ABSTRACT
Excess ammonium and nitrate are associated with physiological disorders in plants; however, these disturbances can be minimized with the use of silicon, especially in plants supplied with ammonium. The objective of this study was to evaluate the effect of silicon on the presence of excess ammonium and nitrate in two cucumber varieties (Cucumis sativus) on physiology and growth of the plants. The experiment was carried out in hydroponic cultivated cucumber plants, at the São Paulo State University, Brazil. A completely randomized design was used with four replications, in a 2 × 3 × 2 factorial corresponding to two sources of nitrogen (ammonium and nitrate) at a concentration of 10 mmol L-1, three silicon concentrations (0, 1 and 10 mmol L-1) and two varieties of cucumber (Tsubasa and Hokushin). At 28 days after treatment application, evaluations were performed for silicon and nitrogen accumulation in the shoots, green color index, number of stomata, nitrate reductase activity, height, leaf number and dry matter mass. Silicon promoted an increase in the growth variables and improved the physiological parameters of the plants only when supplying the ammonium N source. The use of Si, independent of the cucumber variety, mitigated the toxicity of ammonium, resulting in greater total nitrogen accumulation and dry matter of plants; however, it did not benefit the plants under excess nitrate nitrogen.
Key words: Cucumis sativus, nitrogen, nutritional disorder, beneficial element, stress.
Abbreviation:
MSD, minimum significant difference; EDDHMA, ethylenediamine-N, N-bis 2, hydroxy-methyl phenylacetic acid; GCI, green color index; DM, dry matter mass; NS, number of stomata.
Nitrogen (N) can be absorbed by plants in both the ammonium and nitrate forms, and these absorption forms can affect many physiological and biochemical processes including photosynthesis, enzymatic activities of the N metabolism (Lasa et al., 2002; Cruz et al., 2011), development and morphology of the plant (Zhu et al., 2000).
Ammonium excess causes toxicity in plants, changes in intracellular pH, damage to the osmotic balance, metabolism of plant hormones and polyamines, and also induces mineral nutrient deficiency (Gerendas et al., 1997). Summed with these factors, excess ammonium nitrogen also promotes an increase in the content of O2- and H2O2, inducing oxidative stress, lower production of chlorophyll and carotenoids (Wang et al., 2010), and consequently lower photosynthetic rates (Su et al., 2012), resulting in reduced growth, chlorosis and necrosis of leaves and roots (Wong, 2005).
Nitrogen, when absorbed by plants in the nitrate form, even in excess, does not cause damage to plants because most species have a mechanism for nitrate accumulation in the vacuoles (Silveira and Crocomo, 1990), resulting in no signs of toxicity (Lasa et al., 2001, 2002). However, although its excessive accumulation does not harm the plant, consumption of these plants can cause harm to humans and animals (Santamaria, 2006).
Plant species have different tolerances to excess N (Horchani et al., 2011). Some plants are more sensitive to excessive N (ammonium and nitrate), such as C. sativus (Roosta et al., 2009), especially ammonium, presenting toxicity symptoms at concentrations above 5 mmol L-1 ammonium, causing reduced growth and development of plants (Roosta and Schjoerring, 2008; Roosta et al., 2009) and losses in dry matter accumulation (Roosta and Schjoerring, 2007). However, varieties of the same species may present differences regarding tolerance of excess N (Cruz et al., 2011), which could also vary in function of the form of N provided.
To relieve stresses in plants, one option is the selection and the use of genotypes that are tolerant to certain stress. Another alternative is the use of beneficial elements, such as silicon (Si), which can reduce or alleviate stress caused by excess N in plants (Ma and Yamaji, 2006). Therefore, plants that accumulate Si, for example C. sativus (Liang et al., 2005) potentially have greater capacity to alleviate stress caused by absorption of high concentrations of N.
Si is important to mitigate abiotic stresses caused by nutritional imbalances in plants (Ali et al., 2013). Excess N causes lodging and shading of the plants and with the use of Si these effects can be reduced due to deposition of silica in stems and leaf blades (Ma, 2004). Furthermore, the effect of Si on stress mitigation can be attributed to its involvement in metabolic and physiological activities of plants (Shen et al., 2010).
Si promotes alterations in the anatomy of leaves, increasing thickness, leaf area (Farshidi et al., 2012), stomatal conductance (Ali et al., 2013; Shi et al., 2013) and promoting photosynthetic mechanisms (Mateos-Naranjo et al., 2013) in plants under stress conditions.
Associated with this, genotypes with more stomata may promote increases in photosynthesis and consequently the synthesis of carbon skeletons, culminating in increased assimilation of ammonia N and decreasing toxicity due to excess of this ion (Roosta and Schjoerring, 2008).
The beneficial effects of Si may be related to increased antioxidant activity, reducing lipid peroxidation of the leaves (Jiao-Jing et al., 2009) and protecting and helping to maintain them. This may be reflected in the chlorophyll and green color index of plant leaves.
Studies involving Si and excess ammonium and nitrate are still incipient in literature, generated a need to increase research in this area. Therefore, the hypothesis suggested is the use of Si to mitigate the effects of excess N in its ammonium and nitrate forms, independent of the cucumber variety cultivated.
The objective of the present study was therefore to evaluate the effect of silicon on the presence of excess ammonium and nitrate in two cucumber varieties (C. sativus) with regards to physiology and growth of plants.
The experiment was conducted in a greenhouse located at the Department of Soils and Fertilizers, FCAV/UNESP – Jaboticabal Campus, SP, with geographic coordinates of 21° 15' 22'' South, 48° 18' 58'' West and elevation of 575 m, with cucumber plants (Cucumis sativus) grown hydroponically for 43 days.
The experiment was setup in a completely randomized design, with four replications. The treatments were arranged in a 2 × 3 × 2 factorial, corresponding to two nitrogen sources (ammonium and nitrate), applied at a concentration of 10 mmol L-1, three silicon concentrations (0, 1 and 10 mmol L-1) and two cucumber varieties (Hokushin and Tsubasa). Ammonium chloride and calcium nitrate were used as sources of ammonium and nitrate N, respectively, and potassium silicate as the source of Si. Concentrations of potassium and calcium were balanced between treatments so that they were maintained uniform, the sources were potassium chloride and calcium chloride, respectively.
Each experimental unit consisted of a polypropylene pot with a lid, measuring 48 cm long, 11 cm wide at the lower base, 16 cm wide at the upper base and 17 cm tall, containing 8 L of nutrient solution and six cucumber plants.
Seeds were sown in styrofoam trays with 288 cells, in substrate consisting of ground coconut husk. The plants remained in a greenhouse with controlled humidity conditions until presenting three leaves (15 days after germination), and after this period were transplanted to polypropylene pots. To fixate the plants in the holes present on the pot lids a cooler was used, and a string for staking each plant. From then on, the plants were grown in nutrient solution, proposed by Hoagland and Arnon (1950), using the N concentration according to the treatments. The nutrient solution was maintained under continuous oxygenation by means of an air compression system.
The nutrient solution was exchanged weekly and the treatments were added in stages to prevent early toxicity due to excess nitrogen and impair the effect of silicon on the plants. Therefore, in the first week the complete nutrient solution with 50% ionic strength was used, but that the nitrogen concentration was 25% and silicon concentrations were 50%. As of the second week of cultivation the nutrient solution of Hoagland and Arnon (1950) was used with 100% ionic strength, 50% nitrogen concentrations and 100% silicon concentrations. On the third week the nitrogen concentration was increased to 100% and silicon concentration remained at 100% in the nutrient solution.
The nutrient solution used was that proposed by Hoagland and Arnon (1950), modified by changing the iron source to Fe-EDDHMA.
Water used in the hydroponic system was distilled and deionized, where solution levels were completed daily in each vessel with stock solutions corresponding to each treatment. Values of pH were adjusted to between 5.5 and 6.0 using solutions of HCl 1.0 mol L-1 or NaOH 1.0 mol L-1.
After 28 days of treatment application, four plants were selected per experimental unit and the following variables were assessed: height, leaf area, green color index, the nitrate reductase enzyme activity, shoot dry matter mass and a single plant was selected for counting the number of stomata.
Measurements of height were made from the base of the styrofoam to the apical meristem of the main stem with the aid of ruler graduated in centimeters. The leaf area measurements were performed by collecting all the leaves per plant followed by determination using an integrator portable area meter (LI-Cor® LI-3000C model). The green color index was determined on the third developed leaf from the apex, in the middle third of the leaves, during the period between 11:00 and 12:00 h in the morning, obtained with the aid of a green color index meter (Opti-Sciences® CCM-200 Chlorophyll Meter).
The number of stomata was measured on the third developed leaf from the apex between 07:00 and 08:00 h in the morning, followed by preparation of three slides per leaf. Thus, a cyanoacrylate-based adhesive was applied to the median portion of the abaxial leaf surface followed by securing the leaf on the glass slide for two minutes, obtaining impression of the epidermal surface on the slide. For display, we used microscopy with 40x objective lens. On each slide the number of stomata in three fields of vision was counted, where each field corresponded to 0.1 mm2 of the leaf surface.
Activity of the enzyme nitrate reductase was measured in the fourth developed leaf from the apex of the plants with the nitrate N source. For this the leaves were collected between 10:00 and 11:00 h in the morning and soon after the analysis was performed in triplicate with the leaves in vivo according to the methodology proposed by (Cazetta and Villela, 2004).
To obtain the dry mass of the shoots, the plants were collected, the shoots separated, washed, packaged in paper bags, dried in a forced air circulation oven (65°C) until constant weight and after drying the material was weighed on a precision scale. After obtaining the dry mass of the shoots, the material was ground in a Wiley mill and nitrogen concentrations were determined according to the methodology proposed by Bataglia et al. (1983) and silicon according to Kraska and Breitenbech (2010). From the nitrogen and silicon contents and the accumulation of dry shoot mass, the accumulation of these elements in the plant was calculated.
The obtained data was submitted to analysis of variance by the F-test, followed by application of the Tukey test at 5% probability for comparison of the means, using the statistical program SISVAR (Ferreira, 2011).
Accumulation of N and Si
Nitrogen accumulation in cucumber plants was influenced by the effect of the interaction among varieties and nitrogen sources and the interaction between nitrogen sources and silicon concentrations in the nutrient solution (Table 1).
Upon comparison of the two varieties, when nitrogen was supplied via the ammonium source, greater accumulation of the nutrient was observed in the Tsubasa variety. However, when N was supplied in the nitrate form, the Hokushin variety accumulated more N in the shoots (Table 2). Considering only the nitrogen sources, it was found that regardless of the cultivar, when provided as a nitrate source there was greater accumulation of N in the shoots of cucumber plants. Similar results were reported by Cruz et al. (2011) when studying four varieties of pea (Pisum sativum L) subjected to sources of N (ammonium and nitrate), finding that the varieties have different adaptive responses to excess ammonium. This fact may be attributed to distinguishing features already existent among plant species (Lasa et al., 2002) and even varieties (Cruz et al., 2011) with regards to nitrogen fertilization.
The accumulation of nitrogen in plants, independent of N supplied from ammonium or nitrate sources, was greater in the presence of Si with no difference between the concentrations of 1 and 10 mmol L-1 (Table 2). When evaluating the effect of N concentrations (nitrate and ammonium) and addition of Si in Brassica napus L. plants, Bybordi (2010) observed a beneficial ratio of Si with excess NH4+ to promote increases in production of fresh weight and leaf area. According to Ma and Yamaji (2006), the stress caused by high N doses can be reduced or mitigated by use of Si.
The relationship of Si with N has also been verified by Bybordi (2012) and Feng et al. (2010) when reporting that in the presence of this beneficial element there was increased activity of enzymes acting in the N metabolism, such as nitrate reductase, glutamine synthetase, glutamate synthetase and glutamate dehydrogenase. This beneficial effect may be even more significant in Si accumulating plants, such as C. sativus (Liang et al., 2005).
Silicon accumulation in cucumber plants was influenced by the following interactions: Varieties and nitrogen sources, varieties and Si concentrations, and N sources and Si concentrations (Table 1). Accumulation of this beneficial element in shoots of the varieties studied was higher in plants nourished with N in its nitrate form. When submitted to this nitrogen source increases of 111.8 and 65.9% in Si accumulation were observed in the Hokushin and Tsubasa varieties, respectively (Table 2). For the nitrate source, there was no difference in Si accumulation between varieties, however for the ammonium source the Tsubasa variety presented greater accumulation (Table 2).
In the absence of Si, for both N sources the plants presented lower accumulation of the element in relation to the treatment with silicon. No effect was observed on the accumulation of Si among the concentrations of 1 and 10 mmol L-1 of the beneficial element, independent of the variety and N source (Table 2).
Both cucumber varieties showed higher N and Si accumulation when submitted to the nitrate source compared to the ammonium source. This result is attributed to the fact that excess nitrate does not cause toxicity to plants (Lasa et al., 2001, 2002). However, excess ammonium N generates damage to the root system, causing necrosis and reducing growth (Wong, 2005), further resulting in lower absorption of water, nutrients and Si, and therefore reduced plant growth.
Green color index
The green color index (GCI) was influenced by the interactions between varieties and N sources, and N sources and silicon concentrations (Table 1). In both varieties the GCI was higher in treatments where N was provided in the nitrate form. These results can be attributed to excess ammonium causing a decrease in chlorophyll levels (Wang et al., 2010) with consequent chlorosis in the leaves (Wong, 2005) and increased synthesis of putrescine, evolving to necrosis of leaf tissues (Prado, 2008), resulting in the lower green color index found in this study.
Upon comparing the two varieties, when fertilized with nitrate N no differences were observed in the GCI, however when fertilized with ammonium N it was found that Tsubasa presented a higher GCI in relation to Hokushin (Table 2).
The use of Si promoted an increase in the GCI of plants fertilized with ammonium N (Table 2). However, there was no difference between the concentrations of 1 and 10 mmol L-1 of the beneficial element, due to the absence of differences in Si accumulation between these concentrations (Table 2). It is known that Si promotes protective effects to photosynthetic mechanisms, and the balance of nutrients (Mateos-Naranjo et al., 2013), increasing the antioxidant activity of plants and decreasing lipid peroxidation of the leaves (Jiao-Jing et al., 2009). These benefits to the plant metabolism contribute to maintain the leaves photosynthetically active, thus mitigating the harmful effects of ammonium toxicity to the plants, resulting in an increase in chlorophyll synthesis that is reflected in a greater GCI.
Although silicon promoted an increase in the green color index in plants cultivated with excess ammonium N, in plants cultivated with excess nitrate N the use of this beneficial element had no effect on the same variable (Table 2). The use of Si only benefited the plants submitted to excess ammonia, and is explained because excess nitrate does not cause toxicity to C. sativus plants, where toxicity is caused only by the use of ammonium (Roosta and Schjoerring, 2007), thus indicating the benefits of Si on plants under stress conditions.
Enzymatic activity and number of stomata
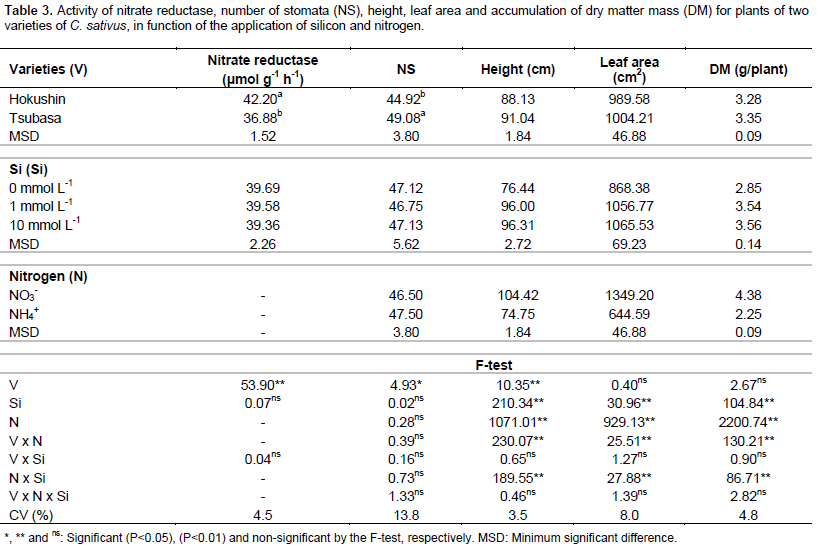
Activity of the nitrate reductase enzyme presented a significant effect (P<0.01) for the cucumber varieties, with higher values in the variety Hokushin than in Tsubasa. No significant effects were evident for Si concentrations or for interaction between varieties and Si concentrations (Table 3). These results are similar to those found by Bybordi (2010) in studies involving Si and N in Brassica napus L. plants.
The number of stomata was influenced only by the varieties, where the variety Tsubasa has a greater number of stomata when compared to the variety Hokushin. Although changes are reported in stomatal conductance with the use of high ammonium concentrations (Lopes and Araus, 2006), there was no effect between N sources and interactions (Table 3).
Despite the fact that Si promoted changes in the anatomy of leaves, increasing the thickness, leaf area (Farshidi et al., 2012) and stomatal conductance in plants under stress conditions (Ali et al., 2013; Shi et al., 2013), no effect of the Si concentrations and interactions was observed regarding the number of stomata.
Growth and production of dry matter mass
The growth of cucumber plants, evaluated by the height, leaf area and dry matter accumulation, was influenced by the interaction between varieties and nitrogen sources and the interaction of nitrogen sources with silicon concentrations (Table 3).
Plant growth evaluated with regards to height, leaf area and accumulated of dry matter mass, when fertilized with ammonium N, was always higher in the variety Tsubasa, however when the N source was nitrate, the highest values for all of these variables were obtained from plants of the variety Hokushin (Table 4).
These results can be attributed to the different responses to excess N by the plant varieties (Cruz et al., 2011) due to the characteristics of each species and variety regarding the site of organic ammonium N incorporation (Lasa et al., 2002), and enzyme activities that participate in the detoxification process of excess N (Doubnerová and Ryslavá, 2011).
There was greater growth of plants under the nitrate N source, promoting increases of 65.3 and 19.2% for height, 141.9 and 82.8% for leaf area, and 136.4 and 63.1% for the accumulated mass of dry matter in the varieties Hokushin and Tsubasa, respectively (Table 4).
This occurred because the excess ammonium N caused toxicity in C. sativus plants, which are characterized as sensitive to excess ammonium (Roosta et al., 2009). Similar results were reported by Roosta and Schjoerring (2007) in studies with C. sativus plants and N sources (nitrate and ammonium), confirming that the accumulated mass of dry matter was lower in plants cultivated with ammonium N in relation to those cultivated with nitrate N.
Excess ammonium in C. sativus plants causes imbalances between nutrients, leading to deficiency of calcium and magnesium (Roosta and Schjoerring, 2007), with harmful consequences to plant nutrition, and therefore growth and production of dry matter. The ammonium concentration of 10 mmol L-1 applied to the nutrient solution is considered high and toxic to the plants tested in this study. According to Roosta and Schjoerring (2008) and Roosta et al. (2009), ammonium concentrations above 5 mmol L-1 cause damage to the growth and development of C. sativus plants.
The low tolerance to excess ammonium by C. sativus plants may be associated with the location of ammonium assimilation, where most is assimilated in shoots (Roosta et al., 2009) and also by enzyme activity (glutamate dehydrogenase and glutamine synthetase) (Cruz et al., 2006). However, excess nitrate is tolerable by most plant species, because it is accumulated in vacuoles (Lasa et al., 2001, 2002), thus showing no symptoms of toxicity.
The use of Si in the nutrient solution at concentrations of 1 and 10 mmol L-1 promoted the best results for the variables of height, diameter, leaf area and accumulation of dry matter mass in plants supplied with ammonium N, however response of the beneficial element on plants supplied with nitrate N was not verified (Table 4). The beneficial effect of silicon on the growth of C. sativus plants subjected to stress conditions was confirmed by Zhu et al. (2004) when establishing that the use of Si (1 mmol L-1) promoted an increase in the growth variables of plants under salt stress, resulting in higher accumulation of dry matter mass. These results, attributed to the fact that Si reduced the oxidative damage of membranes caused by salinity, indicated greater plant growth.
The Si promoted increased antioxidant activity in plants, reducing lipid peroxidation, providing protection to plants against oxidative damage, and increased plant growth and development (Jiao-Jing et al., 2009). These effects on growth induced by Si in conditions of excess ammonium may be associated with its protective effects on the photosynthetic apparatus of plants (Mateos-Naranjo et al., 2013) because excess ammonium has harmful effects on photosynthesis (Britto and Kronzucker, 2002), such as lowering of the green color index (Table 1). Thus the use of Si possibly mitigated this effect of ammonium toxicity.
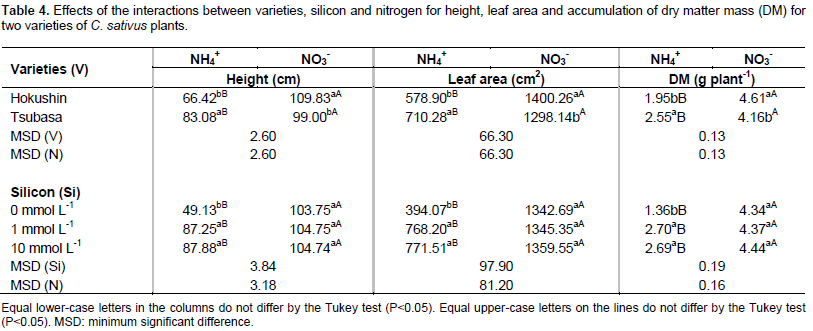
Although silicon promoted growth of the plants when supplied with ammonium N, no difference was observed between the concentrations of 1 and 10 mmol L-1 of this beneficial element, indicating that there were no losses in plant development when cultivated with high concentrations of Si. It was noted that this fact may possibly be explained by the occurrence of Si polymerization in the nutrient solution with increase in its concentration, reducing its absorption. Another explanation is that most of the absorbed Si, about 90%, is immobilized as biogenic opal on the outside of the cell wall (Silva and Bohnen, 2001), with only a small free amount in the plant.
The use of Si independent of the C. sativus variety mitigates the toxicity of ammonium, resulting in higher accumulations of total nitrogen and dry matter of plants; however, it does not benefit plants under excess nitrate N.
The authors have not declared any conflict of interest.
REFERENCES
|
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013). The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 89:66-72.
Crossref
|
|
|
|
Bataglia OC, Furlani AMC, Teixeira JPF, Furlani PR, Gallo JR (1983). Métodos de análise química de plantas. IAC, Campinas. P 48.
|
|
|
|
|
Britto DT, Kronzucker HJ (2002). NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 159(6):567-584.
Crossref
|
|
|
|
|
Bybordi A (2010). Influence of NO3:NH4 ratios and silicon on growth, nitrate reductase activity and fatty acid composition of canola under saline conditions. Afr. J. Agric. Res. 5(15):1984-1992.
|
|
|
|
|
Bybordi A (2012). Effect of ascorbic acid and silicium on photosynthesis, antioxidant enzyme activity, and fatty acid contents in canola exposure to salt stress. J. Integr. Agr. 11(10):1610-1620.
Crossref
|
|
|
|
|
Cazetta JO, Villela, LCV (2004). Nitrate reductase activity in leaves and stems of tanner grass (Brachiaria radicans Napper). Sci. Agric. 61(6):640-648.
Crossref
|
|
|
|
|
Cruz C, Bio AFM, Domínguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Martins-Loução MA (2006). How does glutamine synthetase activity determine plant tolerance to ammonium? Planta. 223(5):1068-1080.
Crossref
|
|
|
|
|
Cruz C, Domínguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Bio A, Martins-Loução MA, Moran JF (2011). Intra-specific variation in pea responses to ammonium nutrition leads to different degrees of tolerance. Environ. Exp. Bot. 70(2-3):233-243.
Crossref
|
|
|
|
|
Doubnerová V, Ryslavá H (2011). What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 180(4):575-583.
Crossref
|
|
|
|
|
Farshidi M, Abdolzadeh A, Sadeghipour HR (2012). Silicon nutrition alleviates physiological disorders imposed by salinity in hydroponically grown canola (Brassica napus L.) plants. Acta Physiol. Plant. 34(5):1779-1788.
Crossref
|
|
|
|
|
Feng J, Shi Q, Wanga X, Wei M, Yang F, Xu H (2010). Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hort. 123(4):521-530.
Crossref
|
|
|
|
|
Ferreira DF (2011). Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 35(6):1039-1042.
|
|
|
|
|
Gerendás J, Zhu Z, Bendixen R, Ratcliffe RG, Sattelmacher B (1997). Physiological and biochemical processes related to ammonium toxicity in higher plants. J. Plant Nutr. Soil Sci. 160(2):239-251.
Crossref
|
|
|
|
|
Hoagland DR, Arnon DI (1950). The water culture method for growing plants without soils. California Agricultural Experimental Station, Berkeley, P 347.
|
|
|
|
|
Horchani F, Hajri R, Aschi-Smiti S (2011). Is the sensitivity to ammonium nutrition related to nitrogen accumulation? Curr. Bot. 2(2):18-22.
|
|
|
|
|
Jiao-Jing L, Shao-Hang L, Pei-Lei X, Xiu-Juan W, Ji-Gang B (2009). Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed Cucumber leaves. Agr. Sci. China 8(9):1075-1086.
Crossref
|
|
|
|
|
Kraska JE, Breitenbeck GA (2010). Simple, robust method for quantifying silicon in plant tissue. Commun. Soil Sci. Plant Anal. 41(17):2075-2085.
Crossref
|
|
|
|
|
|
|
Lasa B, Frechilla S, Aparicio-Tejo PM, Lamsfus C (2002). Role of glutamate dehydrogenase and phosphoenolpyruvate carboxylase activity in ammonium nutrition tolerance in roots. Plant Physiol. Biochem. 40(11):969-976.
Crossref
|
|
|
|
|
Lasa B, Frechilla S, Lamsfus C, Aparicio-Tejo PM (2001). The sensitivity to ammonium nutrition is related to nitrogen accumulation. Sci. Hort. 91(1-2):143-152.
Crossref
|
|
|
|
|
Liang Y, Si J, Romheld V (2005). Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 167(3):797-804.
Crossref
|
|
|
|
|
Lopes MS, Araus JL (2006). Nitrogen source and water regime effects on durum wheat photosynthesis and stable carbon and nitrogen isotope composition. Physiol. Plant. 126(3):435-445.
Crossref
|
|
|
|
|
Ma JF (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50(1):11-18.
Crossref
|
|
|
|
|
Ma JF, Yamaji N (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11(8):392-397.
Crossref
|
|
|
|
|
Mateos-Naranjo E, Andrades-Moreno L, Davy AJ (2013). Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 63:115-121.
Crossref
|
|
|
Prado RM (2008). Nutrição de plantas. UNESP, Jaboticabal. P 407.
|
|
Roosta HR, Sajjadinia A, Rahimi A, Schjoerring JK (2009). Responses of cucumber plant to NH4+ and NO3- nutrition: The relative addition rate technique vs. cultivation at constant nitrogen concentration. Sci. Hortic. 121(4):397-403.
Crossref
|
|
|
|
|
Roosta HR, Schjoerring JK (2007). Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J Plant Nutr. 30(11):1933-1951.
Crossref
|
|
|
|
|
Roosta HR, Schjoerring JK (2008). Effects of nitrate and potassium on ammonium toxicity in cucumber plants. J. Plant Nutr. 31(7):1270-1283.
Crossref
|
|
|
|
|
Santamaria P (2006). Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 86(1):10-17.
Crossref
|
|
|
|
|
Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J (2010). Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 167(15):1248-1252.
Crossref
|
|
|
|
|
Shi Y, Wanga Y, Flowersb TJ, Gong H (2013). Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 170(9):847-853.
Crossref
|
|
|
|
|
Silva LS, Bohnen H (2001). Liberação de nutrientes durante a decomposição de palha de aveia preta (Avena strigosa) com diferentes teores de silício. Rev Bras Ciênc Solo 25(2):515-520.
Crossref
|
|
|
|
|
Silveira JAG, Crocomo OJ (1990). Assimilação de nitrogênio em cana-de-açúcar cultivada em presença de elevado nível de N e de vinhaça no solo. Rev. Bras. Fisiol. Veg. 2(2):7-15.
|
|
|
|
|
Su S, Zhou Y, Qin JG, Wang W, Yao W, Song L (2012). Physiological responses of Egeria densa to high ammonium concentration and nitrogen deficiency. Chemosphere 86(5):538-545.
Crossref
|
|
|
|
|
Wang C, Song HZ, Pei FW, Wei L, Jie L (2010). Effects of ammonium on the antioxidative response in Hydrilla Verticillata (L.f.) Royle plants. Ecotoxicol. Environ. Saf. 73(2):189-195.
Crossref
|
|
|
|
|
Wong M (2005). Visual symptoms of plant nutrient deficiencies in nursery and landscape plants. Soil Crop Manag. 1:1-4.
|
|
|
|
|
Zhu Z, Gerendás J, Bendixen R, Schinner K, Tabrizi H, Sattelmacher B, Hansen UP (2000). Different tolerance to light stress in NO3- and NH4+ grown Phaseolus vulgaris L. Plant Biol. 2(5):558-570.
Crossref
|
|
|
|
|
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004). Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 167(3):527-533.
Crossref
|
|