Vegetables are rich sources of essential nutrients like minerals, vitamins A, C, and E, phytochemicals such as folates, glucosinolates, carotenoids, flavonoids and phenolic acids, lycopene, and dietary fibres (Fasuyi, 2006). India is the second largest producer of vegetables in the world (ranks next to China) and accounts for about15% of the world’s production (Sandhya, 2010). It is estimated that around 20 to 25% of total vegetables are lost due to poor post-harvesting practices and less than 2% of the total vegetables produced in the country are commercially processed (Sandhya, 2010). The consumption of bitter gourd has increased due to awareness about medicinal properties rather than nutritious benefits.
Bitter gourd (Momordica charantia) is a member of Cucubitaceae family and it is one of the most popular vegetables cultivated in China, Taiwan, Pakistan, India, and Philippines for their immature fruits and sometimes for the tender leafy shoots (Yamaguchi, 1983). The immature fruits, called bitter melon, bitter gourd or balsam pear are harvested at developmental stages up to seed hardening. Bitter gourd has an important role as a source of carbohydrate, proteins, vitamins, minerals and other nutrients in human diet (Ali et al., 2008) which are necessary to maintain proper health. The beneficial health effects of bitter gourd have been attributed to the
presence of antioxidants that act as receptors of free radicals. Ascorbic acid and β carotene are the antioxidants present in bitter gourd at high concentrations. Apart from its antioxidant property, it also has many medicinal applications and is used as an hypoglycemic agent for diabetic patients (Cefalu et al., 2008) and it is also beneficial against piles, blood and respiratory disorders and cholera.
Changes in lifestyle patterns has leads to increased demand for cut vegetables as the people do not have time to prepare vegetables at home as well as in hotels. Because of these factors, consumption of minimally processed products has significantly increased (Allende et al., 2006). As a result, the market demands for ‘fresh-cut’ vegetables have increased rapidly (Day, 2001). Wang et al. (2007) found that fresh-cut bitter gourd stored at 2°C had only limited storage of 4 days.
The shelf life of fresh-cut produce under ambient condition is very limited which can be extended by many preservation techniques like low temperature storage, controlled atmosphere, hypobaric and modified atmosphere packaging methods. Modified atmosphere packaging technology is one among them, which is largely used for minimally processed fruits and vegetables including fresh, ‘‘ready-to-use’’ vegetables (Sandya, 2010). Modified atmosphere packaging (MAP) of fresh produce relies on the modification of atmosphere inside the package achieved by the natural interplay between two processes: the respiration rate of the commodity and the permeability of the packaging films. Active and passive modified atmospheric packagings are the two systems generally recognized for packaging of fresh-cut produce (Kitinoja and Gorny, 1998). Passive atmosphere is the modification of the gas composition inside the package due to interplay between the product respiration rate and the gas exchange rate through the package. Active atmosphere is the modification of the gas composition inside the package by replacing, at the moment of packaging, the air with a specific gas mixture either by drawing a vacuum or filling a gas mix.
Though many vegetables are part of our dietary habit, the technology of ready to eat or ready to cook form of minimal processing is available only for few vegetables in India. Thus it is necessary to develop techniques to preserve bitter gourd. Keeping in view the above prospective, the present investigation on preservation of fresh-cut bitter gourd using modified atmosphere packaging with different packaging material has been taken to conduct studies under passive and active modified atmosphere packaging for enhancing the shelf life of fresh-cut bitter gourd.
Raw material
Bitter gourd variety of CO 1 was purchased from the university orchard of Tamil Nadu Agricultural University, Coimbatore. Bitter gourd were harvested to developmental stage to seed hardening. After harvesting (within ½ h) the vegetables were stored at 8±2°C for 2 h before processing. Packaging materials such as Low Density Polyethylene (LDPE) and Poly propylene (PP) of 50, 75 and 100 µ thickness were procured from the local market, Coimbatore. The oxygen and carbon dioxide transmission rates were determined with the help of Manometric permeability tester (M/s. PBI Dansensor, USA).
Permeability of the packaging films
The permeability rates for oxygen and carbon dioxide were determined at a rate at which gases permeate through a film at specified conditions of temperature and relative humidity. The films used for the study should have low permeability to oxygen and carbon dioxide gases. The oxygen and carbon dioxide transmission rates were found with the help of Manometric permeability tester (M/s. PBI Dansensor, USA).
The permeability tester works on the principle of diffusion-Ficks law (Manometric pressure change via gas transmission through film). It consists of two chambers and a provision to keep the sample (film) in between the chambers. In the upper chamber, provision is made to regulate the gas pressure (for which permeability is to be determined) at the required level whereas in the bottom chamber vacuum is maintained. The instrument measures the time required for the pressure in the bottom chamber to increase to a predefined upper limit. The test sample of 10 cm (packaging film) was prepared and placed between the chambers. The gas analyser measures the gas concentration in the chamber and determines the permeability of the film and displayed digitally. The test cycle was repeated until the sample got stabilized and attained equilibrium.
Preparation of fresh-cut bitter gourd
Fresh good quality and uniformly sized bitter gourd were selected and prepared by slicing with sharp sterile stainless steel knives and sliced into 1 cm thick cubes. Fresh-cut bitter gourd were soaked in to sodium hypochlorite solution of 100 ppm dipping was used as pre-treatment for 3 min at room temperature because it acts as a surface disinfectant which reduces the microbial growth. Treated samples were shade dried for 15 min in room temperature to remove the surface moisture and approximately 100 g of slices were packaged in different packaging materials. The treated and control samples (without packaging) were stored under refrigeration condition 8±2°C.
Packaging of fresh-cut bitter gourd
Passive and active modified atmospheric packagings are the two systems used for packaging of fresh-cut bitter gourd. Passive modification (atmospheric condition – 21% O2, 0.01% CO2, 79% N2) was done by placing the fresh-cut vegetables in the package film (LDPE and PP) and then it was sealed using the heat sealer. A desired atmosphere develops naturally inside the package as a consequence of respiration of products and the diffusion of gases through the film. The gas was analyzed using gas analyzer (PBI Dansensor). Active modification was done by placing the fresh-cut vegetables in to the pouch and then the air inside the pouch (LDPE and PP) were replaced by a desired mixture of gases using gas mixing unit (MAP mix 8000 EL, PBI Dansensor). The fresh-cut bitter gourds were flushed with 3% O2, 5% CO2 and 92% N2.
Head space gas analysis
The selected polymeric films (LDPE and PP) were made into pouch of size 20.5 cm length and 15.5 cm breadth. The silicon septum was pasted on the surface of the pouch for drawing the gas samples. The fresh-cut vegetables of approximately 100 g were taken in the pouch and it is sealed for passive atmosphere where as for active atmosphere, the desired mixture of gases are filled and sealed. At particular interval the gas was measured in the package using gas analyser (PBI Dansensor).
Physiological loss in weight (PLW)
Fresh-cut vegetables were weighed with the help of an electronic balance (Make: Avery; Model: OC-51) at regular intervals. The initial and final weights of the samples were recorded and the loss in weight was calculated (Mathad, 2003).
Physiological loss in weight (%) = [(Initial Weight – Final Weight) / Initial weight] × 100 (1)
Physico-chemical and microbiological characteristics of vegetables
Chlorophyll
The chlorophyll content in the fresh-cut bitter gourd at different atmosphere and at storage periods were determined by using 80% acetone (Ranganna, 1995).
Hundred milligrams of finely cut and well mixed representative sample of vegetables were taken into a clean mortar and ground into a fine pulp by adding 20 ml of 80% acetone. The pulp was centrifuged and the supernatant was collected in a 50 ml volumetric flask. The residue obtained was collected and again extracted with acetone. This procedure was repeated till the residue become colorless. The volume was made into 50 ml. The absorbance of the solution at 645 and 663 nm against the solvent (80% acetone) blank was recorded.
The amount of chlorophyll present in the extract (mg chlorophyll per 100 mg tissue) was calculated using the following equation:
Chlorophyll (mg/100 g) = [20.2 (A645) + 8.02 (A663) × V/100 × W]
(2)
Where A = absorbance at specific wavelengths (nm), V = final volume of the chlorophyll extract in 80% acetone and W = fresh weight of sample (g).
Ascorbic acid content
Ascorbic acid content were determined by visual titration using 2,6-dichlorophenol- indophenol (Ranganna, 1995). One gram of vegetable was made in to pulp and extracted using 10 ml of 4% oxalic acid solution, made up to 50 ml. Then 5 ml of this made up solution was pipetted out in to a concial flask and titrated against dye. The titration was repeated for the concordant values.
Quantity of ascorbic acid (mg) present in 100 g of sample was calculated as follows.
Ascorbic acid (mg / 100 g) = [0.5/V1 × V2/5 ml × 50 ml/ wt of sample × 100] (3)
V1= volume of dye occupied by the working standard (ascorbic acid); V2= volume of dye occupied by the sample (bitter gourd).
Titratable acidity
Titratable acidity was determined by titrating a known volume of vegetable juice (by extracting the juice from 10 g of the sample using pestle and motor) with 0.1 N NaOH to an end point of permanent pale pink color using phenolphthalein as indicator. The NaOH required to neutralize the juice and the titratable acidity was calculated and expressed as % citric acid.
Titratable acidity = (N× V× Equivalent weight of acid× 100) / (Weight of the sample taken ×100) (4)
N= Normality of the NaOH; V= Volume of the NaOH required to neutralize the juice (bitter gourd).
Microbial analysis
The qualities of fresh-cut vegetables are based on the number and kind of microorganisms present, which was assessed by standard plate count method (Allen, 1953). Commonly used media for the enumeration of bacteria and fungi are nutrient agar medium and Martin’s Rose Bengal Agar medium. One gram of the fresh-cut vegetable was taken and added into a test tube containing 10 ml of sterile water. The test tubes were shaken well for 10 to 15 min for uniform distribution of microbial cell in the water blank. This will give a dilution of 1:10 (10-1). One ml from (10-1) dilution was transferred to 9 ml of sterile water with a sterile one ml pipette, which gave a dilution of 10-2. The process was repeated up to 10-5 dilutions with the serial transfer of the diluents. One ml aliquots from 10-3 and 10-5 dilutions were transferred to the sterile petri dishes for the enumeration of fungi and bacteria, respectively. Three replications were maintained for calculating the population as a mean of four replications.
Sensory evaluation
Sensory evaluation of the vegetables was done by the panel of semi-trained judges (10 members) for appearance, color, flavor, texture, taste and overall acceptability using 9-point Hedonic scale varying from like extremely (rated as 9) to dislike extremely (rated as 1).
Statistical analysis
All the analysis was carried out in four. Statistical analysis was carried out to study the effect of different parameters on all the dependent variables. Analysis of variance (ANOVA) was conducted with Factorial Completely Randomized block Design (FCRD) using the software AGRES version 7.01.
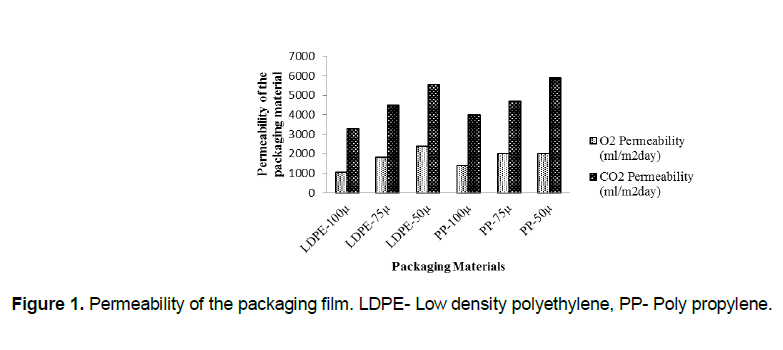
Permeability of the packaging material
The permeability of low density polyethylene (LDPE) and polypropylene (PP) packaging materials of different thickness was assessed and is shown in the Figure 1. From the figure it was observed that, the permeability decreased with thickness of the packaging film for both O2 and CO2. The permeability of O2 in the films leads to more respiration and deteriorate the quality of the product. eHence to maintain the quality of the product, the films (LDPE-100 µ, PP-100 µ) with low permeability to O2 and CO2 were selected for further study. Ati and Hotchkiss, (2002) reported that LDPE and PP films with a thickness of 25 to 100 µ are most commonly used for storage of minimally processed vegetables.
Respiration rate of fresh-cut bitter gourd
During respiration, the fresh-cut bitter gourd consumes O2 and produces CO2 a result of metabolic activity. Meyer et al. (1973) reported that the plant materials during respiration takes oxygen and break the organic reserves to simpler molecules of CO2 and water with release of energy. The respiration rate of pre-treated fresh-cut bitter gourd at 8±2°C was determined experimentally in a closed system.
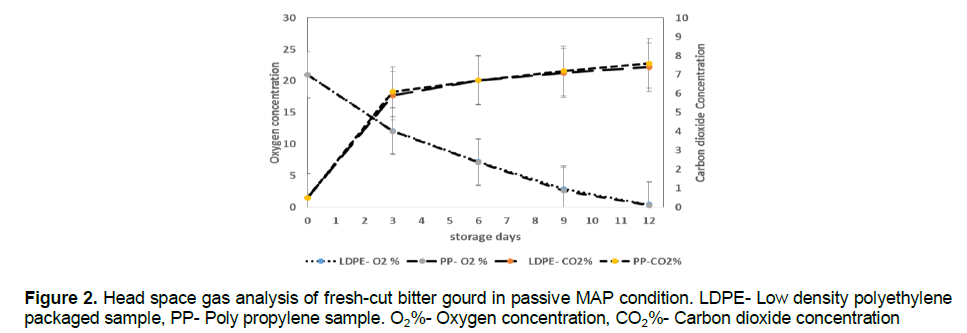
Head space gas analysis
The effect of gas concentration on the head space of the pouches (LDPE and PP) containing fresh-cut bitter gourd stored at 8±2?C are shown in the Figure 2. The initial concentration of passive MAP were 21% O2 and 0.01% CO2 and at active MAP were 3% O2, 5% CO2 and 92% N2.
The gas concentration of fresh-cut bitter gourd packaged under passive MAP in a LDPE pouch (Figure 2) showed the decrease of O2 concentration from 21 to 0.423% and increase of CO2 from 0.01 to 7.4% whereas in PP pouches, the O2 concentration was decreasing from 21 to 0.321% and CO2 production was increasing from 0.01 to 7.6% till the 12th day of storage. The PP film showed more concentration of O2 (0.321%) and CO2 (7.6%) than LDPE owing to higher permeability to the gases. In active MAP, the fresh-cut bitter gourd stored in LDPE pouch showed the decrease of O2 from 3 to 0% (Figure 2) and increase of CO2 from 5 to 13.5% whereas in PP pouches the decrease of O2 from 3 to 0% and increase of CO2 from 5 to 13.8%.
After 12 days of storage in passive MAP and 15 days of storage in active MAP, it started producing off-odor. This may be due to low O2 content which would have facilitated anaerobic condition. Low oxygen concentrations, in combination with temperature fluctuations, have been reported to result in the production of off-flavour (Forney and Jordan, 1999). Mannapperuma et al. (1989) reported that 3 to 4% of O2 and 4 to 5% of CO2 were more suitable for maintaining the quality and extending shelf life of fresh-cut produces at refrigerated condition.
The shelf life of fresh-cut bitter gourd extended up to 15 days when it was stored under active MAP whereas in passive MAP it was only 12 days. The increase in shelf life was due to the lesser respiration rate at lower concentration of O2. Fonseca et al. (2002) also reported the increase of shelf life in shredded galega kale under active MAP.
Physiological loss in weight for MAP of fresh-cut bitter gourd
The effects of storage period on physiological loss in weight of fresh-cut bitter gourd stored in MAP conditions are shown in the Table 1. There was a significant effect of treatment (packaging material × active and passive MAP) at p≤0.01 on physiological loss in weight. The respiration and the transpiration of water from the product attributed to PLW of the samples (Wills et al., 1989).
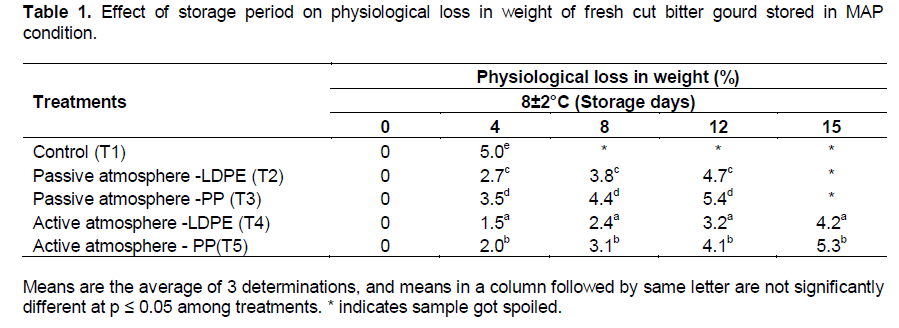
The maximum loss of about 5% was found in control sample after 4 days. This may be due to less humidity in atmospheric air. The minimum loss in weight with maximum storage of 15 days was observed as 4.2% in LDPE pouches under active MAP. This may be due to less respiration rate at lower temperature and more humidity inside the package which would have resulted in minimum loss in weight. In passive atmosphere, loss in weight was 3.2% in LDPE pouches which can be stored up to 12 days. The LDPE showed 4.2 % (T4) loss in weight at 8±2°C under active atmosphere in 15 days which was found to be less than PP pouch with 5.3%. This may be due to less permeability of LDPE to the water vapor transmission. Kudachikar et al. (2011) reported that for robusta banana packaged in LDPE pouches showed lesser PLW due to low water vapor transmission rate. By comparing both active and passive MAP it was concluded that minimum weight loss (4.2%) was in active MAP of LDPE pouches with maximum storage period.
Physico-chemical characteristics for MAP of fresh-cut bitter gourd
The physico-chemical analysis of fresh-cut bitter gourd was carried out for color, chlorophyll, ascorbic acid, titratable acidity, bacterial and fungal growth.
Changes in chlorophyll content of MAP fresh-cut bitter gourd
The effect of storage period on chlorophyll content of fresh-cut bitter gourd stored in MAP condition are shown in the Table 2. There was significant effect of treatment (packaging material × active and passive MAP) at (p≤0.01) on the chlorophyll content. The initial chlorophyll content of the fresh-cut bitter gourd was 19.56 mg/100 g. The control sample (T1) had maximum loss of chlorophyll content (12.3 mg/100 g) in 4 days of storage. The chlorophyll content decreased with increase of storage period. Roura et al. (2000) reported that processing induced the decrease of chlorophyll content during storage in swiss chard leaves.
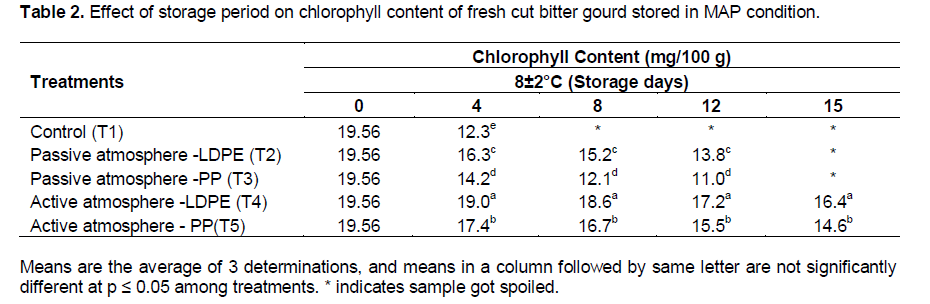
Minimum loss of chlorophyll was observed in T4 (16.4 mg/100g) under 8±2°C in 15 days of storage. The active MAP showed less loss of chlorophyll content in LDPE (17.2 mg/100 g) and PP pouches (15.5 mg/100 g) at 8±2°C compared to passive MAP for both LDPE (13.8 mg/100 g) and PP (11.0 mg/100 g) in 12 days of storage. Zagory and Kader (1988) reported that low O2 concentration reduced the breakdown of chlorophyll to phaeophytin. The loss of chlorophyll content in fresh-cut bitter gourd was 19.2%. Similar trend of result was also reported by Wang et al. (2007) for the fresh-cut bitter gourd with a loss of 20% chlorophyll content in 7 days of storage under 8±2°C. The reduction in the loss of chlorophyll content in the present study may be due to the variation in the variety of the bitter gourd selected.
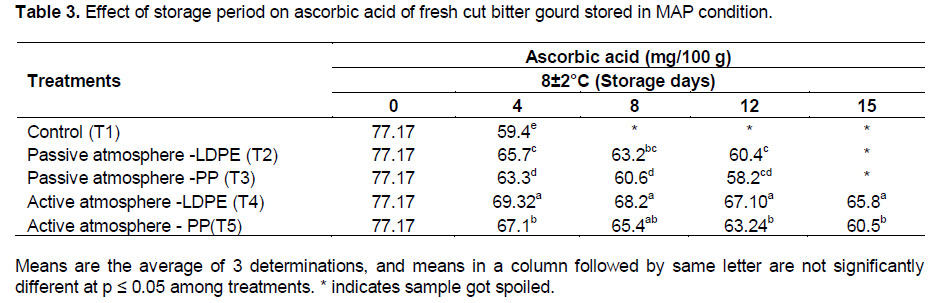
Changes in ascorbic acid for MAP of fresh-cut bitter gourd
The effect of storage period on ascorbic acid content of fresh-cut bitter gourd stored in MAP condition is presented in the Table 3. There was significant effect of treatment of fresh-cut bitter gourd (packaging material × active and passive MAP) at p≤0.01 on the ascorbic acid content. The initial ascorbic acid content of fresh-cut bitter gourd was 77.17 mg/100 g. The minimum loss of ascorbic acid was seen in T4 (54.4 mg/100 g) in 15 days of storage compared to other treatment and the maximum loss was observed in control (57.4 mg/100 g) after 4 days of storage. In 8±2°C, the shelf life of fresh-cut bitter gourd was 15 days under active MAP and it was only 12 days under passive condition. The active MAP showed a minimum loss of 17.2% in 15 days of storage whereas in passive condition, the loss was 15% in 12 days. The reduction in loss of ascorbic acid in active MAP over the passive system may be due to low O2 content that would have prevented the oxidation of ascorbic acid to dehydro-ascorbic acid. Peterson and Berends, (1993) reported that for sweet green peppers, the low O2 content in the package retarded the dehydro-ascorbic acid during storage.
Means are the average of 3 determinations, and means in a column followed by same letter are not significantly different at p ≤ 0.05 among treatments. * indicates sample got spoiled.
The ascorbic acid content of fresh-cut bitter gourd under MAP decreased with storage period. This result is in agreement with Wang et al. (2007) for minimally processed bitter gourd where there was a decrease in ascorbic acid content (58%) over storage under 8±2°C.
Changes in Titratable acidity for MAP of fresh-cut bitter gourd
The effects of storage period on titratable acidity of fresh-cut bitter gourd stored in MAP condition are shown in the Table 4. It was observed that the treatments (packaging material × active and passive MAP) had significant effect on the titratable acidity at p≤0.01. The initial titratable acidity of fresh-cut bitter gourd was 0.68 mg/100 g. The titratable acidity increased with storage period. Wang et al. (2007) also reported that cutting of bitter gourd lead to the accumulation of titratable acidity. The minimum increase of titratable acidity was observed in T4 (2.0%) in 15 days of storage under 8±2°C compared to other treatments and maximum increase was seen in T1 (3.2%) after 4 days of storage.
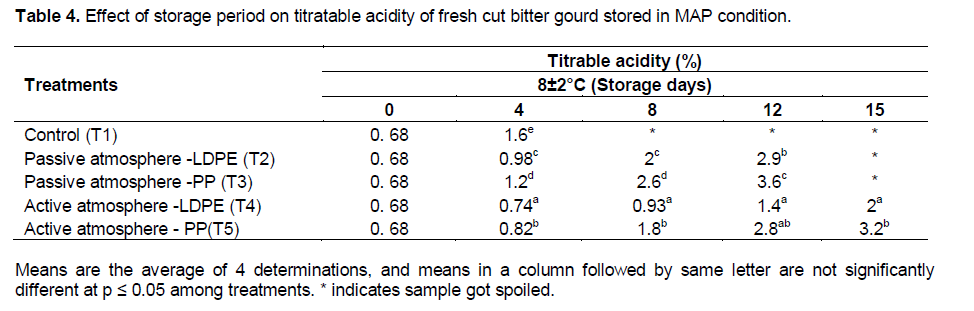
Increase in acidity will lead to sour taste of bitter gourd. The titratable acidity of fresh-cut bitter gourd in LDPE and PP pouches increased under passive MAP by 11.7 and 16. 1% respectively at the end of 12 days of storage whereas in active MAP, the increase were 66% (LDPE) and 78.7% (PP) at end of 15th day.
From the results, it was concluded that the samples stored under 8±2°C in LDPE with active MAP was the best with respect to minimum increase (66% in T4) of titratable acidity. Piga et al. (2003) also reported increase of titratable acidity (59%) during the storage of fresh-cut bitter gourd under 8±2°C (10°C) for 7 days. The variation in the increase of titratable acidity reported may be due to different variety that was used for the study.
Microbial growth in MAP fresh-cut bitter gourd on storage
The effect of storage period on microbial growth for MAP of fresh-cut bitter gourd is shown in Tables 5 and 6. There was significant effect on treatment (packaging material × active and passive MAP) of fresh-cut bitter gourd at (p≤0.01) on the bacterial and fungal growth. The initial load of bacteria and fungi seen in fresh-cut bitter gourd after pre-treatment was 3.12 × 105 cfu/g and 4.2 × 103 cfu/g respectively. Nguyen and Carlin (1994) also reported that bacterial (103-106 cfu/g) and fungi counts (103-104 cfu/g) were seen in fresh-cut vegetables. It was evident from the figure that the bacteria and fungi count were maximum in control sample for fresh-cut bitter gourd. This may be due to cross contamination of product from the atmospheric air and the higher temperature would have facilitated the growth of micro-organism.
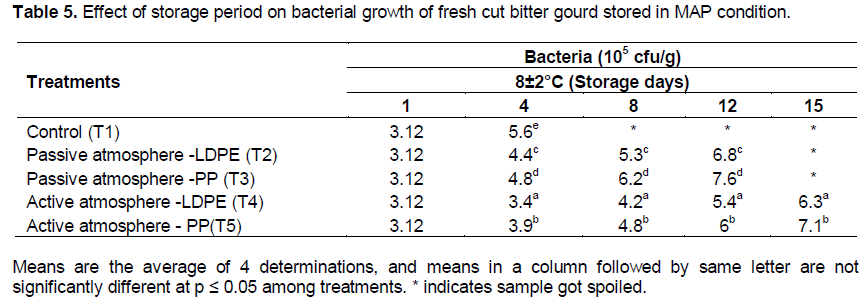
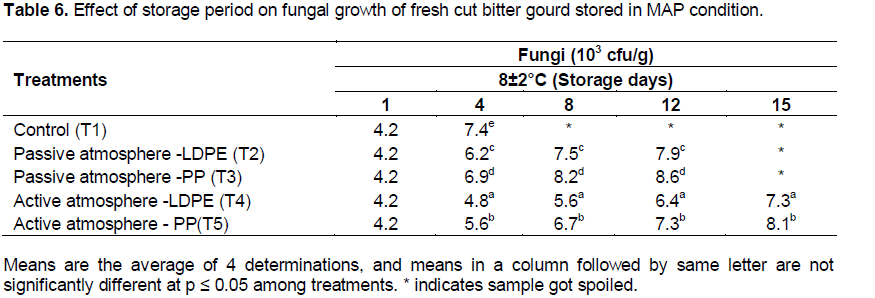
The less bacterial (6.3 × 105cfu/g) and fungi count (7.3 × 103cfu/g) were found in T4 (LDPE packaged sample) under active atmosphere at 8±2°C. Corbo et al. (2004) reported that for cactus pear fruit, lower storage temperature significantly reduced the microbial growth. Active atmosphere showed lesser bacterial and fungal growth than passive atmosphere. Oliu et al. (2008) reported that in fresh-cut melon, the microbial growth was less in active atmosphere than passive. In the present study, the shelf life was 15 days in active MAP whereas in passive condition it was only 12 days. Atmospheres with low O2 (1 to 5%) and high CO2 (5 to 10%) have shown the maximum shelf-life of fresh-cut fruits (Gorny et al., 2002).
Sensory evaluation for MAP of fresh-cut bitter gourd
The shelf life of the product is mainly depending on the microbial and sensory quality. Based on the results obtained for the microbial analysis, it was observed that the samples (T2, T3, T4 and T5) were within the safe microbial limit and hence the samples were taken for conducting the sensory evaluation. The dishes were prepared from the selected samples after keeping them for a maximum period of storage and sensory evaluation was done using a 9-point hedonic scale and the results of the study are presented in the Table 7.
From the Table 7, it was observed that, the fresh sample scored overall acceptability of 8.2. Among the samples, active MAP of fresh-cut bitter gourd packaged in LDPE (T7-7.88) was the best followed by T3, T4, and T5. The active MAP extended the product shelf life by lowering the O2 concentration and retained the color and texture. Gomez and Arte (2005) also reported that MAP packaged cerely sticks scored maximum in sensory attributes. There was significant effect on treatment (packaging material × active and passive MAP) of fresh-cut bitter gourd at p≤0.01 on sensory evaluation.
The fresh-cut bitter gourd packaged in LDPE film extended the shelf life up to 15 days whereas in PP film it was 12 days by storing it at low temperature (8±2?C) with minimum change in its quality by modified atmospheric packaging.
The authors have not declared any conflict of interest.