ABSTRACT
The objective of this study was to evaluate the effects of nutrition and salt stress on the growth and accumulation of mineral elements in Jatropha curcas plants. The work was conducted in greenhouse with 100% solar radiation interception, at Goiás State University, Ipameri Campus, Goiás, Brazil. The experiment was set up in five-liter containers following the completely randomized design in 4 x 2 factorial arrangement (I: Clark’s complete nutrient solution, Clark’s nutrient solution modified to prevent contents of ii: nitrogen, iii: potassium or iv: magnesium, and two salinity treatments, distilled water and saline water [2 dS m–1] obtained by NaCl addition). On even days, the plants received nutrient solution and on odd days, they received saline or distilled water. The plants were irrigated with complete nutrient solution up to the 20th day after germination and the treatments were applied from the 21st to the 60th day. The results show that there was no toxic effect of salt on J. curcas plants; however, salinity caused nutritional unbalance and significantly hindered Ca2+ and K+ absorption and slightly reduced vegetative growth due to the small change in water status. J. curcas plants are moderately tolerant to irrigation water salinity.
Key words: Mineral nutrition, biofuel, oleaginous, osmotic stress, water restriction.
Concern for natural resources has intensified the search for renewable energy alternatives, like biofuels. Brazil stands out in the world scenario for its growing, vigorous renewable energy matrix, since 45% of the energy and 18% of the fuels consumed in Brazil are renewable, while in the rest of the world, only 14% of consumed energy comes from renewable source (ANP, 2015).
The Brazilian program for production and use of biofuel has stimulated the cultivation of raw materials and marketing of vegetable oil. However, the program is founded on only one raw material, soybeans, accounting for 73.92% of all vegetable oil for biodiesel production (ANP, 2014). Therefore, diversifying the sources of raw material is required in order to make the system less vulnerable to weather and economic conditions, by introducing promising species such as Jatropha curcas L.
J. curcas is a perennial lactiferous Euphorbiaceae, native to Mexico in Central America, where high genetic diversity was reported (Henning, 2008). The plants are deciduous with juicy stem and fast growth. The species has great economic potential, either in landscaping as hedge, pharmacology, and especially in the production of biodiesel oil. The seeds are oleaginous with about 40% of the oil easily processed into biodiesel. J. curcas seed biodiesel has 84% of diesel oil calorific value (Matos, 2010; Ferreira, 2015). The oil has about 80% of unsaturated fatty acids and low freezing point (-10°C) and can be marketed in various regions of the world (Matos, 2010), which represents great advantage, especially in colder regions. These features have contributed to increased commercial interest in this culture.
The J. curcas plant develops in several kinds of soils, including sandy, stony, saline, alkaline and rocky ones, which, from the physical nutritional point of view, are restrictive to full root development (Carvalho et al., 2013). It is a fast-developing shrub and can start production already in the first year of planting and remain productive for about 40 years. Its productive climax occurs at the fourth year in the field (Dias et al., 2007). Commercial plantations of Jatropha in Brazil are still in early stages due to the low scientific knowledge of the plant. With the development of new research, the culture is expected to turn from potential to actual raw material for the biodiesel market (Andréo-Souza et al., 2010). J. curcas species lacks genetic improvement, in that it shows significantly uneven growth, architecture, development and fruit ripening. Although, rustic and tolerant to drought, the crop needs agronomic knowledge, as basic information such as fertilizing recommendation, salinity tolerance and productivity is not available on the species (Matos et al., 2014a).
Although, it is drought tolerant and can survive with 200 mm per year for three years using water stored in stems and roots, it is presumed that its maximum genetic potential is expressed in adequate conditions of water availability. Adequate water supply enables significant productivity gains. However, water scarcity in many inland reservoirs intensifies the need for research to assess the possibility of the species cultivation with saline water irrigation. The use of saline water for irrigation has become an important alternative given the scarcity of good quality water worldwide. The quality of many water sources is low, especially water wells and surface reservoirs. Because it contains soluble salts, water used for irrigation entails periodic salt incorporation to the soil profile. In the absence of leaching, the salt is deposited in the root area and soil surface due to water evaporation (Veras et al., 2011).
Recent studies point to reasonable sensitivity of J. curcas to salinity with significant reduction in biomass, leaf number and stem diameter (Oliveira et al., 2015; Sousa et al., 2011). The adverse effects of salinity are the result of three primary factors: i) inhibition of water absorption caused by the solution low water potential; ii) toxic effects of Na+ and Cl– ions at the cellular level; and iii) changes in nutritional balance altering the absorption of K+, Ca2+, Mg2+, NO3–, Cl–, Na+ and other chemical elements (Diaz-Lopez et al., 2012; Mansour, 2013). The combination of these factors causes changes in cell metabolism with significant reduction in grain yield. Plants develop innumerable salt stress tolerating mechanisms that include osmotic adjustment, toxic ion compartmentalization, and absorption inhibition and/or exclusion. Their salinity tolerance is difficult to measure due to the dependence on many factors such as weather, growth stage and soil fertility (Taiz and Zeiger, 2013). Besides, many genetical and environmental effects were reported in growth of different plant species from seed collection to harvest (Yazici, 2010; Dilaver et al., 2015; Tebes et al., 2015; Cercioglu and Bilir, 2016).
Under natural conditions, abiotic stresses may occur simultaneously, sometimes causing irreversible damage to the plants. With a view to better physiological understanding of salinity and nutrient deficiency occurring simultaneously, this study aimed at evaluating the effects of nutrition and salt stress on the growth and accumulation of mineral elements of J. curcas plants.
Experimental design
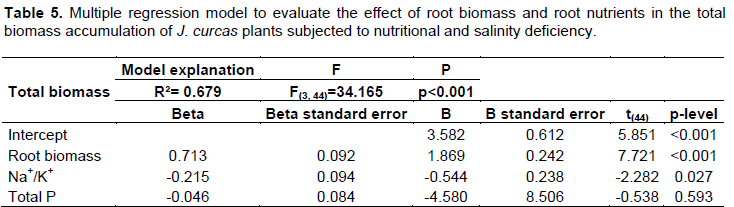
The work was conducted in greenhouse with 100% solar radiation interception at Goiás State University, Ipameri Campus (17°43’19’’ S, 48°09’35’’ W, 773 m), Ipameri, Goiás. According to Köppen classification, the region has tropical savanna climate (Aw), with rainy summers and dry winters. The experiment was carried out in five-liter containers following the completely randomized design in a 4 × 2 factorial arrangement (i: Clark complete nutrient solution, Clark’s nutrient solution modified to prevent contents of ii: nitrogen, iii: potassium or iv: magnesium, and two salinity treatments, distilled water and saline water [2 dS m–1] obtained by NaCl addition). On even days, the plants were treated with nutrient solution and on odd days, with saline or distilled water. The plants were irrigated with the complete nutrient solution until the 20th day after germination and the treatments from the 21st to the 60th days using the salts as shown in Table 1.
The solution volume to be applied was determined based on the container weight differences. Initially, the solution was placed in the containers until drainage started and 60 min later, the same containers were weighed for the first time. Exactly 24 h after the first weighing, the containers were weighed again and the difference in mass between the first and second weighing corresponded to the solution volume to be applied daily per container (150 ml). 60 days after germination, the following analyses were performed: leaf number, plant height, stem diameter, transpiration, leaf area, relative water content, total biomass and nitrogen concentration (N), calcium (Ca) potassium (K), magnesium (Mg) and sodium (Na) in leaves and roots.
Growth variables
Th enumber of leaves was obtained by counting. The plant height and stem diameter were measured using a graduated ruler and a digital pachymeter, respectively. For leaf area evaluation, a graduated tape (cm) was used, measuring the length and width of all leaves in each plant and then the leaf area was calculated following the equation proposed by Severino et al. (2007). The leaves, roots and stems were plucked and oven-dried at 72°C to reach constant dry mass and then weighed separately. The mass data enabled calculation of the leaf mass ratio (LMR), root mass ratio (RMR), stem mass ratio (SMR), shoot/root system ratio (S/RS) and total biomass.
Leaf relative water content (RWC)
In order to obtain the relative water content, twenty 12-mm leaf discs were taken, weighed, and saturated for 24 h in Petri dishes with distilled water. Then, the discs were weighed again and dried at 70°C for 72 h. Subsequently, the dry matter was obtained.
Photosynthetic pigments
In order to determine the total chlorophyll and carotenoid concentration, leaf discs were taken from known areas and put into jars containing dimethyl sulfoxide (DMSO). Afterwards, extraction was made in 65°C water bath for one hour. Aliquots were taken for spectrophotometric reading at 480, 649, 1 and 665 nm. Chlorophyll a (Chl a) and chlorophyll b (Chl b) contents were determined according to the equation proposed by Wellburn (1994).
Nutritional analyses
Samples of roots and fully expanded leaves were collected, and then total N concentration was determined according to Kjeldahl sulfuric digestion method. In order to determine the contents of potassium, calcium, magnesium and sodium, the methodology proposed by Johnson and Ulrich (1959) was used.
Statistical procedures
The experiment was set up in five-liter containers following the completely randomized design in a 4 × 2 factorial arrangement and six replications. The experimental plot corresponded to one plant per container. In order to evaluate the effects of nutritional deficiency and salinity on the growth and mineral accumulation in plants, variance analysis was used (ANOVA). Assumptions of normal distribution and variance homogeneity were always tested. Whenever the ANOVA results were significant, further comparisons of Newman-Keuls were performed to detect differences between the treatment averages.
In order to evaluate the effect of the analyzed variables on root biomass, multiple regression analyses were performed using the forward stepwise selection model (Sokal and Rolf, 1994). The forward stepwise model selects the independent variables in the regression model according to the amount of explained variation in the dependent variable; the first independent variable introduced in the model explains most of the variation; the second one, most of the remaining variation, and so forth. Adding variables to the model ends when the next variable to be included does not have a significant partial correlation (p>0.05). The same analytical procedure was performed to evaluate the effect of variables on the plant total biomass. All statistical analyzes used in this work were performed under the procedures of R software (R CORE TEAM, 2015).
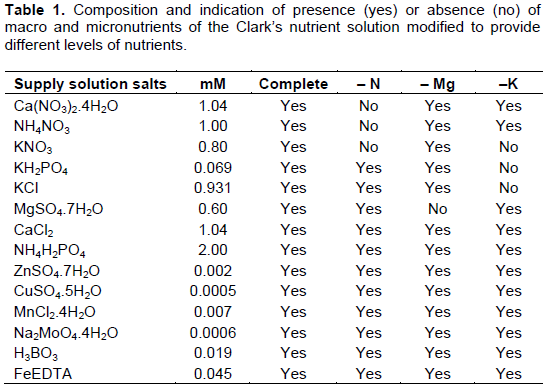
Biomass, stem diameter and relative water content were, respectively, 16, 9 and 11% lower in the plants irrigated with saline solution as compared to plants irrigated with non-saline solution. Only plant height showed statistical significance due to the nutritional treatment applied. This variable was approximately 9% lower in treatment lacking nitrogen, when compared with the average of the other treatments containing nitrogen. The summary of the variance analysis for growth and water status variables is shown in Table 2.
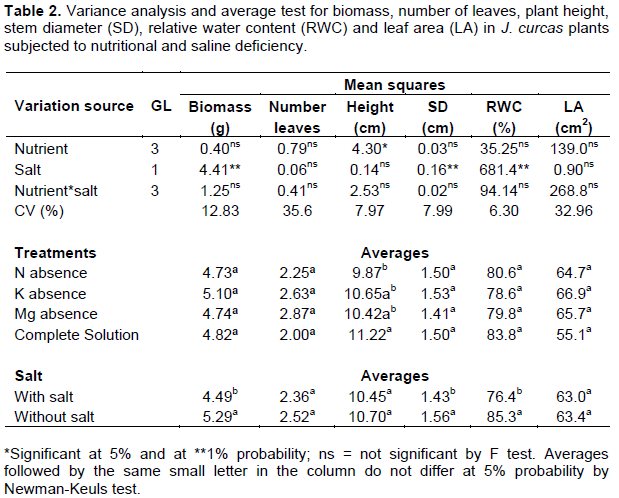
Potassium concentration (K+) was 18% higher in the roots of plants treated with the complete nutrient solution when compared with the average of plants treated with lacking nutrients. Calcium concentration (Ca2+) was 17% lower in the roots of plants treated with nutrient solution without nitrogen when compared with the average of other treatments. Sodium concentration (Na+) was 28% higher in the roots of plants treated with the complete nutrient solution when compared with the average of plants treated without nutrients. K+ and Ca2+ concentrations were, respectively, 26 and 22% lower in plants irrigated with saline solution as compared to plants irrigated with non-saline water. Na+ concentration and Na+/K+ ratio were, respectively, 50 and 63% lower in plants irrigated with non-saline water when compared with the plants irrigated with saline solution. The summary of the variance analysis for nutrient concentrations in the root system is shown in Table 3.
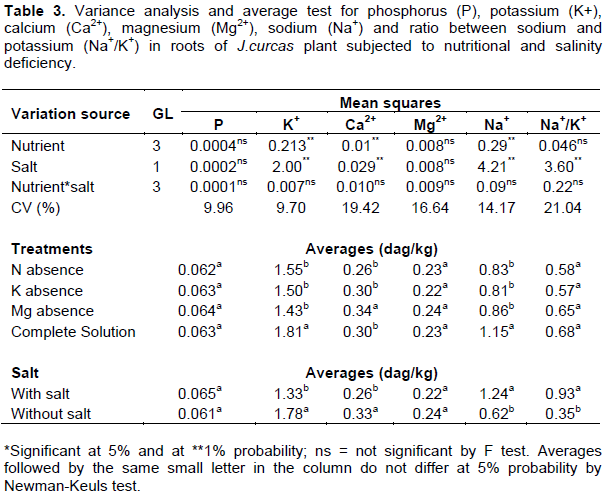
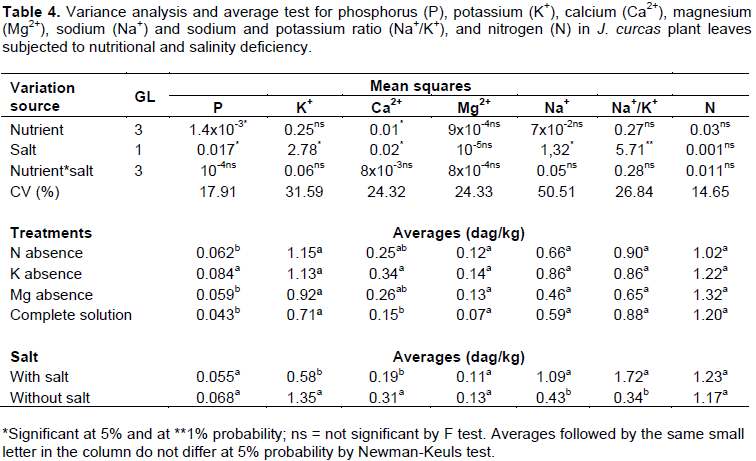
Phosphorus concentration was 35% higher in the leaves of plants treated with nutrient solution without K+
when compared with the average of the other treatments. Ca2+ concentration was 47% lower in the leaves of plants treated with the complete nutrient solution when compared with the average of the other nutrient-deficient treatments. Sodium concentration (Na+) was 28% higher in the roots of plants treated with the complete nutrient solution when compared with the average of plants treated with those lacking nutrients. K+ and Ca2+ concentrations were, respectively, 68 and 49% lower in plants irrigated with saline solution as compared to plants irrigated with non-saline water. Na+ concentration and Na+/K+ ratio were, respectively, 61 and 81% lower in plants irrigated with non-saline water when compared with the plants irrigated with saline water. The summary of the variance analysis for nutrient concentrations in leaves is shown in Table 4.
Multivariate regression showed that the nutrients present in roots and root biomass explained 67% of the total biomass variation (model R2: 0.738; p<0.001) (Table 5). The partial coefficients indicate that the values were significant only for root biomass and Na+/K+, the relation being positive for the former and negative for the latter.
Under natural conditions, abiotic stresses often occur simultaneously and involve complex damage and repair mechanisms, causing either reversible or irreversible harm to the plants. The nutritional unbalance caused by salinity in irrigation water shows that J. curcas is moderately tolerant to irrigation water salinity.
The absence of significant changes in root mass, stem and leaf ratios, total carotenoid and chlorophyll leaf concentrations (data not shown) caused by irrigation water salinity and/or nutritional deficiency points to the absence of toxic effects on chloroplasts. On the other hand, variations in the concentration of photosynthetic pigments in plants exposed to salinity are indicative of toxicity in chloroplasts (Souza et al., 2015). Salinity promoted small changes to vegetative growth (biomass, height, diameter, number of leaves and leaf area) of J. curcas plants. These variations were mainly due to low osmotic potential of irrigation solution, which slightly changed the plant water status. The reduction in the relative water content in plants grown unsder salinity possibly limited (even mildly) cell expansion and vegetative growth. Approximately, 2/3 of plant growth is due to cell expansion, which depends on cell turgor provided by tissue hydration (Taiz and Zaiger, 2013). Reduction in growth variables in J. curcas plants grown under salinity and/or water limitation has been reported in literature in numerous studies (Carvalho et al., 2013; Carvalho Junior et al., 2014; Oliveira et al., 2010, 2015). Despite the long treatment time, it is not possible to assert that there was water deficit, but rather a small change in water status. The succulent stem of J. Curcas plants works as a water buffer, supplying the leaves under reduced water availability (Matos et al., 2014b). Moreover, J. curcas plants absorb NaCl from saline solutions and use Na+ and Cl– to accomplish osmotic adjustment and continue absorbing water (Da Silva et al., 2009). Lower plant height under nitrogen deficiency is due to the importance of this nutrient to a vigorous vegetative stage. Nitrogen is essential for J. curcas plant vegetative growth (Do Carmo et al., 2014). Quite possibly, a longer treatment would result in significant variations in other growth variables.
Lower concentrations of K+, Ca2+ and Na+ in the roots of plants grown under nutrient deficiency when compared with plants treated with the complete solution are due to the importance of K+ and Ca2+ for the balance of organic and inorganic anions in the plant and to increased presence of salts in the complete solution. Higher foliar Ca2+ concentration in plants grown under nutritional deficiency can be associated with Ca2+ secondary messenger role in the transfer of information to the response elicitor target. Despite the reduction in foliar Ca2+ concentration under salt stress, this nutrient is essential for membrane stability and maintenance of Na+/K+ relationship at suitable levels for the plants (Lacerda et al., 2004). The chemical similarity between phosphorus and cadmium can result in greater absorption of the latter because of changes to membrane selectivity in the presence of Na+ (Taiz and Zaiger, 2013). Higher concentration of total P in leaves under K+ deficiency and no difference in the concentration of this nutrient in leaves and roots of plants treated with saline solution are indicative of maintenance of plasma membrane integrity as to total P absorption.
Salinity caused nutritional unbalance in J. curcas plants. High intracellular Na+ concentration has possibly enhanced the competition for absorption sites with low and/or high K+ affinity and reduced concentrations of this chemical element in plant tissues (root and leaves).
According to Taiz and Zaiger (2013), Na+ high denaturing potential hinders membrane proteins and contributes to the reduction of K+ internal concentration due to the competition with Na+ for the same absorption site. In addition, intracellular reductions of Ca2+ in plants grown under salinity may be associated with increased influx of Na+ through nonselective cationic channels. Epstein and Bloom (2005) stated that excessive Na+ reduces Ca2+ influx and prevents Na+ efflux cellular detoxification mechanism activated by Ca2+.
The Na+/K+ ratio (0.93 and 1.72, respectively) in leaves and roots of J. curcas plants irrigated with saline solution was always higher than that of plants irrigated with non-saline water due to Na+ high accumulation and K+ reduced absorption. According to Dias-López et al. (2012), it is common for the Na+/K+ ratio in J. curcas to be higher than 1.2 and lower than 2.5, but for optimal cellular metabolism, it should always be lower than 0.6. Multiple regressions indicate that the root biomass had a positive and fundamental importance for total biomass accumulation. However, Na+ root concentration and Na+/K+ ratio were the variables that most negatively contributed to the plant total biomass accumulation. Na+/K+ ratio is specifically an important indicator of salt stress tolerance or susceptibility of J. curcas plants.
1. In the present study, it was not possible to identify the toxic effects of salt in J. curcas. However, there was nutritional unbalance due to high accumulation of Na+ in the tissues and mild vegetative growth reduction caused by small variation in water status.
2. J. curcas plants are moderately tolerant to the salinity of irrigation water.
3. Salinity causes nutritional unbalance in J. curcas plants with significant damage to Ca2+ and K+ absorption.
The authors have not declared any conflict of interests.
REFERENCES
|
Andréo-Souza Y, Pereira AL, Silva FFS, Ribeiro-Reis RC, Evangelista MRV, Castro RD, Dantas BF (2010). Efeito da salinidade na germinação de sementes e no crescimento inicial de mudas de pinhão-manso. Rev. Bras. Sementes 32:83-92.
Crossref
|
|
|
|
ANP (Agency national of oil, gas and biofuels) (2014). Oil, natural gas and biofuels statistical yearbook Brazil, Brasilia. Available at
View
|
|
|
|
|
ANP (Agency national of oil, gas and biofuels) (2015). Energy Balance Brazil, Brasilia. Available at:
View. Accessed in April 2015.
|
|
|
|
|
Carvalho CM, Viana TVA, Marinho AB, Lima Júnior LA, Valnir Júnior M (2013). Pinhão-manso: crescimento sob condições diferenciadas de irrigação e de adubação no semiárido nordestino. Rev. Bras. Eng. Agríc. Amb. 17:487-496.
|
|
|
|
|
Carvalho Junior GS, Lima MSR, Rocha MS, Beltrão NEM, Negreiros KV (2014). Crescimento de Jatropha curcas sob diferentes níveis de salinidade e silício. Rev. Caatinga 27:39-46.
|
|
|
|
|
Cercioglu M, Bilir N (2016). Seed source effect on quality and morphology of Turkish red pine (Pinus brutia Ten.) seedlings. Reforesta 2:1-5.
Crossref
|
|
|
|
|
Da Silva EN, Silveira JAG, Rodrigues CRF, Lima CS, Viégas A (2009). Contribuição de solutos orgânicos e inorgânicos no ajustamento osmótico de pinhão-manso submetido à salinidade. Pesq. Agropec. Bras. 44:437-445.
Crossref
|
|
|
|
|
Dias LAS, Leme LP, Laviola BG, Pallini A, Pereira OL, Carvalho M, Manfio CE, Santos AS, Sousa LCA, Oliveira TS, Dias DCFS (2007). Cultivo de pinhão-manso (Jatropha curcas L.) para produção de óleo combustível. Viçosa, MG. 1:1-40.
|
|
|
|
|
Diaz-López LD, Gimeno V, Lidón V, Simón I, Martínez V, Sánchez FG (2012). The tolerance of Jatropha curcas seedlings to NaCl: An ecophysiological analysis. Plant Physiol. Biochem. 54:34-42.
Crossref
|
|
|
|
|
Dilaver M, Seyedi N, Bilir N (2015). Seedling quality and morphology in seed sources and seedling type of Brutian pine (Pinus brutia Ten.). World J. Agric. Res. 3:83-85.
|
|
|
|
|
Do Carmo MS, Borges LP, Torres Junior HD, Santos PGF, Matos FS (2014). Efeito da disponibilidade de nitrogênio e déficit hídrico no crescimento inicial de plantas de pinhão manso. Rev. Agrotecnol. 5(2):33-49.
Crossref
|
|
|
|
|
Epstein E, Bloom AJ (2005). Mineral nutrition of plants. Principles and perspectives, 2nd ed. Sinauer associates, Inc., Sunderland, M.A.
|
|
|
|
|
Ferreira FA (2015). Avaliação da toxicidade e do potencial angiogênico do látex de pinhão manso. Dissertação de Mestrado, Universidade Estadual de Goiás, Ipameri, Goiás. 46p.
|
|
|
|
|
Henning RK (2008). The Jatropha system in Zambia- Evaluation of the existing Jatropha activities and proposals for an implementation strategy in Southem province of Zambia.
|
|
|
|
|
Johnson CM, Ulrich A (1959). Analytical methods for use in plants analyses. In: Bulletin 766. University of California Experiment Station, Berkeley, Los Angeles. pp. 32-33
|
|
|
|
|
Lacerda CF, Cambraia J, Oliva MA, Ruiz HA (2004). Influência do cálcio sobre o crescimento e solutos em plântulas de sorgo estressadas com cloreto de sódio. Ver. Bras. Ciênc. do Solo 28:289-295.
Crossref
|
|
|
|
|
Mansour MMF (2013). Plasma Membrane permeability as na indicator of salt tolerance in plants. Biol. plants 57:1-10.
|
|
|
|
|
Matos FS (2010). Caracterização Fisiológica da Senescência Foliar de Populações de Jatropha curcas L... Universidade Federal de Viçosa, Viçosa, Minas Gerais. PhD. thesis 48p.
|
|
|
|
|
Matos FS, Rosa VR, Borges LFO, Ribeiro RP, Cruvinel CKL, Dias LAS (2014a). Response of Jatropha curcas Plants to Changes in the Availability of Nitrogen and Phosphorus in Oxissol. Afr. J. Agric. Res. 9:3581-3586.
|
|
|
|
|
Matos FS, Torres Junior HD, Rosa VR, Santos PGF, Borges LFO, Ribeiro RP, Neves TG, Cruvinel CKL (2014b). Estratégia morfofisiológica de tolerância ao déficit hídrico de mudas de pinhão manso. Magistra 26:19-27.
|
|
|
|
|
Oliveira FA, Guedes RAA, Gomes LP, Bezerra FMS, Lima LA, Oliveira MKT (2015). Interação entre salinidade e bioestimulante no crescimento inicial de pinhão manso. Ver. Bras. Eng. Agríc. e Amb. 19:204-2010.
|
|
|
|
|
Oliveira IRS, Oliveira FN, Medeiros MA, Torres SB, Teixeira FJV (2010). Crescimento inicial de Jatropha curcas em função da salinidade da água de irrigação. Rev. Caatinga 23:40-45.
|
|
|
|
|
R core team (2015). A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. Available at:
View.
|
|
|
|
|
Severino LS, Vale LS, Beltrao NEM (2007). Método para medicáo da área foliar do pinháo manso. Rev. Bras. Oleaginosas Fibrosas 8(1):753-762.
|
|
|
|
|
Sokal RR, Rohlf FJ (1994). Biometry. New York: W. H. Freeman 880pp
|
|
|
|
|
Sousa AEC, Gheyi HR, Correia KG, Soares FAL, Nobre RG (2011). Crescimento e consumo hídrico de pinhão manso sob estresse salino e doses de fósforo. Ver. Ciênc. Agronôm. 42:310-318.
|
|
|
|
|
Souza BR, Freitas IAS, Lopes VA, Rosa VR, Matos FS (2015). Growth of Eucalyptus plants irrigated with saline water. Afr. J. Agric. Res. 10:1091-1096.
Crossref
|
|
|
|
|
Taiz L, Zeiger E (2013). Fisiologia vegetal. 5.ed. Artmed, Porto Alegre, Santa Catarina.
|
|
|
|
|
Tebes NT, Seyedi N, Bilir N (2015). Morphological characteristics and quality in Black pine (Pinus nigra Arnold. subsp. pallasiana) seedlings. Int. J. Latest Res. Sci. Technol. 4:7-9.
|
|
|
|
|
Veras RP, Laime EMO, Fernandes PD, Soares FAL, Freire EA (2011). Altura de planta, diâmetro caulinar e produção do pinhão-manso irrigado sob diferentes níveis de salinidade. Rev. Bras. Eng. Agríc.e Amb. 15:582-587.
|
|
|
|
|
Wellburn AR (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 144:307-313.
Crossref
|
|
|
|
|
Yazici N (2010). Determination of water consumption of some coniferous seedlings and its relation with meteorological parameters, Graduate School of Natural and Applied Science, Suleyman Demirel University, Isparta, Turkey. PhD. Thesis.
|
|