was as high as 28°C with average relative humidity of up to 73.2%. Environment conditions characterized with high temperatures and drought stress have an impact on the physiology of both the host and fungus, with high temperature which favour fungal growth may affect the infected plant. In addition, there are compounds present within the kernel that are induced when infected by Aspergillus spp. which affect its development hence the low severity (Chanda et al., 2009, Atehnkeng et al., 2008). Studies have demonstrated that kernel pericarp wax has been associated with resistance to Aspergillus infection and inhibited growth (Gembeh et al., 2001; Brown et al., 2013). In addition Gas chromatography/mass spectroscopy (GC/MS) analysis of the whole was component showed a higher percentage of phenol-like compounds in the resistant genotypes than in the susceptible lines (Gembeh et al., 2001). Another study which examined kernel proteins revealed differences between genotypes resistant and susceptible to aflatoxins contaminations (Huang et al., 1997)
Earlier studies have indicated that A. ?avus in maize may differed between the different agro-ecological zones due to the prevailing climatic conditions (Cotty and Jaime-Garcia, 2007), the cultivars grown in each zone, the cultural practices and / or the storage methods (Sétamou et al., 1997). Land management strategies and, particularly, crop rotation systems and factors such as genotype may in?uence crop infestation by Aspergillus section Flavi and the a?atoxin content of maize.
Although rainfall received was low but temperature were high during the seasons when this study was carried out (Table 1), it created an environment characteristic of warm and humid. Such conditions are conducive for Aspergillus growth. Similar results were reported for maize in the USA (Anderson et al., 1975) but are contrary to results by Sisson (1987) which revealed that minimum temperature above 21°C was negatively correlated with aflatoxin incidence in maize in the USA. Aspergillus strains have been reported to survive in a wider range of temperature from 19 to 35°C (Northolt and Van Egmond, 1981), although 28°C is the most conducive for aflatoxin production (Sanchis and Magan, 2004; Simyung et al., 2013). The mean temperature in the districts covered by the study was 28°C for most of the districts, and this probably enhanced the development of A. flavus. This is exemplified by the high incidence and severity in Pallisa district which experienced moderate rainfall, high temperatures (28°C) and high relative humidity (71%) (Table 1).
In addition to temperature, availability of moisture has a significant impact on A. flavus growth and aflatoxin production. This combination of susceptible genotypes and good weather (warm-humid) supports high pathogen severity and incidence. Besides the climatic factors, the high incidence of A. flavus in samples from Pallisa, Bugiri, Kiryandongo, and Iganga, could be explained by the common farming practice of leaving maize in the field for more than three weeks after physiological maturity, which leads to increased incidence of A. flavus (Kaaya et al., 2005).
These results provide for the first time, key evidence about the presence and distribution of A. flavus in the key maize producing regions in Uganda. The implication of these results is that maize grain from these districts may place consumers at risk of exposure to a?atoxin. It is necessary to establish the levels of a?atoxin contamination in maize grain from farmers’ fields and maize grain dealers in these districts and correlate these with the incidence and severity of A. flavus. This information will be useful for the formulation of guidelines on aflatoxin management in Uganda. From the earlier studies (Gardner et al., 1987; Campbell and White, 1995), combined use of management control strategies should be considered to reduce Aspergillus infection and hence aflatoxin control.
Incidences of over 50% in samples from central and eastern regions suggest that A. flavus occurrence is widespread in some of the important maize production regions in Uganda. Our study demonstrates presence of A. flavus, and these are useful resources for identifying and developing biological control technology to manage a?atoxins (Atehnkeng et al., 2008). However, more research is required to evaluate these atoxigenic strains for identifying a few effective and adapted atoxigenic vegetative compatibility groups that can be pursued in developing biological control technology in Uganda. Also further work is needed to understand the relationship between pathogen occurrence and aflatoxin levels along the maize supply chain.
The authors have not declared any conflict of interest.
This work was financed by Rural Development Administration (RDA) under Research Program for Korean-African Food and Agricultural Cooperation Initiative (KAFACI) and Agricultural Science and Technology Development (Project No. PJ009654), National Academy of Agricultural Science, Rural Development Administration, Republic of Korea and also Government of Uganda through the Agricultural Technology and Agribusiness Advisory Services (ATAAS) project.
REFERENCES
Anderson HW, Nehring EW, Wichser WR (1975). Aflatoxin contamination of corn in the field. J. Agric. Food Chem. 23:775-782. J. Agric. Food Chem. 23:775-782.
Crossref |
|
|
Atehnkeng J, Ojiambo PS, Donner M, Ikotun T, Sikora RA, Cotty PJ, Bandyopadhyay R (2008). Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 122:74-84.
Crossref |
|
|
Brown LR, Chen ZY, Abebe M (2013). Aflatoxins - Recent Advances and Future Prospects. ISBN 978-953-51-0904-4
Crossref |
|
|
|
Calvo AM, Hinze LL, Gardner HW, Keller NP (1999). Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 65:3668–3673. |
|
|
Campbell KW, White DG (1995). Evaluation of corn genotypes for resistance to Aspergillus ear-rot, kernel infection, and aflatoxin production. Plant Dis. 79:1039–1042.
Crossref |
|
|
Chanda A, Roze LV, Kang S, Artymovich KA, Hicks GR, Raikhel NV, Calvo AM, Linz JE (2009). A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. U.S.A. 106:19533-19538.
Crossref |
|
|
Corkidi G, Balderas-Ruíz KA, Taboada B, Serrano-Carreón L, Galindo E (2006). Assessing mango anthracnose using a new three-dimensional image-analysis technique to quantify lesions on fruit. Plant Pathol. 55:250–257.
Crossref |
|
|
Cotty PJ (1997). Aflatoxin producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 101:698-704.
Crossref |
|
|
Cotty PJ, Jaime-Garcia R (2007). Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 119:109-15.
Crossref |
|
|
Cotty PJ, Bayman P, Egel DS, Elis KS (1994). Agriculture, aflatoxins and Aspergillus. In: Powell KA, Renwick A,Peberdy JF, eds. The Genus Aspergillus: From Taxonomy and Genetics to Industrial Applications, . Plenum Press, New York pp. 1–27.
Crossref |
|
|
|
Fao (2002). Evaluation of Certain Mycotoxins in Food: Fifty-sixth Report of the Joint, FAO/WHO Expert Committee on Food Additives, World Health organization, Geneva. |
|
|
Gardner C, Darrah L, Zuber M, Wallin J (1987). Genetic Control of Aflatoxin Production in Maize. Plant Dis. 71:426-429.
Crossref |
|
|
Gembeh SV, Brown RL, Grimm C, Cleveland TE (2001). Identification of chemical components of corn kernel pericarp wax associated with resistance to Aspergillus flavus infection and aflatoxin production. J. Agric. Food Chem. 49:4635-4641.
Crossref |
|
|
|
Horn BW, Dorner JW (1999). Regional differences in production of aflatoxin B and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl. Environ. Microbiol. 65:1444-1449. |
|
|
Huang Z, White D, Payne G (1997). Maize Seed Proteins Inhibitory to Aspergillus flavus and Aflatoxin Biosynthesis. Phytopathology 87:622-627.
Crossref |
|
|
Kaaya AN, Warren LH, Kyamanywa S, Kyamuhangire W (2005). The effect of delayed harvest on moisture content, insect damage, moulds and aflatoxin contamination of maize in Mayuge district of Uganda. J. Sci. Food Agric. 85:2595–2599.
Crossref |
|
|
|
Kaaya NA, Warren HL (2005). A review of past and present research on aflatoxin in Uganda. Afr. J. Food Agric. Nutr. Dev. 5:1. |
|
|
|
Klich MA (2002). Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. p. 116 |
|
|
Krishnan S, Manavathu EK, Chandrasekar PH (2009). Aspergillus flavus: an emerging non-fumigatus Aspergillus species of signiï¬cance. . Mycoses 52:206–222.
Crossref |
|
|
|
MFPED (2014). Minister of Agriculture, Animal Industry and Fisheries: POLICY STATEMENT For the Financial Year 2013/14. |
|
|
Moreno OJ, Kang MS (1999). Aflatoxins in maize: the problem and genetic solutions. Plant Breed. 118:1-16.
Crossref |
|
|
|
Northolt MD, Van Egmond HP (1981). Limits of water activity and temperature for the production of some mycotoxins. 4th Meeting Mycotoxins in Animal Disease. pp. 106-108. |
|
|
Rodrigues I, Naehrer KA (2002). Three-years survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins (Basel) 4:663–675.
Crossref |
|
|
|
Sanchis V, Magan N (2004). Environmental conditions affecting mycotoxins. In N. Magan & M. Olsen (Eds.), Mycotoxins in Food. Detection and Control. CRC Press, Boca Raton 496 p |
|
|
|
Sétamou M, Cardwell KF, Schulthess F, Hell K (1997). Aspergillus flavus infection and aflatoxin contamination of preharvest maize in Benin Plant Dis. 81:1323-1327. |
|
|
Simyung L, Soohyung L, Sserumaga JP, Narae H, Sooyoun P, Yunsoo Y, Hyojin K, Hyunsuk C (2013). Survey for Contamination of Aflatoxin in Ugandan Maize. Kor. J. Int. Agric. 25:335-340.
Crossref |
|
|
|
Sisson PF (1987). The effect of climatic conditions on the incidence and severity of aflatoxin in the USA. in: M.S. Zuber et al. (ed.) Aflatoxin in maize: Proceedings of the workshop. CIMMYT, Mexico, D.F. pp. 172–177. |
|
|
Zhang Y, Kang MS, Magari R (1997). Genetics of resistance to kernel infection by A. flavus in maize. Plant Breed. Plant Breed.116:146-152.
Crossref |